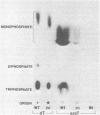
Abstract
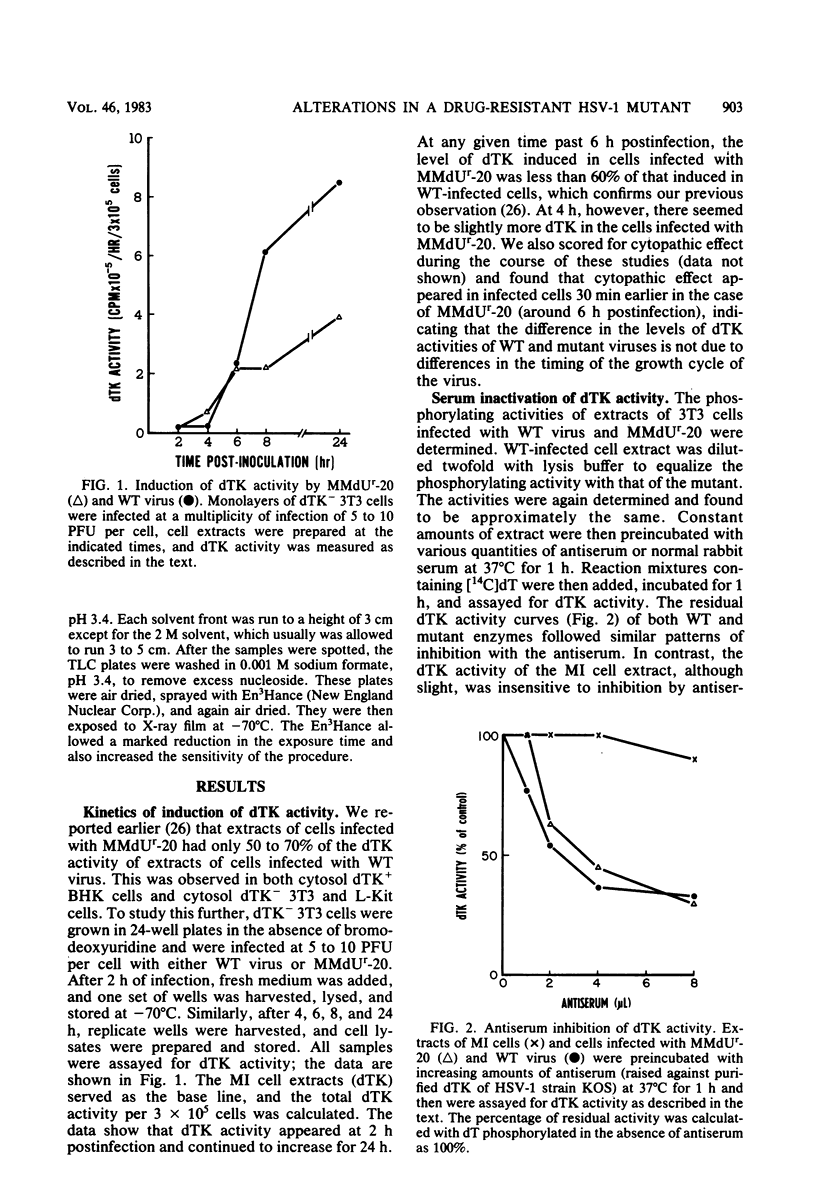
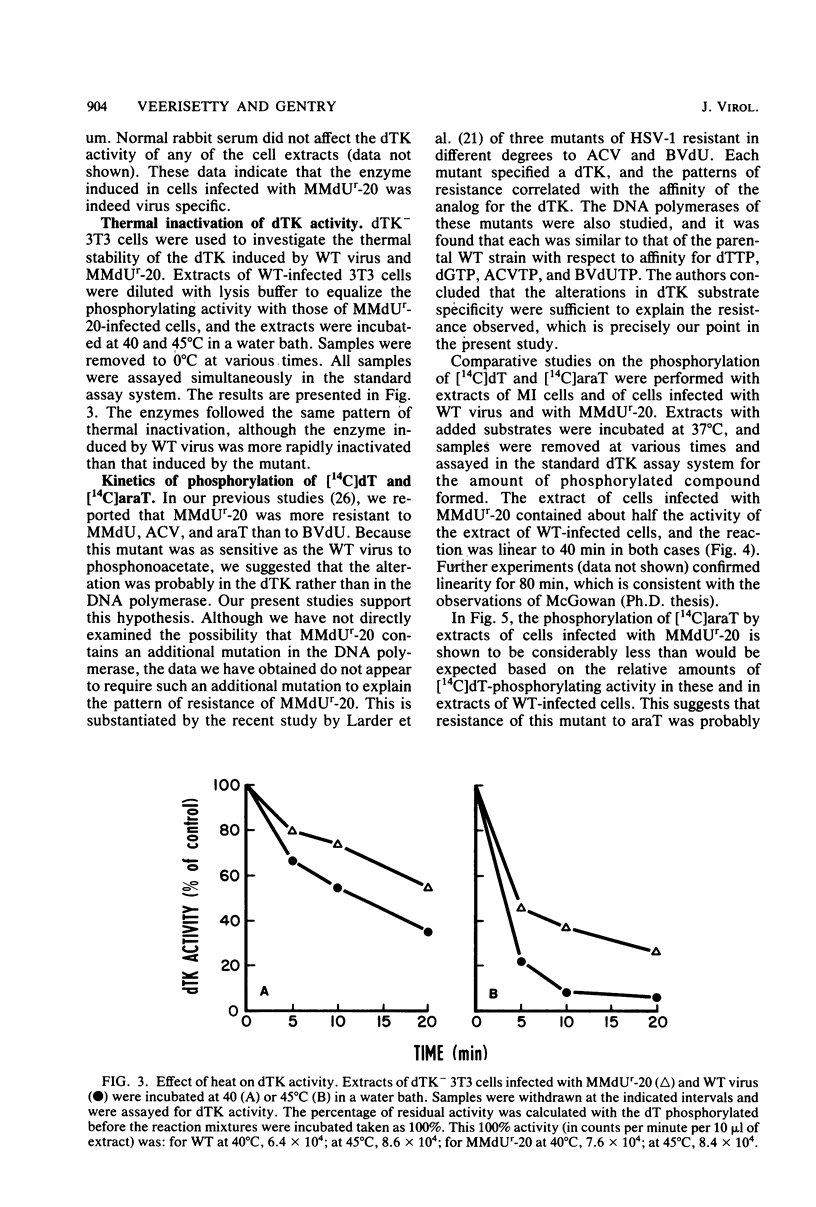
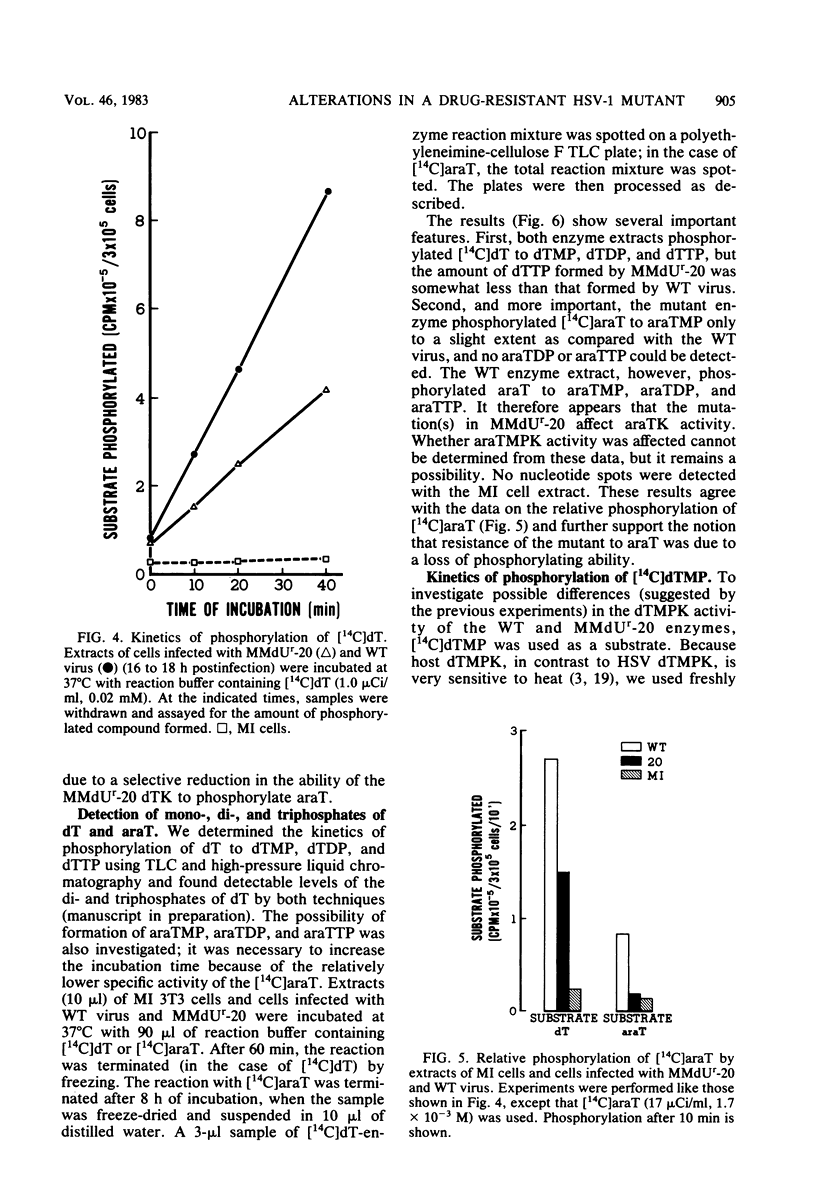
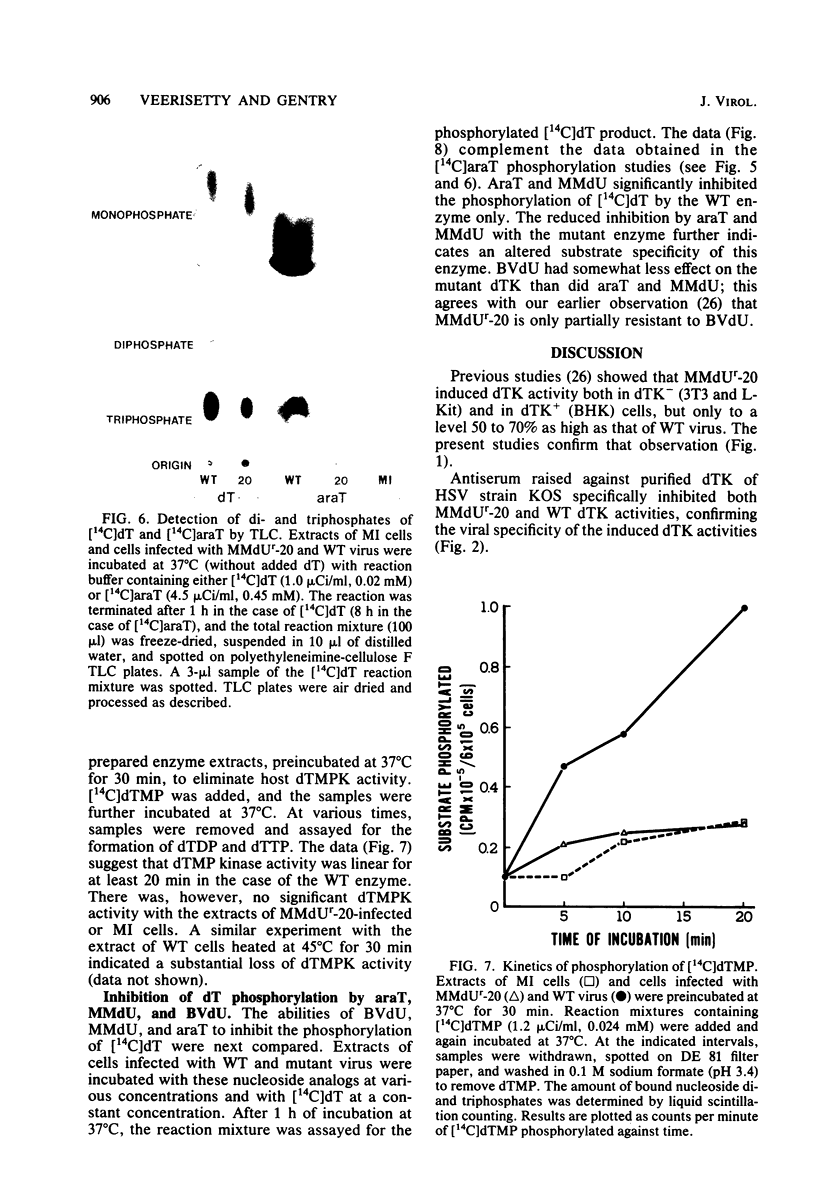
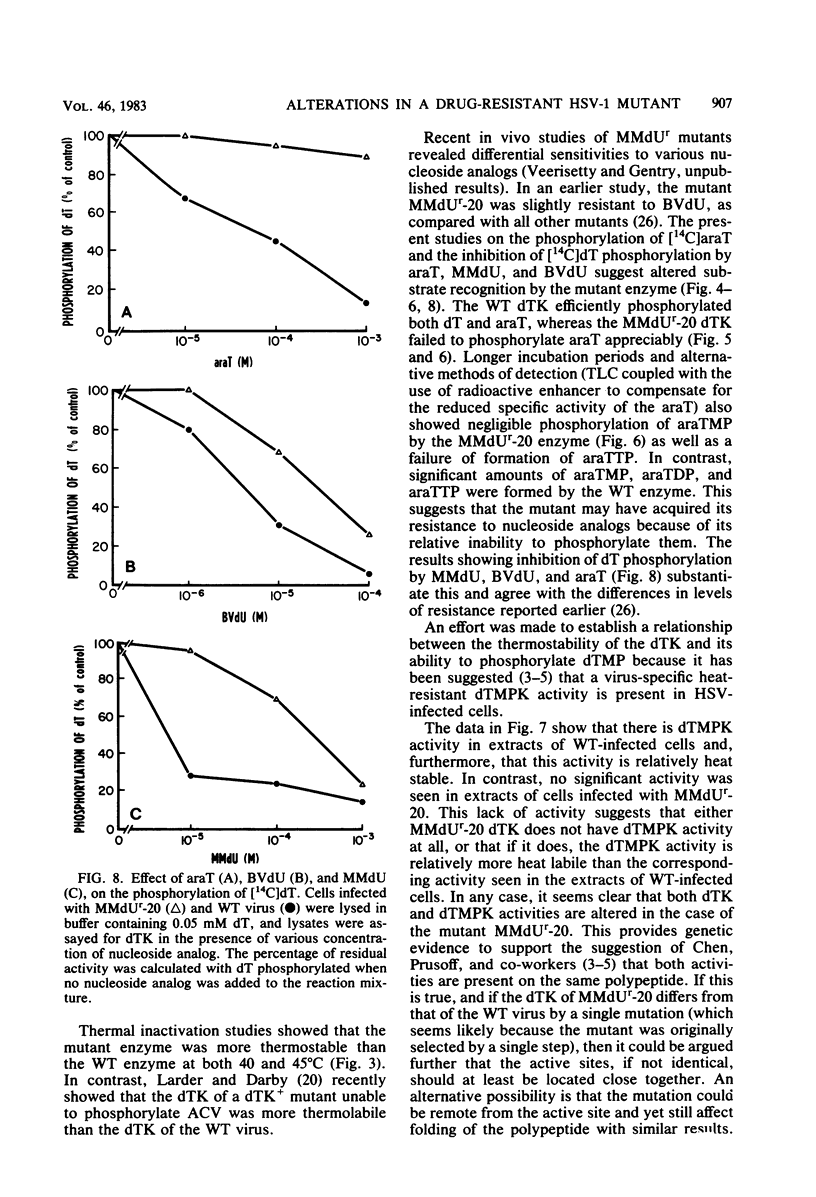
The deoxythymidine kinase (dTK) activity of a 5-methoxymethyldeoxyuridine-resistant mutant (MMdUr-20) of herpes simplex virus type 1 was compared with that of the parental wild-type (WT) virus. The dTK activity induced by the mutant was consistently less than that induced by the WT virus, was inhibited by antibody specific for herpes simplex virus dTK, and was more thermostable than the WT dTK. Further, it was inhibited to a lesser degree than the WT dTK by the nucleoside analogs MMdU and arabinosylthymine (araT), which suggests that one of the effects of the mutation was a selective alteration in substrate recognition by the dTK. The loss of ability to inhibit the mutant dTK by E-(2)-5-bromovinyldeoxyuridine was not as great as that seen with araT and MMdU. This agrees well with our previous observation that the MMdUr-20 mutant of herpes simplex virus is only partially resistant to this analog, as compared with araT and MMdU (V. Veerisetty and G. A. Gentry, Virology 114:576-579, 1981). [2-14C]araT was used to explore further the resistance to araT. Extracts of cells infected with the mutant, although producing a small amount of [14C]araTMP, were unable to produce [14C]araTTP, in contrast to extracts of cells infected with the WT virus. Both extracts, however, produced [14C]dTTP from [14C]deoxyribosylthymine. Finally, the ability of the extracts to phosphorylate [14C]dTMP was examined. It was found that this activity was greatly reduced relative to dTK activity in the case of the mutant. These findings suggest that a mutation in the dTK polypeptide has affected recognition not only of nucleoside substrates but of the nucleotide substrate dTMP as well, which agrees with the suggestion of Chen et al. that both activities are located on the same polypeptide (M. S. Chen and W. H. Prusoff, J. Biol. Chem. 253:1325-1327, 1978; M. S. Chen, J. Walker, and W. H. Prusoff, J. Biol. Chem. 254:10747-10753, 1979; M. S. Chen, W. P. Summers, J. Walker, W. C. Summers, and W. H. Prusoff, J. Virol. 30:942-945, 1979).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allaudeen H. S., Kozarich J. W., Bertino J. R., De Clercq E. On the mechanism of selective inhibition of herpesvirus replication by (E)-5-(2-bromovinyl)-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1981 May;78(5):2698–2702. doi: 10.1073/pnas.78.5.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns W. H., Saral R., Santos G. W., Laskin O. L., Lietman P. S., McLaren C., Barry D. W. Isolation and characterisation of resistant Herpes simplex virus after acyclovir therapy. Lancet. 1982 Feb 20;1(8269):421–423. doi: 10.1016/s0140-6736(82)91620-8. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Prusoff W. H. Association of thymidylate kinase activity with pyrimidine deoxyribonucleoside kinase induced by herpes simplex virus. J Biol Chem. 1978 Mar 10;253(5):1325–1327. [PubMed] [Google Scholar]
- Chen M. S., Summers W. P., Walker J., Summers W. C., Prusoff W. H. Characterization of pyrimidine deoxyribonucleoside kinase (thymidine kinase) and thymidylate kinase as a multifunctional enzyme in cells transformed by herpes simplex virus type 1 and in cells infected with mutant strains of herpes simplex virus. J Virol. 1979 Jun;30(3):942–945. doi: 10.1128/jvi.30.3.942-945.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. S., Walker J., Prusoff W. H. Kinetic studies of herpes simplex virus type 1-encoded thymidine and thymidylate kinase, a multifunctional enzyme. J Biol Chem. 1979 Nov 10;254(21):10747–10753. [PubMed] [Google Scholar]
- Cheng Y. C. Deoxythymidine kinase induced in the HELA TK- cells by herpes simplex virus type I and type II. Substrate specificity and kinetic behavior. Biochim Biophys Acta. 1976 Dec 8;452(2):370–381. doi: 10.1016/0005-2744(76)90186-8. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Marlowe S. I., Kowalsky P. N., Hershey B. J., Levin M. J. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N Engl J Med. 1982 Feb 11;306(6):343–346. doi: 10.1056/NEJM198202113060606. [DOI] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. MUTANT STRAINS OF HERPES SIMPLEX DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Apr;22:493–502. doi: 10.1016/0042-6822(64)90070-4. [DOI] [PubMed] [Google Scholar]
- Darby G., Field H. J., Salisbury S. A. Altered substrate specificity of herpes simplex virus thymidine kinase confers acyclovir-resistance. Nature. 1981 Jan 1;289(5793):81–83. doi: 10.1038/289081a0. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., Verhelst G., Walker R. T., Jones A. S., Torrence P. F., Shugar D. Comparative efficacy of antiherpes drugs against different strains of herpes simplex virus. J Infect Dis. 1980 May;141(5):563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]
- De Clercq E. New trends in antiviral chemotherapy. Arch Int Physiol Biochim. 1979 May;87(2):353–395. [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Darby G. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob Agents Chemother. 1980 Feb;17(2):209–216. doi: 10.1128/aac.17.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Darby G., Wildy P. Isolation and characterization of acyclovir-resistant mutants of herpes simplex virus. J Gen Virol. 1980 Jul;49(1):115–124. doi: 10.1099/0022-1317-49-1-115. [DOI] [PubMed] [Google Scholar]
- Gentry G. A., Allen G. P., Holton R., Nevins R. B., McGowan J. J., Veerisetty V. Thymine salvage, mitochondria, and the evolution of the herpesviruses. Intervirology. 1983;19(2):67–76. doi: 10.1159/000149340. [DOI] [PubMed] [Google Scholar]
- Gentry G. A., Aswell J. F. Inhibition of herpes simplex virus replication by araT. Virology. 1975 May;65(1):294–296. doi: 10.1016/0042-6822(75)90034-3. [DOI] [PubMed] [Google Scholar]
- Hirano A., Yumura K., Kurimura T., Katsumoto T., Moriyama H., Manabe R. Analysis of herpes simplex virus isolated from patients with recurrent herpes keratitis exhibiting "treatment-resistance" to 5-iodo-2'-deoxyuridine. Acta Virol. 1979 May;23(3):226–230. [PubMed] [Google Scholar]
- IVES D. H., MORSE P. A., Jr, POTTER V. R. Feedback inhibition of thymodine kinase by thymodine triphosphate. J Biol Chem. 1963 Apr;238:1467–1474. [PubMed] [Google Scholar]
- Larder B. A., Darby G. Properties of a novel thymidine kinase induced by an acyclovir-resistant herpes simplex virus type 1 mutant. J Virol. 1982 May;42(2):649–658. doi: 10.1128/jvi.42.2.649-658.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Derse D., Cheng Y. C., Darby G. Properties of purified enzymes induced by pathogenic drug-resistant mutants of herpes simplex virus. Evidence for virus variants expressing normal DNA polymerase and altered thymidine kinase. J Biol Chem. 1983 Feb 10;258(3):2027–2033. [PubMed] [Google Scholar]
- Parris D. S., Harrington J. E. Herpes simplex virus variants restraint to high concentrations of acyclovir exist in clinical isolates. Antimicrob Agents Chemother. 1982 Jul;22(1):71–77. doi: 10.1128/aac.22.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. P., Wagner M., Summers W. C. Possible peptide chain termination mutants in thymide kinase gene of a mammalian virus, herpes simplex virus. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4081–4084. doi: 10.1073/pnas.72.10.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Veerisetty V., Gentry G. A. 5-Methoxymethyldeoxyuridine-resistant mutants of herpes simplex virus type 1. Virology. 1981 Oct 30;114(2):576–579. doi: 10.1016/0042-6822(81)90239-7. [DOI] [PubMed] [Google Scholar]