Abstract
The prototype strain of minute virus of mice and the immunosuppressive strain are unable to grow lytically in each other's murine host cell type. To characterize these strain-dependent virus-host cell interactions further, we have compared the early events of both productive and restrictive infections. Each virus binds to specific receptors on the surface of both productive and restrictive cell types. Competition experiments show that both viruses recognize the same receptor on each cell type. Penetration and uncoating are presumed to be similar in both productive and restrictive infections, since incoming viral genomes are converted to parental replicative form DNA independent of the final outcome of the virus-host cell interaction. In contrast to the majority of other systems studied to date, these differences in minute virus of mice target cell specificity are not mediated at the cell surface, but by the interaction of a strain-specific viral determinant with intracellular host factors that are expressed in particular cell types as a function of differentiation. These cellular factors catalyze a step in viral replication which occurs after the initiation of viral DNA synthesis, but before the detectable expression of the viral capsid polypeptide genes.
Full text
PDF

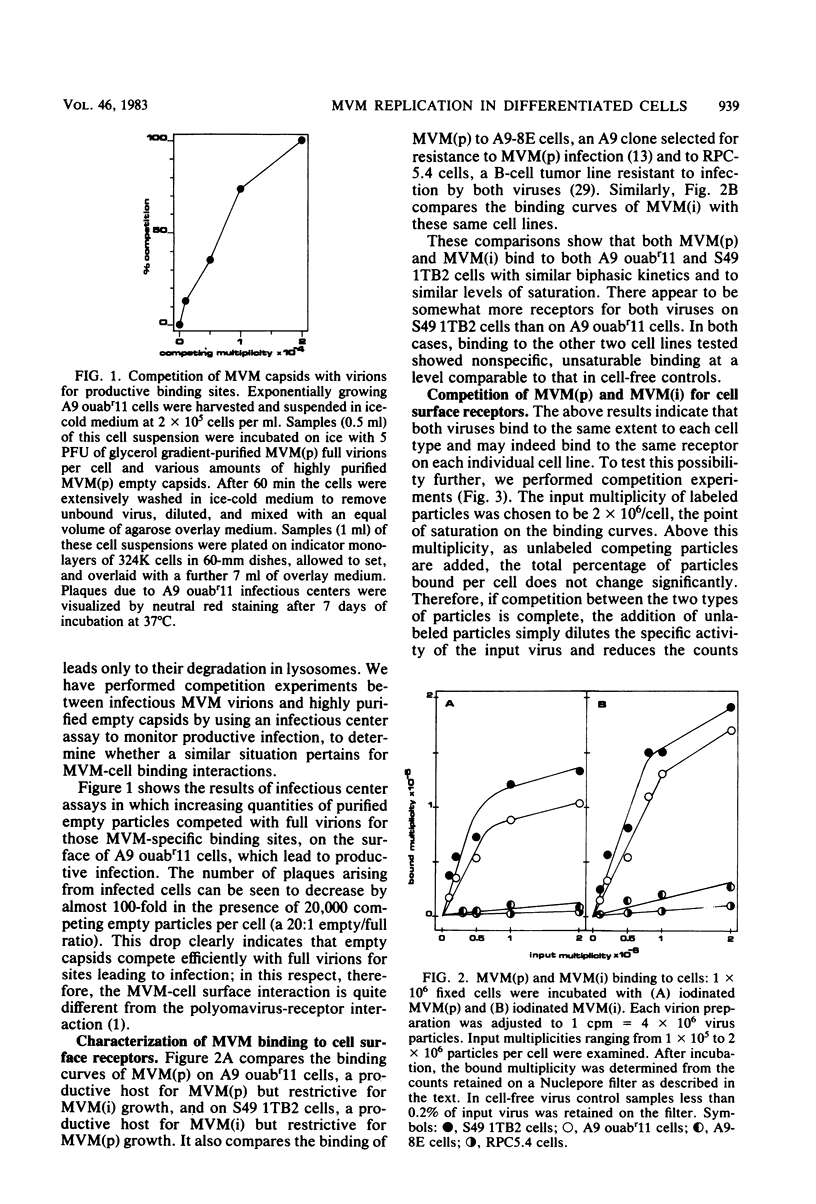
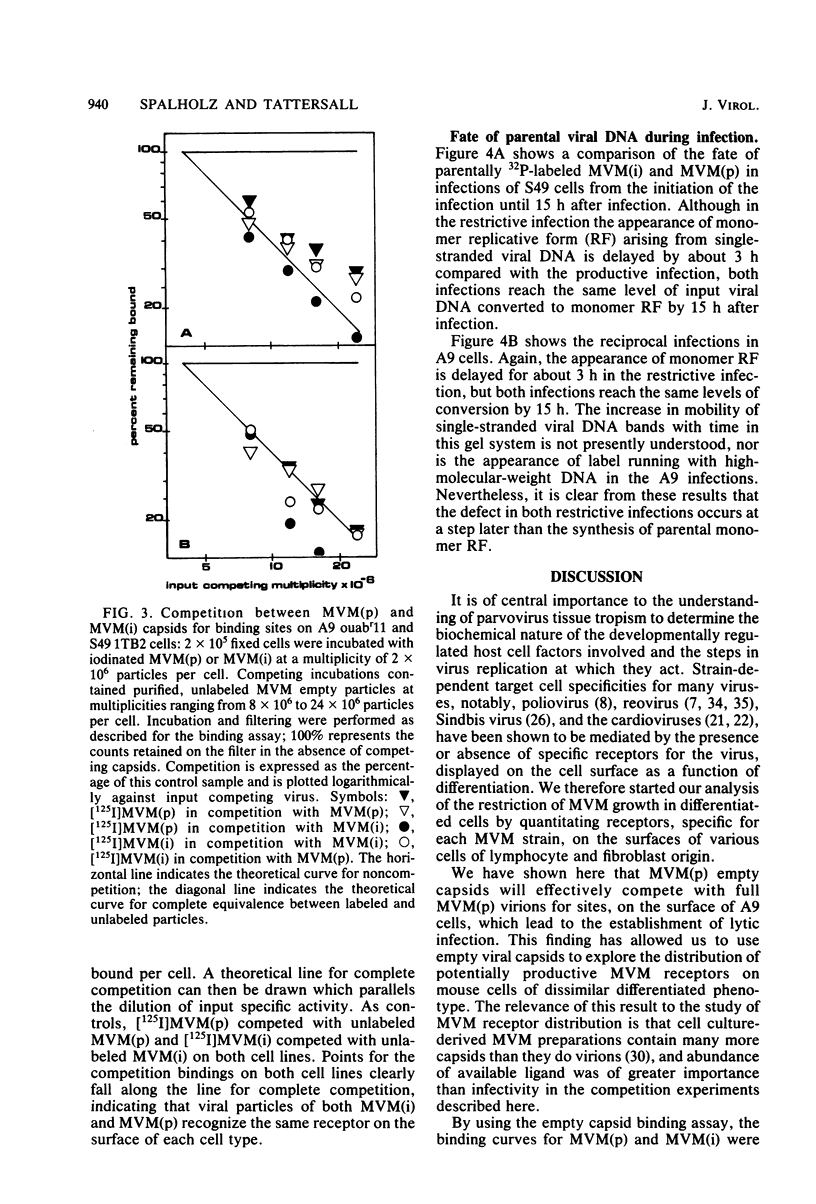
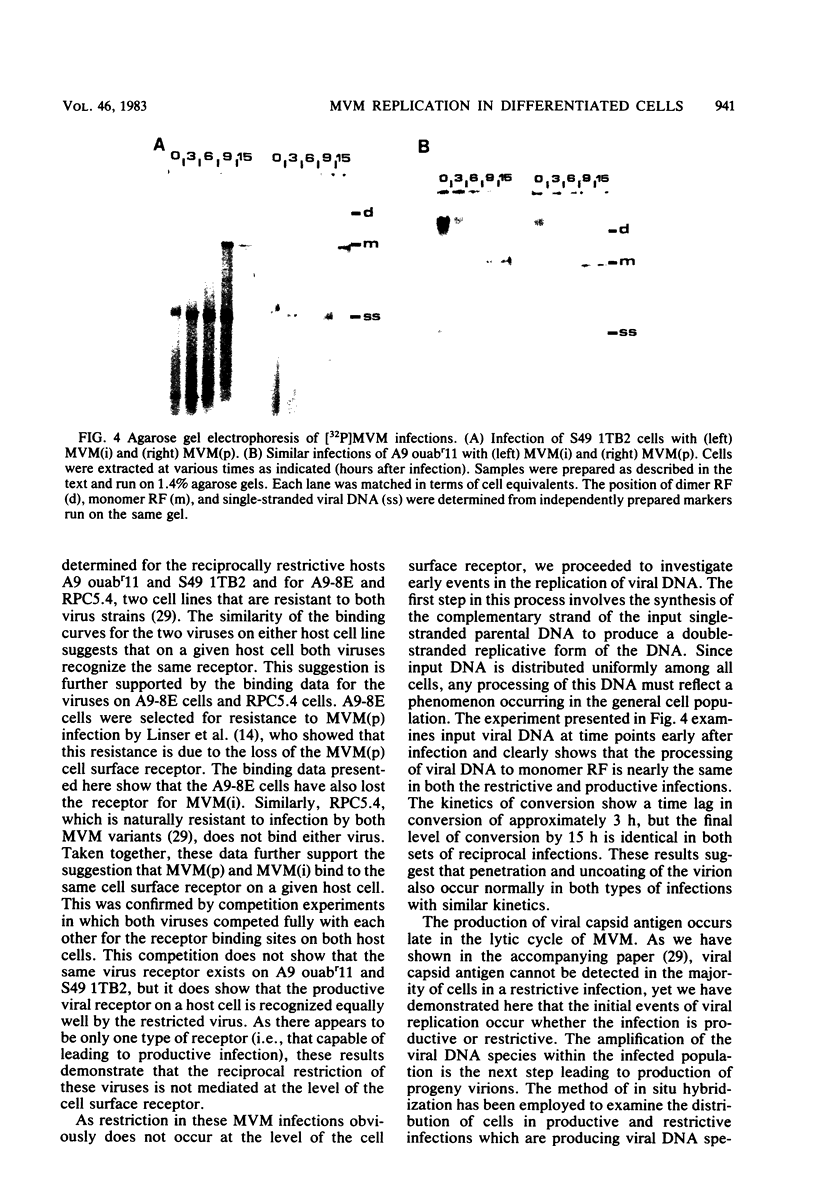


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolen J. B., Consigli R. A. Differential adsorption of polyoma virions and capsids to mouse kidney cells and guinea pig erythrocytes. J Virol. 1979 Nov;32(2):679–683. doi: 10.1128/jvi.32.2.679-683.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnard G. D., Manders E. K., Campbell D. A., Jr, Herberman R. B., Collins M. J., Jr Immunosuppressive activity of a subline of the mouse EL-4 lymphoma. Evidence for minute virus of mice causing the inhibition. J Exp Med. 1976 Jan 1;143(1):187–205. doi: 10.1084/jem.143.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D., Brownlee S. M. Peptide mapping of proteins from acrylamide gels. Anal Biochem. 1973 Sep;55(1):213–221. doi: 10.1016/0003-2697(73)90306-0. [DOI] [PubMed] [Google Scholar]
- Campbell D. A., Jr, Staal S. P., Manders E. K., Bonnard G. D., Oldham R. K., Salzman L. A., Herberman R. B. Inhibition of in vitro lymphoproliferative responses by in vivo passaged rat 13762 mammary adenocarcinoma cells. II. Evidenceth Kilham rat virus is responsible for the inhibitory effect. Cell Immunol. 1977 Oct;33(2):378–391. doi: 10.1016/0008-8749(77)90166-6. [DOI] [PubMed] [Google Scholar]
- Engers H. D., Louis J. A., Zubler R. H., Hirt B. Inhibition of T cell-mediated functions by MVM(i), a parvovirus closely related to minute virus of mice. J Immunol. 1981 Dec;127(6):2280–2285. [PubMed] [Google Scholar]
- Feunteun J., Benjamin T. L. Isolation of transformation-defective host-range mutants of polyoma virus on normal mouse cells. Virology. 1982 Jun;119(2):310–316. doi: 10.1016/0042-6822(82)90091-5. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Greene M. I. Genetic and molecular mechanisms of viral pathogenesis: implications for prevention and treatment. Nature. 1982 Nov 4;300(5887):19–23. doi: 10.1038/300019a0. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. Receptor affinities as major determinants of enterovirus tissue tropisms in humans. Virology. 1961 Nov;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- Kilham L., Margolis G. Problems of human concern arising from animal models of intrauterine and neonatal infections due to viruses: a review. I. Introduction and virologic studies. Prog Med Virol. 1975;20:113–143. [PubMed] [Google Scholar]
- Kilham L., Margolis G. Spontaneous hepatitis and cerebellar "hypoplasia" in suckling rats due to congenital infections with rat virus. Am J Pathol. 1966 Sep;49(3):457–475. [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The nature of the host range restriction of SV40 and polyoma viruses in embryonal carcinoma cells. Curr Top Microbiol Immunol. 1982;101:1–30. doi: 10.1007/978-3-642-68654-2_1. [DOI] [PubMed] [Google Scholar]
- Linser P., Bruning H., Armentrout R. W. Specific binding sites for a parvovirus, minute virus of mice, on cultured mouse cells. J Virol. 1977 Oct;24(1):211–221. doi: 10.1128/jvi.24.1.211-221.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Johnson R. T. The pathogenesis of rat virus infections in the newborn hamster. Lab Invest. 1972 Nov;27(5):508–513. [PubMed] [Google Scholar]
- Lum G. S. Serological studies of rat viruses in relation to tumors. Oncology. 1970;24(5):335–343. doi: 10.1159/000224534. [DOI] [PubMed] [Google Scholar]
- Margolis G., Kilham L. Problems of human concern arising from animal models of intrauterine and neonatal infections due to viruses: a review. II. Pathologic studies. Prog Med Virol. 1975;20:144–179. [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. A., Ward D. C., Ruddle F. H. Embryonal carcinoma cells (and their somatic cell hybrids) are resistant to infection by the murine parvovirus MVM, which does infect other teratocarcinoma-derived cell lines. J Cell Physiol. 1977 Jun;91(3):393–401. doi: 10.1002/jcp.1040910309. [DOI] [PubMed] [Google Scholar]
- Mohanty S. B., Bachmann P. A. Susceptibility of fertilized mouse eggs to minute virus of mice. Infect Immun. 1974 Apr;9(4):762–763. doi: 10.1128/iai.9.4.762-763.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima T., McClintock P. R., Aulakh G. S., Billups L. C., Notkins A. L. Genomic and receptor attachment differences between mengovirus and encephalomyocarditis virus. Virology. 1982 Oct 30;122(2):461–465. doi: 10.1016/0042-6822(82)90245-8. [DOI] [PubMed] [Google Scholar]
- Morishima T., McClintock P. R., Billups L. C., Notkins A. L. Expression and modulation of virus receptors on lymphoid and myeloid cells: relationship to infectivity. Virology. 1982 Jan 30;116(2):605–618. doi: 10.1016/0042-6822(82)90152-0. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Tignor G. H. Host cell receptors for two strains of Sindbis virus. Arch Virol. 1980;66(1):11–26. doi: 10.1007/BF01315041. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983 Jun;46(3):944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Layman K. R., Hand R. E. Effect of cell physiological state on infection by rat virus. J Virol. 1969 Dec;4(6):872–878. doi: 10.1128/jvi.4.6.872-878.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Ault K. A., Fields B. N. Interaction of reovirus with cell surface receptors. I. Murine and human lymphocytes have a receptor for the hemagglutinin of reovirus type 3. J Immunol. 1980 May;124(5):2143–2148. [PubMed] [Google Scholar]
- Weiner H. L., Drayna D., Averill D. R., Jr, Fields B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]