Abstract
Cocaine is known to enhance nucleus accumbens dopamine (NAcc DA), serve as a positive reinforcer and produce negative effects, such as anxiety. The influence of diazepam on cocaine intake, cocaine-stimulated behavioral activity and NAcc DA was investigated using self-administration and experimenter-administered intravenous (i.v.) cocaine. In Experiment 1, rats were pretreated with diazepam (0.25 mg/kg) or saline (0.1 ml) 30 minutes prior to 20 daily 1-hr cocaine (0.75 mg/kg/inj) self-administration sessions. Cocaine intake increased for all animals across sessions, but was highest in diazepam-pretreated animals. Diazepam rats also self-administered their first cocaine injection of each session faster than controls. Experiment 2 utilized in vivo microdialysis to assess NAcc DA levels before and after experimenter-administered i.v. cocaine injections (0.75 mg/kg/injection X 2; 10-min interval) in diazepam- and saline-pretreated rats. Group differences were not revealed across basal and cocaine-stimulated NAcc DA assessments, indicating that diazepam did not decrease NAcc DA during cocaine self-administration. Findings that diazepam enhances cocaine self-administration and decreases cocaine response latency support the notion that cocaine-induced anxiety limits voluntary cocaine intake. It is further suggested that individual variations in cocaine-induced aversive effects may determine whether cocaine use is avoided or repeated.
Keywords: Anxiety, drug abuse, in vivo microdialysis, behavior, addiction
Dopamine (DA) neural activation is associated with pleasurable effects of natural and drug-induced reward (Carelli, 2004; Kelley and Berridge, 2002) as well as aversive emotional states, such as fear and stress (Adinoff, 2004; Pezze and Feldon, 2004; Pruessner et al., 2004). This dichotomy may be epitomized during cocaine use, as the motivational effects of cocaine seeking are highly correlated with elevated nucleus accumbens (NAcc) terminal DA (Kiyatkin et al., 2000; Phillips et al., 2003), yet positive effects are often accompanied by an opposing state of anxiety. Indeed, human users have commonly reported that anxiety and panic attacks occurred after initial pleasurable feelings elicited by cocaine (Bystritsky et al., 1991; Cox et al., 1990; Geracioti and Post, 1991; Gunnarsdottir et al., 2000; Walfish et al., 1990). Cocaine-induced anxiogenic effects are also demonstrated with animal behavioral models, where certain conditions of cocaine administration result in approach/avoidance and escape, as well as defensive postures and activities, such as crouching and directed sniffing (Blanchard and Blanchard, 1999; Blanchard et al., 1998; Devries and Pert, 1998; Ettenberg, 2004; Ettenberg and Geist, 1991; Paine et al., 2002).
Anxiety and fear denote expectations of danger (Delgado et al., 2006) and lead to danger avoidance, as a means for increasing survival. In regards to cocaine use, self-protective feelings, including cocaine-induced anxiety and panic, may be defense mechanisms promoting anti-addictive behavior (David et al., 2001). Therefore, an individual’s emotional response to cocaine may determine whether initial cocaine use is followed by repeated, increased or termination of drug-taking behavior. If anxiety works to decrease cocaine intake, it is therefore conceivable that treatments blocking anxiogenic cocaine effects may lead to repetitive cocaine use, and serve as “pro-addictive” agents that could facilitate the transition from recreational user to cocaine abuser.
Benzodiazepines, a class of drugs with sedative-hypnotic, muscle-relaxant, anxiolytic and anticonvulsant properties (Charney et al., 2001) have been used for the treatment of cocaine-induced toxicity and seizures (Smith and Landry, 1990; Spivey and Euerle, 1990). Benzodiazepines can also reduce cocaine-induced anxiogenic behaviors in animals (David, et al., 2001; Ettenberg and Geist, 1991; Paine, et al., 2002) and are known to be co-administered with cocaine in human users (Wolf et al., 2005). Though benzodiazepine use alone is rarely lethal, post-mortem evidence indicates that benzodiazepine and cocaine co-administration can result in increased cocaine intake, toxicity and mortality (Wolf, et al., 2005).
The present study was conducted to determine the influence of the benzodiazepine, diazepam, on cocaine self-administration and cocaine-induced DA enhancement in the NAcc. In Experiment 1, cocaine self-administration rates, latency to self-administer the first cocaine injection of the session and behavioral activity were assessed across twenty (20) daily cocaine self-administration sessions. Experiment 2 utilized in vivo microdialysis procedures to determine whether diazepam affects basal or cocaine-stimulated NAcc DA levels.
1. Materials and methods
1.1 Animals
Male Sprague Dawley rats, weighing approximately 250 g at the start of each experiment were used. Ad libitum access to food and water was provided, except during acquisition of food-reinforced operant training. The temperature in the colony was maintained at 20° C and animals were kept on a 12-hour reverse light/dark cycle (lights off at 9 am). Rats were handled for 10 min daily for 2 weeks prior to the start of operant training. The experimental protocol was approved by the University of Texas Institutional Animal Care and Use Committee (IACUC) in compliance with NIH standards.
1.2 Apparatus
Food training, self-administration and in vivo microdialysis sessions for Experiments 1 and 2 were conducted in identical one-lever operant chambers (28 x 22 x 21 cm) within sound-attenuating boxes (Med Assoc, St. Albans, VT). The Plexiglas chambers contained a single retractable lever on the right wall, with a stimulus light directly above the lever and a house light on the opposite wall. Houselights were illuminated to signal the start of the sessions and availability of reinforcement. The stimulus light above the lever was activated with each lever response. Three pairs of photobeams monitored locomotor activity: one in the center and two others, each located 5 cm from the chamber walls. Interruptions between photobeam pairs were assessed as units of locomotor activity. Cocaine injections were delivered via 10 ml syringe positioned in a pump that was connected by tubing to a single-channel swivel mounted on a counterbalanced arm above each chamber. The tubing was connected to the animal using a spring-covered tether that screwed onto the jugular catheter endpiece located on the animal’s head (Plastics One, Roanoke, VA). Operant programs and data collection was controlled by a MED P4 Intel computer system using MED-PC Software (Med Assoc, St. Albans, VT).
1.3 Food Training
Following a handling period of two weeks, all animals were food restricted and trained in the operant chambers to lever press for sucrose pellets (45 mg, P. J. Noyes, Lancaster, NH) on a fixed ratio-1 (FR-1) schedule of reinforcement. After the lever press response was acquired, 10-min daily operant training sessions were continued for a minimum of 6 days without food restriction.
1.4 Surgical procedures
After completion of operant training, all animals were implanted with a jugular catheter. Animals in Experiment 2 were also implanted with an intracranial guide cannula aimed above the NAcc to accommodate the insertion of a microdialysis probe one day before the test day. Rats were anesthetized with pentobarbital sodium (Nembutal®; 50 mg/kg, i.p.). Atropine sulfate (80 μg/rat, s.c.) was given prophylactically to prevent respiratory secretions. Supplemental chloral hydrate (100 mg/kg, i.p.) was given, if necessary, to prolong anesthesia. A Silastic catheter (0.625 mm o.d.) was inserted into the right jugular vein and advanced into the right atrium. The distal end of the catheter was connected to a cannula endpiece (Plastics One, Roanoke, VA), which was routed subcutaneously along the side of the neck and out an incision on the head. In Experiment 2, rats were implanted with unilateral cannula (22 g; Plastics One, Roanoke, VA) using the following stereotaxic coordinates for the NAcc in relation to bregma (flat skull position): AP: +1.7 mm; ML: ±1.7 mm; DV: −2.5 mm. These NAcc coordinates were chosen from experiments previously conducted in our laboratory (D’Souza and Duvauchelle, 2006). The cannula end of the jugular catheter and the NAcc cannula were both affixed to the skull with dental acrylic. Stainless steel obturators were placed into the NAcc cannulae and moved daily to prevent obstruction. Gentamicin sulfate (50 mg/ml) was topically applied into the jugular incision to prevent infection. Rimadyl (5 mg/kg, s.c.) was given as a post-surgical analgesic. On days 1–7 post-surgery, jugular catheters were flushed daily with 0.1 ml of a saline solution containing 1U/ml heparin, 1000 U/ml streptokinase (Streptase®), and 67 mg/ml of the antibiotic Timentin. After day 7, catheters were flushed daily with the same solution minus the Timentin component. Animals were allowed to recover for one week prior to cocaine self-administration sessions.
1.5 Drugs and Groups
Diazepam (0.25 mg/kg; Abbot Laboratories, North Chicago, IL) was delivered through the intravenous catheter in volumes ranging from 14–19 μl followed by a 0.1 ml heparin/saline flush. Cocaine HCl (0.75 mg/kg/inj; RTI International, Triangle Park, NC) was diluted in sterile 0.9% sodium chloride. Concentrations were adjusted according to individual weights so that each self-administered cocaine injection was delivered in a volume of 0.1 ml. Experiments 1 and 2 each consisted of two drug treatment groups: 1) Vehicle + Cocaine, and 2) Diazepam + Cocaine. These groups were treated with either saline (0.1 ml, i.v.) or diazepam (0.25 mg/kg, i.v.) prior to cocaine administration. Pilot work in our lab found that this particular diazepam dose reduced rat abdominal muscle tension, but did not visibly affect baseline locomotor activity.
1.6 Experiment 1: Cocaine Self-Administration
One week after surgery, animals in Experiment 1 underwent daily self-administration sessions. Animals were taken from their home cage, given their assigned pretreatment and placed back into the home cage for 30 min. Animals were then placed into the operant chamber and the session commenced immediately with the illumination of the houselight. Cocaine self-administration was maintained on a FR-1 schedule of reinforcement, with each response resulting in a 0.75 mg/kg cocaine infusion through the intravenous catheter. Each cocaine injection was infused over 6 sec, during which time the stimulus lamp was illuminated. After each infusion, there was a 20-sec “time-out” period, during which time the lever was retracted and no infusions could be delivered. Self-administration sessions were one hour in duration with a limit of 29 responses per session. In cases that maximum lever responses were obtained prior to the end of the 1-hr session, the house light turned off and the animal was removed from the chamber. After each session, the indwelling jugular catheters were flushed with 0.05 ml solution of streptokinase/heparin (see Surgery). Self-administration sessions were conducted between 10 am and 2 pm daily, 5 days/week, for a total time of 20 days (4 weeks). For Experiment 1, the number of lever responses, response latency (for first cocaine injection), and locomotor activity measures (photobeam breakages) were assessed daily and recorded by MED-PC software.
1.7 Experiment 2: Cocaine-stimulated extracellular NAcc DA
1.7.1 In Vitro Recovery Calibration
Microdialysis probes were constructed as previously described (Duvauchelle et al., 2000), with an active membrane length of 2.5 mm at the probe tip. Prior to probe recovery, all probes were flushed with nanopure water. On the day of probe calibration, 1.0 ml gastight Hamilton 1000 series syringes were filled with freshly prepared filtered Ringer’s solution (128.3 mM NaCl, 1.35 mM CaCl2, 2.68 mM KCl, and 2.0 mM MgCl2), and pumped through the probe at 1.6 μl/min. The probe tips were kept in a beaker with Ringer’s solution containing ascorbic acid (1.0%) and 5 nM DA, maintained at 37° C. Two 10-min samples from each probe were collected and assayed by high performance liquid chromatography with electrochemical detection (HPLC-EC). Probe recoveries were calculated by comparing the average peak heights of the two probe samples to those from a 25% dilution standard (1.25 nM DA). The mean (± SEM) recovery of probes used in the experiment was 14.75 (±0.51).
1.7.2 Microdialysis Probe Implantation
After a 1-week recovery from jugular catheter/stereotaxic surgery, cocaine-naïve animals in Experiment 2 were briefly anesthetized with 1.5% isoflurane and implanted with a microdialysis probe through the previously implanted NAcc guide cannula. Each microdialysis probe was connected to a 1.0 ml gastight Hamilton 1000 series syringe mounted on a syringe pump (Razel®, Model A), and freshly prepared Ringer’s solution (pH=7.3) was pumped through the probe. Animals implanted with the probe remained in a holding chamber overnight with the syringe pump speed set at 0.2 μl/min. Bedding, food, and water were available in the holding chamber. Two hours prior to the test session, the pump speed was increased to 1.6 μl/min.
1.7.3 NAcc DA Test Session
For the in vivo microdialysis experiment (Experiment 2), microdialysis samples were collected at 10-min intervals for the duration of the 120-min test session. The session progressed as follows: Animals were placed in the operant chamber and three baseline dialysate samples (30-min total) were collected. Animals then received their assigned pretreatment (diazepam or saline through the i.v. catheters), followed by the collection of three pretreatment dialysate samples (30-min total). The final hour of the session consisted of programmed intravenous injections of cocaine (0.75 mg/kg, i.v. X 2; time 0 and 10), with dialysate samples continuously collected.
1.7.4 Assay of dialysate and recovery samples
The dialysate and recovery samples were analyzed for DA using HPLC-EC equipped with Shizeido capcell C-18 narrow bore column, ESA Model 5200 A Coulochem II Detector, a Model 5020 Guard Cell and a Model 5041 amperometric analytical cell. The mobile phase contained 150 mM Na2HPO4, 50 μM EDTA, 4.5 mM ~ 6.0 mM sodium dodecyl sulfate, 4.76 mM citric acid, 12.5 % (v/v) acetonitrile, 12.5 % (v/v) methanol (pH=5.6). The analytical cell potential was set at +200 mV (oxidation). The detection limit for DA was calculated at 0.05 pg with a signal/noise ratio of 3:1. Flow rate was set at 0.2 ml/min and 10 μl samples were manually injected. The amount of DA within each dialysate sample was determined by comparison with standards (DA HCl, Sigma, St. Louis) prepared and analyzed on the day of sample analysis. Data were collected and analyzed using an ESA Model 500 Data station.
1.7.5 Histological analysis
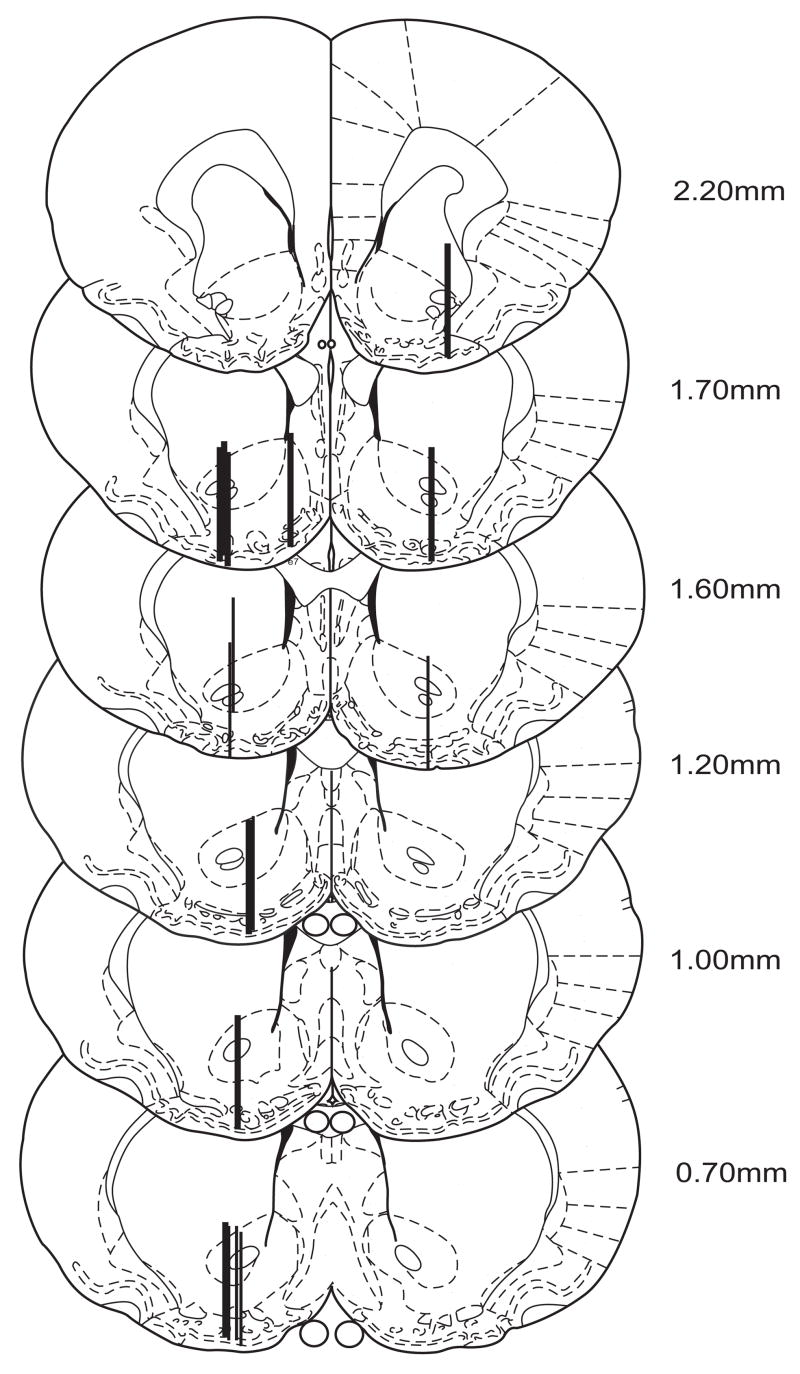
Animals in the dialysis experiment were euthanized by an overdose of pentobarbital sodium and perfused with isotonic saline and 10% formalin. Brains were removed and stored in 10% formaldehyde/30% sucrose solution. The probe placements within NAcc (see Fig 1) were verified from coronal sections (60 μm) stained with cresyl violet using the atlas of Paxinos and Watson (Paxinos and Watson, 1998).
Figure 1.
Locations of dialysis probe membranes within the NAcc (n=16)/Experiment 2. Thickest lines represent probe locations for diazepam-pretreated animals.
1.8 Statistic Analyses
For Experiment 1, the number of lever responses, locomotor activity and cocaine response latency (e.g., elapsed time between lever availability and first self-administered cocaine injection) across daily cocaine self-administration sessions in the diazepam- and saline- pretreated groups were compared using two-way repeated measures ANOVAs. Mean totals (sessions 2–20) of cocaine response latencies were also compared between groups using a two-sample t-test (two-tailed probability). For Experiment 2, extracellular NAcc DA concentrations (nM concentrations corrected according to probe recovery values) were analyzed using a two-way ANOVA with repeated measures at each 10-min interval across the 120-min test session. Since two-way interaction effects were not observed in any of the above ANOVA comparisons, posthoc tests were not performed.
2. Results
2.1 Experiment 1: Lever Responses
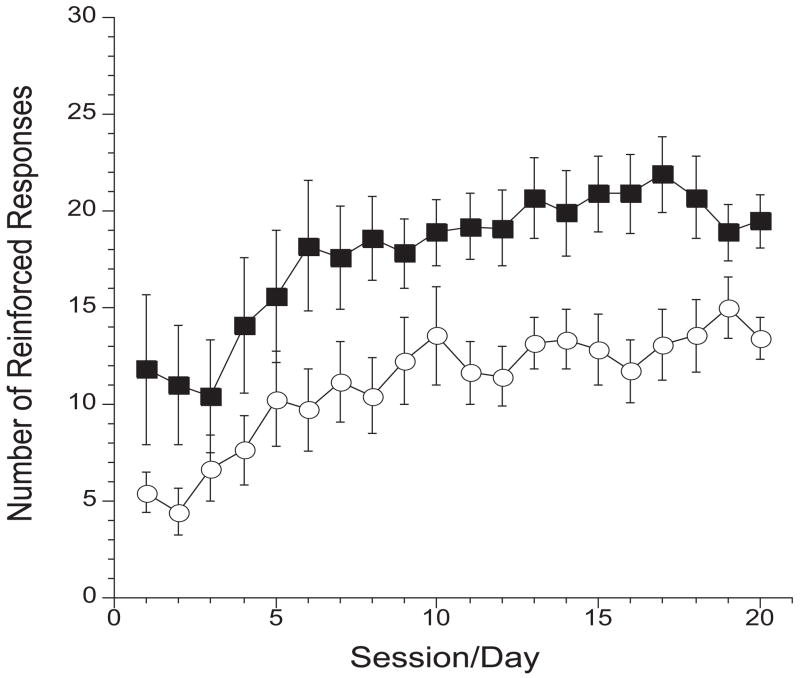
A two-way ANOVA performed on number of lever responses across the 20 daily cocaine self-administration sessions showed significant Group [F(1,19)=8.0368; p=0.01] and Time effects [F(19,361)= 10.8; p<0.0001], but no significant Group X Time Interaction [F(19,361)=0.64; n.s.] (see Fig 2).
Figure 2.
Daily lever responses during cocaine self-administration sessions/Experiment 1. Data represents mean ± SEM of cocaine-reinforced responses (0.75 mg/kg/inj) in diazepam- (filled square; n=10) and saline- (open circle; n=11) pretreated animals.
2.2 Experiment 1: Locomotor Activity
A two-way repeated measures ANOVA across the 20 self-administration sessions showed no significant Group [F(1,19)=1.150; n.s.] or Interaction effects [F(19,361)=1.069; n.s.], but significant effects of Time [F(19,361)=3.75; p<0.0001] (see Fig 3).
Figure 3.
Locomotor activity during cocaine self-administration sessions/Experiment 1. Data represents mean ± SEM of locomotor activity (recorded as number of photobeam breakages within operant chamber) in diazepam- (filled square; n=10) and saline- (open circle; n=11) pretreated animals. No significant group differences in locomotor activity were observed across sessions.
2.3 Experiment 1: Response Latency
This measure was defined as the time interval between the start of each self-administration session (e.g., houselight on and lever available) and the first lever press for cocaine. Latencies were assessed from sessions 2–20, as animals were cocaine-naïve prior to session 1. A two-way repeated measures ANOVA across sessions 2–20 showed significant Group effects [F(1,19)=5.68; p=0.02], but no significant effects of Time or Group X Time Interaction [F(18,342)=1.14 and F(18, 342)=0.78, respectively, both n.s.]. A two-sample t-test for independent groups revealed that the mean response latency for diazepam-pretreated animals was significantly lower than the vehicle-pretreated group [t(19)=2.18; p=0.042] (see Fig 4).
Figure 4.
Cocaine response latency/Experiment 1. Data represents mean ± SEM of elapsed time prior to the first operant response of cocaine self-administration sessions 2–20 in diazepam- (filled square; n=10) and saline- (open circle; n=11) pretreated animals. Insert: Data depicted as mean ± SEM of sessions 2–20. Diazepam pretreated rats responded significantly faster for the first cocaine injection of each session than control animals. * = significant difference at p<0.05.
2.4 Experiment 2: NAcc DA levels
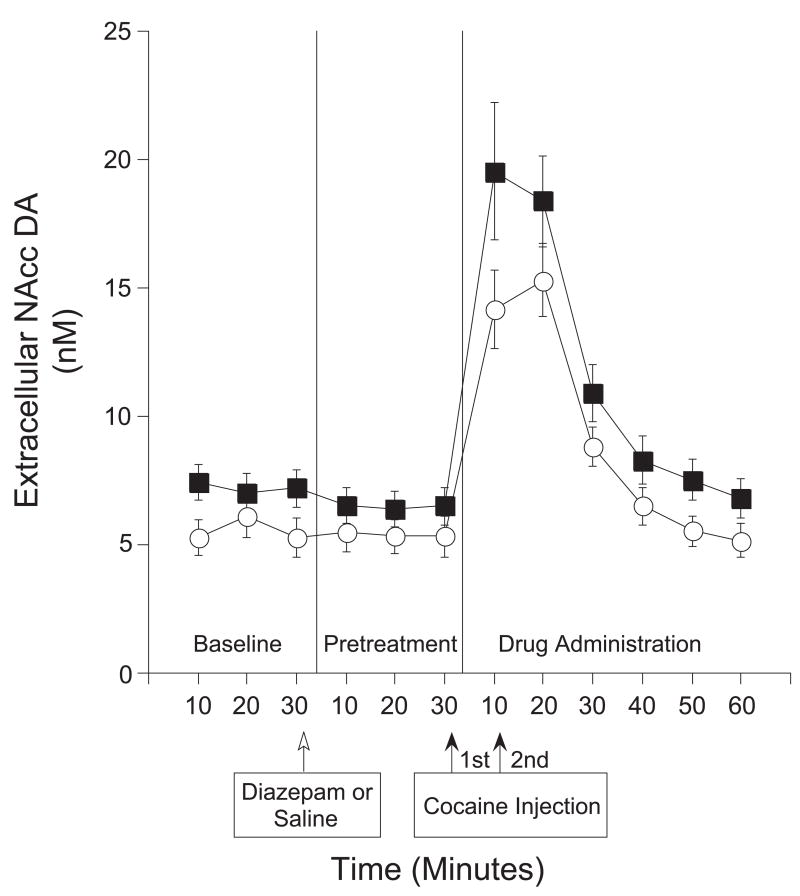
A two-way ANOVA with repeated measures on the 10-min intervals of the test session showed no significant Group [F(1,14)=2.37; n.s] or Group X Time Interaction [F(11,154)=1.53; n.s.], but significant effects of Time [F(11,154)=68.89; p<0.0001] (see Fig 5).
Figure 5.
NAcc DA response to experimenter-administered cocaine injections/Experiment 2. DA expressed in nM concentrations (mean ± SEM). No significant group differences in NAcc DA levels were detected for diazepam (filled square; n=9) and saline (open circle; n=7) pretreated animals.
3. Discussion
Diazepam alleviates cocaine-induced anxiogenic-like behavior in animals (Blanchard and Blanchard, 1999; Blanchard, et al., 1998; Devries and Pert, 1998; Ettenberg and Geist, 1991; Tarr and Macklin, 1987; Wesson and Smith, 1985). In Experiment 1, diazepam-pretreated animals made significantly more lever responses and responded significantly faster to obtain their first cocaine injection of each session compared to control animals. As suggested previously (Ettenberg and Geist, 1991; Geist and Ettenberg, 1997), diazepam treatment may shift the mixed emotional effects of cocaine to the positive side by alleviating negative, anxiogenic effects. Indeed, differences in response rates have often been attributed to changes in reward value and/or reinforcement efficacy (Caine et al., 1995; Hubner and Moreton, 1991; Olmstead et al., 2000). However, based on past findings, increased levels of cocaine-maintained responding, as observed in diazepam-pretreated rats in the present study, may be interpreted as reflecting a decrease (Caine, et al., 1995) or increase (Goeders, 1997) in cocaine reinforcement efficacy. Though it has been argued that reinforcement efficacy cannot be determined through self-administration rates alone (Arnold and Roberts, 1997), if diazepam acting to decrease negative effects of cocaine, the mechanisms for those effects may be distinct from those acting on the positive reinforcing aspects of cocaine. For instance, the tendency of control animals to hesitate longer than diazepam-treated animals in responding for their first cocaine injection during self-administration sessions suggests cocaine-induced anxiety acts to increase response latency. Therefore, as diazepam is proposed to attenuate the anxiogenic properties of cocaine inhibiting self-administration, diazepam treatment makes it possible for a greater number of lever responses to be completed during a session. Diazepam has also been shown to increase responding for punishment-suppressed food (Johnson, 1978; Thiebot et al., 1979) and brain stimulation (Moriyama et al., 1984) and to decrease anxiety associated with open fields and novel objects (Hoplight et al., 2005). Though we observed marked variability in response latency for vehicle-pretreated rats self-administering cocaine (as depicted in Fig 4), taken together, past and present data imply that diazepam enhances behaviors suppressed by a variety of anxiogenic factors.
The present study also observed that both the diazepam- and the saline-pretreated groups progressively increased lever responses for cocaine across sessions. These data concur with previous findings of enhanced cocaine responding during long-term self-administration (Emmett-Oglesby et al., 1993; Liu et al., 2005b). Profound escalation in cocaine responding can also be observed over fewer sessions with longer daily access than provided in the current experiment (Ben-Shahar et al., 2004).
Diazepam is therapeutically used as a sedative/hypnotic agent and is thereby associated with attenuation in motion and performance. Some findings show acute use of diazepam is associated with decreased locomotor activity, and chronic use leads to increased locomotion (Djeridane et al., 2005) or behavioral tolerance to these effects (Fernandes et al., 1996; Mediratta et al., 2001). Others have found a range of effects on locomotor activity, including enhancement at lower doses of diazepam (0.25 mg/kg) (Soderpalm et al., 1991), no effects (Kiyatkin and Bae, 2007) and attenuation at higher doses (0.5 mg/kg)(Soderpalm, et al., 1991). However, in the present study, locomotor activity increased progressively in both groups, with no significant differences between control and diazepam-pretreated rats.
The diazepam-pretreated group of Experiment 1 showed differences in cocaine intake during self-administration sessions. Therefore, Experiment 2 was conducted to determine whether diazepam influenced extracellular NAcc DA. Diazepam has been shown to affect ventral tegmental area DA neuronal firing rates and has dose-dependent decreasing effects on basal (Invernizzi et al., 1991) and cocaine-stimulated DA levels in the NAcc shell (Giorgetti et al., 1998). Therefore, it could be argued that, rather than decreasing cocaine-induced anxiety, the enhancement of lever responses observed in the diazepam group might reflect a compensatory behavioral response aimed at increasing DA levels to an “optimal” range achieved by the control animals. However, our data indicate this was not the case, since we found that cocaine-stimulated extracellular NAcc DA levels in the diazepam-pretreated group were not lower than controls. Our findings that basal NAcc DA is not affected by a low dose of diazepam has been previously reported (Invernizzi, et al., 1991). Yet, previous work to date showing inhibitory effects of GABA agonists on VTA DA neuronal activity (Giorgetti, et al., 1998; Liu et al., 2005a) would logically support predictions that NAcc DA, behavioral activity and abuse liability would all be decreased in subjects treated with diazepam in combination with cocaine. In the present study, our findings do not support such predictions. However, since the combined diazepam and cocaine dosages utilized in the current work have not been examined previously, the effects on behaviors and NAcc DA reported here are novel contributions to the literature.
It should be noted that although differences in the magnitude of DA response between self-administered and experimenter-administered drugs have been reported (Hemby et al., 1995), there are no reports indicating opposing effects of cocaine on DA responses between the different modes of intake. Therefore, Experiment 2 provides reasonable evidence that cocaine-stimulated NAcc DA responses were enhanced for both groups during Experiment 1, thus comparably influencing operant behavior. Though not empirically addressed in the present experiment, it is possible that other neurotransmitters and neuromodulators involved in stress, such as corticotropin releasing hormone (CRH), adrenocorticotropic hormone (ACTH), norepinephrine (NE) and serotonin (5-HT) which interact with GABAergic neurotransmission, may have contributed to the differences in behaviors observed between the two treatment groups (Carrasco and Van De Kar, 2003).
Findings from the present study suggest that anxiety alters cocaine self-administration behavior in a manner that is responsive to enhanced GABA activation. Though the negative effects of cocaine are less examined than the positive rewarding effects, these findings suggest that cocaine intake may be increased in individuals co-administering sedative drugs. Correspondingly, therapeutic interventions that maximize the aversive effects of cocaine may be a novel means of decreasing cocaine use and abuse.
Acknowledgments
Authors acknowledge Elizabeth Garcia for collecting preliminary data forming the basis of this study and a University of Texas Undergraduate Research Award (E.G.). This study was assisted by a University of Texas College of Pharmacy Hispanic Center of Excellence Fellowship (R. T. L.) and supported by NIH/NIDA Grant 14640 (C.L.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12:305–20. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–7. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev. 1999;23:981–91. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hebert MA, Dulloog L, Kaawaloa N, Nishimura O, Blanchard DC. Acute cocaine effects on stereotype and defense: an ethoexperimental approach. Neurosci Biobehav Rev. 1998;23:179–88. doi: 10.1016/s0149-7634(98)00019-0. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Ackerman DL, Pasnau RO. Low dose desipramine treatment of cocaine-related panic attacks. J Nerv Ment Dis. 1991;179:755–8. doi: 10.1097/00005053-199112000-00008. [DOI] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing and rapid dopamine signaling during goal-directed behaviors in rats. Neuropharmacology. 2004;47 Suppl 1:180–9. doi: 10.1016/j.neuropharm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–72. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Charney D, Mihic S, Harris R. Hypnotics and sedatives. In: Hardman J, Limbird L, Gilman A, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001. pp. 399–427. [Google Scholar]
- Cox BJ, Norton GR, Swinson RP, Endler NS. Substance abuse and panic-related anxiety: a critical review. Behav Res Ther. 1990;28:385–93. doi: 10.1016/0005-7967(90)90157-e. [DOI] [PubMed] [Google Scholar]
- D’Souza MS, Duvauchelle CL. Comparing nucleus accumbens and dorsal striatal dopamine responses to self-administered cocaine in naive rats. Neurosci Lett. 2006;408:146–50. doi: 10.1016/j.neulet.2006.08.076. [DOI] [PubMed] [Google Scholar]
- David V, Gold LH, Koob GF, Cazala P. Anxiogenic-like effects limit rewarding effects of cocaine in balb/cbyj mice. Neuropsychopharmacology. 2001;24:300–18. doi: 10.1016/S0893-133X(00)00205-0. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006 doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Pert A. Conditioned increases in anxiogenic-like behavior following exposure to contextual stimuli associated with cocaine are mediated by corticotropin-releasing factor. Psychopharmacology (Berl) 1998;137:333–40. doi: 10.1007/s002130050627. [DOI] [PubMed] [Google Scholar]
- Djeridane Y, Lemmer B, Touitou Y. Diazepam affects both level and amplitude of rat locomotor activity rhythm but has no effect on core body temperature. Chronobiol Int. 2005;22:975–85. doi: 10.1080/07420520500395094. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Ikegami A, Castaneda E. Conditioned increases in behavioral activity and accumbens dopamine levels produced by intravenous cocaine. Behav Neurosci. 2000;114:1156–66. doi: 10.1037//0735-7044.114.6.1156. [DOI] [PubMed] [Google Scholar]
- Emmett-Oglesby MW, Peltier RL, Depoortere RY, Pickering CL, Hooper ML, Gong YH, Lane JD. Tolerance to self-administration of cocaine in rats: time course and dose-response determination using a multi-dose method. Drug Alcohol Depend. 1993;32:247–56. doi: 10.1016/0376-8716(93)90089-9. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–8. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology (Berl) 1991;103:455–61. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Fernandes C, File SE, Berry D. Evidence against oppositional and pharmacokinetic mechanisms of tolerance to diazepam’s sedative effects. Brain Res. 1996;734:236–42. [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. Concurrent positive and negative goalbox events produce runway behaviors comparable to those of cocaine-reinforced rats. Pharmacol Biochem Behav. 1997;57:145–50. doi: 10.1016/s0091-3057(96)00300-0. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Post RM. Onset of panic disorder associated with rare use of cocaine. Biol Psychiatry. 1991;29:403–6. doi: 10.1016/0006-3223(91)90227-d. [DOI] [PubMed] [Google Scholar]
- Giorgetti M, Javaid JI, Davis JM, Costa E, Guidotti A, Appel SB, Brodie MS. Imidazenil, a positive allosteric GABAA receptor modulator, inhibits the effects of cocaine on locomotor activity and extracellular dopamine in the nucleus accumbens shell without tolerance liability. J Pharmacol Exp Ther. 1998;287:58–66. [PubMed] [Google Scholar]
- Goeders NE. A neuroendocrine role in cocaine reinforcement. Psychoneuroendocrinology. 1997;22:237–59. doi: 10.1016/s0306-4530(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Gunnarsdottir ED, Pingitore RA, Spring BJ, Konopka LM, Crayton JW, Milo T, Shirazi P. Individual differences among cocaine users. Addict Behav. 2000;25:641–52. doi: 10.1016/s0306-4603(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE. The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis. J Pharmacol Exp Ther. 1995;273:591–8. [PubMed] [Google Scholar]
- Hoplight BJ, Vincow ES, Neumaier JF. The effects of SB 224289 on anxiety and cocaine-related behaviors in a novel object task. Physiol Behav. 2005;84:707–14. doi: 10.1016/j.physbeh.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hubner CB, Moreton JE. Effects of selective D1 and D2 dopamine antagonists on cocaine self-administration in the rat. Psychopharmacology (Berl) 1991;105:151–6. doi: 10.1007/BF02244301. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Pozzi L, Samanin R. Release of dopamine is reduced by diazepam more in the nucleus accumbens than in the caudate nucleus of conscious rats. Neuropharmacology. 1991;30:575–8. doi: 10.1016/0028-3908(91)90075-m. [DOI] [PubMed] [Google Scholar]
- Johnson DN. Effect of diazepam on food consumption in rats. Psychopharmacology (Berl) 1978;56:111–2. doi: 10.1007/BF00571417. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–11. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, Bae D. Behavioral and brain temperature responses to salient environmental stimuli and intravenous cocaine in rats: effects of diazepam. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-007-0965-y. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Kiyatkin DE, Rebec GV. Phasic inhibition of dopamine uptake in nucleus accumbens induced by intravenous cocaine in freely behaving rats. Neuroscience. 2000;98:729–41. doi: 10.1016/s0306-4522(00)00168-8. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005a;437:1027–31. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Roberts DC, Morgan D. Effects of extended-access self-administration and deprivation on breakpoints maintained by cocaine in rats. Psychopharmacology (Berl) 2005b;179:644–51. doi: 10.1007/s00213-004-2089-y. [DOI] [PubMed] [Google Scholar]
- Mediratta PK, Sharma KK, Rana J. Development of differential tolerance to the sedative and anti-stress effects of benzodiazepines. Indian J Physiol Pharmacol. 2001;45:111–5. [PubMed] [Google Scholar]
- Moriyama M, Ichimaru Y, Gomita Y. Behavioral suppression using intracranial reward and punishment: effects of benzodiazepines. Pharmacol Biochem Behav. 1984;21:773–8. doi: 10.1016/s0091-3057(84)80018-0. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Parkinson JA, Miles FJ, Everitt BJ, Dickinson A. Cocaine-seeking by rats: regulation, reinforcement and activation. Psychopharmacology (Berl) 2000;152:123–31. doi: 10.1007/s002130000498. [DOI] [PubMed] [Google Scholar]
- Paine TA, Jackman SL, Olmstead MC. Cocaine-induced anxiety: alleviation by diazepam, but not buspirone, dimenhydrinate or diphenhydramine. Behav Pharmacol. 2002;13:511–23. doi: 10.1097/00008877-200211000-00001. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–20. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–31. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Landry MJ. Benzodiazepine dependency discontinuation: focus on the chemical dependency detoxification setting and benzodiazepine-polydrug abuse. J Psychiatr Res. 1990;24 Suppl 2:145–56. doi: 10.1016/0022-3956(90)90046-s. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Svensson L, Hulthe P, Johannessen K, Engel JA. Evidence for a role for dopamine in the diazepam locomotor stimulating effect. Psychopharmacology (Berl) 1991;104:97–102. doi: 10.1007/BF02244561. [DOI] [PubMed] [Google Scholar]
- Spivey WH, Euerle B. Neurologic complications of cocaine abuse. Ann Emerg Med. 1990;19:1422–8. doi: 10.1016/s0196-0644(05)82612-5. [DOI] [PubMed] [Google Scholar]
- Tarr JE, Macklin M. Cocaine. Pediatr Clin North Am. 1987;34:319–31. doi: 10.1016/s0031-3955(16)36217-4. [DOI] [PubMed] [Google Scholar]
- Thiebot MH, Jobert A, Soubrie P. [Effects of muscimol and diazepam: a comparative study on behavioral inhibiton induced by novelty, punishment, and nonreward (author’s transl)] Psychopharmacology (Berl) 1979;61:85–9. doi: 10.1007/BF00426816. [DOI] [PubMed] [Google Scholar]
- Walfish S, Massey R, Krone A. Anxiety and anger among abusers of different substances. Drug Alcohol Depend. 1990;25:253–6. doi: 10.1016/0376-8716(90)90150-d. [DOI] [PubMed] [Google Scholar]
- Wesson DR, Smith DE. Cocaine: treatment perspectives. NIDA Res Monogr. 1985;61:193–203. [PubMed] [Google Scholar]
- Wolf BC, Lavezzi WA, Sullivan LM, Middleberg RA, Flannagan LM. Alprazolam-related deaths in Palm Beach County. Am J Forensic Med Pathol. 2005;26:24–7. doi: 10.1097/01.paf.0000153994.95642.c1. [DOI] [PubMed] [Google Scholar]