Abstract
Genetic and early environmental factors interact to influence ethanol’s motivational effects. To explore these issues, a reciprocal cross-fostering paradigm was applied to Fischer and Lewis rats. The adult female offspring received vehicle or the kappa opioid antagonist nor-BNI (1 mg/kg) followed by assessments of conditioned taste aversion (CTA), blood alcohol concentrations (BACs) and hypothermia induced by 1.25 g/kg intraperitoneal ethanol. CTA acquisition in the in-fostered Fischer and Lewis animals did not differ; however, the Fischer maternal environment produced stronger acquisition in the cross-fostered Lewis rats versus their in-fostered counterparts. CTAs in the Fischer rats were not affected by cross-fostering. In extinction, the in-fostered Lewis animals displayed stronger aversions than the Fischer groups on two trials (of 12) whereas the cross-fostered Lewis differed from the Fischer groups on nine trials. Despite these CTA effects, Lewis rats exhibited higher BACs and stronger hypothermic responses than Fischer with no cross-fostering effects in either strain. No phenotypes were affected by nor-BNI. These data extend previous findings dissociating the aversive and peripheral physiological effects of ethanol in female Fischer and Lewis rats, and highlight the importance of genetic and early environmental factors in shaping subsequent responses to alcohol’s motivational effects in adulthood.
Keywords: Alcohol, Gene-Environment Interaction, Conditioned Taste Aversion, Blood Alcohol, Hypothermia, Kappa Opioid System
Introduction
Like other drugs of abuse, alcohol acts on multiple targets in brain and body to produce a complex array of effects, including both rewarding and aversive subjective effects (Carr and White, 1986; Cunningham, 2007; Evans and Levin, 2004; Foltin et al., 1981; Vansickel et al., 2007; Zacny and Gutierrez, 2003). As such, it may be argued that vulnerability to alcohol abuse is not only influenced by reward processes, but may be a function of the balance between the contrasting motivational effects produced by the drug (Griffiths et al., 2003; Lynch and Carroll, 2001; Riley and Simpson, 2001). However, individuals also differ in the constituent biological systems mediating alcohol’s affective properties, which in turn interact with experiential influences to shape individual patterns of alcohol use (Dick et al., 2006; Enoch and Goldman, 2001; Zimmermann et al., 2007). Indeed, a more comprehensive understanding of how genetic and environmental factors act and interact is a major goal of preclinical research on neuropsychiatric disorders such as drug and alcohol abuse (Caspi and Moffitt, 2006; Ellenbroek et al., 2005; Gunzerath and Goldman, 2003).
Over the last 20 years, a number of laboratories have explored genetic factors in drug-induced phenotypes using the inbred Fischer and Lewis rat strains as an animal model (Kosten and Ambrosio, 2002), with our laboratory in particular often utilizing these strains to study the aversive effects of abused drugs within the conditioned taste aversion (CTA) paradigm. In relation to alcohol, Fischer males develop more robust ethanol-induced CTAs than Lewis males (Roma et al., 2006), behavioral findings that are consistent with the negative correlation generally observed between ethanol CTA and self-administration in rodents (see Green and Grahame, 2008 and Riley et al., in press for reviews). Although published self-administration data in female Fischer and Lewis rats are less conclusive (Taylor et al., 2006), it is interesting to note that the strains develop equivalent CTAs despite two-fold higher blood alcohol concentrations (BACs) in the Lewis animals (Roma et al., 2007a).
Sex differences notwithstanding, a general assumption in research using selected lines and inbred strains is that the observed effects on physiology and behavior are due to differences in genotype; however, cross-fostering work on stress reactivity and inflammatory disease susceptibility in adult Fischer and Lewis rats has shown that genotype alone simply cannot account for all strain differences (Gomez-Serrano et al., 2001; Gomez-Serrano et al., 2002). Cross-fostering effects can vary in symmetry and magnitude, with certain combinations of strain and maternal environment even producing effects on phenotypes in which the strains did not initially differ, which further underscores the importance of accounting for both genetic and environmental factors in research using inbred rodent strains. Additional work in Fischer and Lewis rats has supported the relevance of such an approach for animal models of drug abuse, as cross-fostering effects have been observed in response to the aversive properties of morphine and cocaine (Gomez-Serrano, 2005; Riley et al., in press; Roma et al., 2007b; Roma and Riley, 2007); however, it is unknown if or how genotype and early maternal environment interact to affect subsequent responses to alcohol’s aversive effects.
The primary goal of the present study was to determine the contributions of genetic and early environmental factors to several ethanol-induced phenotypes in Fischer and Lewis rats. To this end, a reciprocal cross-fostering procedure was employed which yielded litters representing all four combinations of genotype and maternal environment. Using previously established parameters (Roma et al., 2007a; Roma et al., 2006), the adult female offspring then underwent assessments of CTA acquisition and extinction, BACs and hypothermia induced by acute injection(s) of 1.25 g/kg ethanol. We chose the female offspring for this experiment because of increasing interest in the biological bases of substance abuse in females (el-Guebaly, 1995; Lynch et al., 2002), especially given human epidemiological reports of significant increases in alcohol abuse among adult women over the last decade (Grant et al., 2004a) and other research indicating greater health risks in alcohol-abusing women versus men (Hommer, 2003). Although female Fischer and Lewis rats do not differ in ethanol-induced CTAs, they do differ dramatically in BACs in response to acute ethanol (Roma et al., 2007a), and more importantly, females of these strains are sometimes more receptive to cross-fostering effects than are males in behavioral assays (Gomez-Serrano et al., 2001; Roma et al., 2007b).
Another goal of the present study was to explore potential neurobiological mechanisms mediating gene-environment interaction effects on ethanol CTA. A system of long-standing interest regarding alcohol is the endogenous opioids (Davis and Walsh, 1970; Gianoulakis, 2004; Oswald and Wand, 2004), which has focused mostly on the mu opioid system in relation to alcohol’s rewarding and reinforcing effects. However, in addition to gastrointestinal disturbance and acute toxicity (Adinoff et al., 1988; Sanders and Berry, 1985), ethanol administration provokes the release of dynorphins, the family of endogenous kappa opioid ligands, in the central nervous system (Marinelli et al., 2006). Administration of kappa opioid agonists also conditions robust avoidance responses in taste and place conditioning assays (McLaughlin et al., 2005; Mucha and Herz, 1985), decreases dopamine release in the nucleus accumbens and caudate (Carlezon et al., 2006; Di Chiara and Imperato, 1988; Thompson et al., 2000), and produces pro-depressant and anhedonic behavioral effects (Carlezon et al., 2006; Mague et al., 2003; Pfeiffer et al., 1986; Todtenkopf et al., 2004). Given these interrelationships between ethanol, dynorphin and aversive motivational states, it is reasonable to suppose that such aversive effects are part of the stimulus complex produced by alcohol, and that the magnitude of these effects may contribute to ethanol CTA. For the present study, a potential role for the kappa opioid system was determined pharmacologically by systemic administration of the selective, potent and long-lasting kappa opioid antagonist nor-BNI. We hypothesized that reducing available binding sites for dynorphin would attenuate ethanol-induced CTAs.
Method
Subjects
A total of 74 female rats contributed to this study (26 dams and 48 offspring). Of these, 14 dams and 29 offspring were of the Fischer strain (F344/NHsd) and 12 dams and 19 offspring were of the Lewis strain (LEW/SsNHsd). The primiparous dams were purchased from Harlan Sprague-Dawley (Indianapolis, IN, USA) and arrived at our facility on Day 14 of gestation. Dams were housed in clear plastic bins (25.9 × 47.6 × 20.9 cm) filled with 3 cm of wood shavings and were provided with two 3.5 × 7 cm paper towel strips as supplementary nesting material. The animal housing room operated on a 12-hr light/dark schedule (lights on at 0800 hr) and was maintained at an ambient temperature of 23°C. All experimental procedures were conducted during the light portion of the cycle and all animals had free access to food and water. All procedures described in this report were in compliance with the US Animal Welfare Act and National Research Council guidelines and were approved by the Institutional Animal Care and Use Committee at American University.
Drugs and Solutions
Ethanol (95% stock solution) and sodium saccharin were purchased from Sigma-Aldrich (St. Louis, MO). Saccharin was prepared as a 1 g/L (0.1%) solution with tap water. All fluids were presented at room temperature in 50-ml graduated cylinders, and consumption was measured to the nearest 0.5 ml. Ethanol was prepared as a 15% solution (v/v) with saline and administered via IP injection at a dose of 1.25 g/kg. Nor-binaltorphimine dihydrochloride (nor-BNI) was synthesized by the Chemical Biology Research Branch at the National Institute on Drug Abuse. The drug was prepared as a 1 mg/ml solution in distilled water and administered via subcutaneous (SC) injection at a dose of 1 mg/kg. This dose of nor-BNI was chosen because of the drug’s potency and previous demonstrations of its effectiveness in behavioral assays (Beardsley et al., 2005; Steinmiller and Young, 2008). All non-drug injections were matched by vehicle, volume and route to their respective drug injections as described below.
Cross-Fostering, Rearing and Housing
Within 24 hr of parturition (post-natal day [PND] 0), pups were assigned to unrelated dams of either their own genotype (in-fostered) or the other (cross-fostered). This manipulation created the following four rearing groups: Fischer offspring raised by Fischer dams (F/F, n = 8 litters), Fischer offspring raised by Lewis dams (F/L, n = 6 litters), Lewis offspring raised by Lewis dams (L/L, n = 6 litters) and Lewis offspring raised by Fischer dams (L/F, n = 6 litters). Each foster litter contained no more than three related pups; insofar as was possible, litters were culled to eight same-strain animals per dam and were sex-balanced. All litters were left undisturbed except for cage-cleaning and weighing on PNDs 11 and 22.
Upon weaning on PND 22, all pups were group-housed with same-sex littermates until PND 60. Beginning on PND 60, all rats were housed in individual hanging wire cages (24 × 19 × 18 cm) until initiation of the CTA procedures on PND 82. To control for litter effects, assignment of animals ensured that rats from each foster litter were represented in both the vehicle and nor-BNI pretreatment groups, although assignment of individuals from the same litter to either condition was random.
Ethanol-Induced Conditioned Taste Aversion
Previous work from our laboratory has shown that Fischer and Lewis females acquire equivalent dose-dependent CTAs to 1.0 and 1.5 g/kg (Roma et al., 2007a), so an intermediate dose of 1.25 g/kg under identical parameters was chosen to allow potential cross-fostering and/or nor-BNI effects to emerge. The groups were composed as follows: F/F, n = 8/pretreatment; F/L, n = 6–7/pretreatment; L/L, n = 4–5/pretreatment; L/F, n = 5/pretreatment.
Habituation
All rats were first habituated to 20-min access to a single water bottle for 12 consecutive days. Five hours after the 12th day’s consumption period, half of the animals from each rearing group received an injection of vehicle or 1 mg/kg nor-BNI; habituation then continued for two more days.
Acquisition
On Day 1 of conditioning, water was replaced with a 0.1% saccharin solution, and the 20-min fluid access period was immediately followed by an intraperitoneal (IP) injection of 1.25 g/kg ethanol. On Day 2, the animals had 20-min access to water followed by IP saline injection. This pattern of saccharin + ethanol injection on Day 1 followed by water + vehicle injection on Day 2 was repeated for three consecutive cycles over the course of six days and culminated in a final one-bottle aversion test (saccharin + no injection) on the seventh day. These first seven days constituted the Acquisition phase of the CTA experiment.
Extinction
The Extinction phase began the day after the final one-bottle aversion test and consisted of 12 consecutive daily presentations of both saccharin and water during the 20-min consumption period followed by no injections. Locations of the saccharin and water bottles relative to each other on the home cage were counterbalanced within each group and alternated daily throughout Extinction.
Blood Alcohol Assessment
Fischer and Lewis females exhibit different BACs in response to acutely administered alcohol (Roma et al., 2007a). In order to account for this pharmacokinetic variable, all animals were returned to ad libitum food and water for a week after CTA Extinction and then administered a single IP injection of 1.25 g/kg ethanol followed by tail-blood sampling in a separate room at 15, 60 and 120 min post-injection. For the sampling procedure, each rat’s tail was soaked in warm water for 60–75 sec and wiped dry with a paper towel. The rat was then held in an oversized restraint tube (Plas-Labs, Lansing, MI, USA) while approximately 1 mm of the tip of the tail was cut with surgical scissors. For subsequent samplings, the tail was re-soaked and dried, with further incisions and the restraint tube employed on an as-needed basis. For all samplings, approximately 200 μl of whole blood were collected in heparinized capillary tubes (Drummond Scientific, Broomall, PA, USA) and the contents immediately transferred to microcentrifuge vials. Each sample was centrifuged at 3000 rpm for 20 min; the plasma was then transferred via micropipette to new vials and kept frozen until ready for assay. Undiluted plasma was assayed using the HP 6890 Series headspace gas chromatography/mass spectrometry system (Hewlett-Packard, Palo Alto, CA, USA) according to protocols developed by the Laboratory of Clinical and Translational Studies at the National Institute on Alcohol Abuse and Alcoholism.
Ethanol-Induced Hypothermia
Systemic ethanol produces hypothermia in rodents, the magnitude of which positively correlates with the strength of ethanol-induced CTAs in outbred rats (Cunningham et al., 1992; Rinker et al., 2008). To take this physiological response into account without the competing hyperthermic reaction to the stress of tail-blood sampling, body temperatures were recorded at 15, 60 and 120 min after each injection throughout the Acquisition phase of the CTA experiment. Core body temperatures were measured via digital thermometer (Vicks Speed-Read model V911; Kaz USA, Inc., Southborough, MA, USA). During temperature readings, each individual rat was cradled by an experimenter while the lubricated probe of the thermometer was gently inserted 3 cm into the rectum for 5–10 sec.
Data Analysis
Data were statistically evaluated using various Analysis of Variance (ANOVA) models with Fisher’s least significant difference (LSD) contrasts and independent samples t tests for post-hoc comparisons. Genetic and environmental contributions were tested as separate two-level factors of offspring genotype (Fischer or Lewis) and maternal environment (Fischer or Lewis) as were the effects of nor-BNI pretreatment (vehicle or 1 mg/kg). Additional details regarding data analysis for each variable are provided below. Statistical significance for all analyses was set at α = .05.
Results
Ethanol-Induced Conditioned Taste Aversion
Acquisition
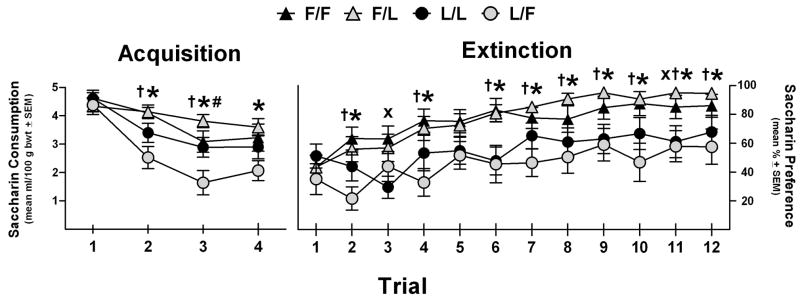
A preliminary 2 × 2 × 2 univariate ANOVA with between groups factors of genotype, maternal environment and nor-BNI pretreatment was performed on the raw consumption data from the first acquisition trial. The only significant term in this analysis was a main effect of genotype (F(1,40) = 15.0, p < 0.001; all other F(1,40)s < 2.4, ps > 0.10). As in previous CTA assessments in these strains, rats of the Fischer genotype consumed significantly less saccharin than those of the Lewis genotype (mean ± SD = 6.4 ± 1.6 ml vs. 8.1 ± 1.3 ml, respectively). For formal analyses, individual saccharin consumption values during the four one-bottle acquisition trials were transformed to ml consumed per 100 g body weight and were analyzed by a 4 × 2 × 2 × 2 mixed ANOVA with a repeated-measures factor of trial and between groups factors of genotype, maternal environment and nor-BNI pretreatment. This analysis revealed significant effects of trial (F(3,120) = 27.3, p < 0.001), genotype (F(1,40) = 9.9, p < 0.01) and maternal environment (F(1,40) = 4.1, p < 0.05) as well as a significant trial × genotype interaction (F(3,120) = 3.8, p < 0.05). No other terms, including all those involving nor-BNI, achieved statistical significance (Fs < 1.7, ps > 0.09). Given the significant contributions of genotype and maternal environment across trials, post-hoc tests collapsed across nor-BNI pretreatment groups were carried out and confirmed equivalent baseline consumption among all groups on the first trial (ps > 0.40) and equivalent aversions among the in-fostered Fischer and Lewis animals at all trials (ps > 0.09). The cross-fostered Fischers did not differ from their in-fostered counterparts at any trial (ps > 0.10); however, the cross-fostered Lewis rats exhibited significantly stronger aversions than both Fischer groups at trials 2 and 3 (ps < 0.01) and stronger aversions than all other groups at trial 3 (ps < 0.05; L/L vs. L/F p = 0.051).
Extinction
A 12 × 2 × 2 × 2 mixed ANOVA with a repeated-measures factor of trial and between groups factors of genotype, maternal environment and nor-BNI pretreatment was performed on the % saccharin preference data (saccharin as a percentage of total fluid consumed) over the course of the two-bottle extinction phase. This analysis revealed significant main effects of trial and genotype and a trial × genotype interaction (Fs > 3.0, ps < 0.01). No other terms approached significance (Fs < 1.6, ps > 0.10) except for a trend towards a trial × maternal environment interaction (F(11,440) = 1.6, p = 0.089). Given the group differences observed in acquisition, the extinction data were also collapsed across nor-BNI pretreatment conditions and subjected to post-hoc analyses across trials. Unlike in acquisition, the in-fostered Lewis rats showed significantly stronger, albeit sporadic, avoidance responses versus both groups of the Fischer genotype (trials 6 and 11, ps < 0.05). However, as in acquisition, the cross-fostered Lewis animals consistently showed significantly stronger aversions than both groups of Fischer rats (trials 2, 4, 6, 7–12, ps < 0.05). The results of the CTA analyses are depicted in Figure 1.
Figure 1.
Acquisition and extinction of conditioned taste aversion (CTA) induced by 1.25 g/kg IP ethanol (15% v/v) in adult female in-fostered and cross-fostered Fischer and Lewis rats pretreated with vehicle or 1 mg/kg nor-BNI. There were no effects of nor-BNI pretreatment, so each data point represents the collapsed mean ± SEM within each rearing group. The Acquisition data depict ml of saccharin consumed per 100 g of body weight (one-bottle tests), whereas the Extinction data depict relative saccharin preference (saccharin as a % of total fluid consumed per two-bottle tests). F/F = Fischer rats raised by Fischer dams (n = 16), F/L = Fischer rats raised by Lewis dams (n = 13), L/L = Lewis rats raised by Lewis dams (n = 9), and L/F = Lewis rats raised by Fischer dams (n = 10). The cross-fostered Lewis animals (L/F) generally displayed stronger avoidance responses than all other groups. Significant difference between L/L vs. F/F and F/L (x), L/F vs. F/F (†), L/F vs. F/L (*) or L/F vs. L/L (#).
Blood Alcohol Assessment
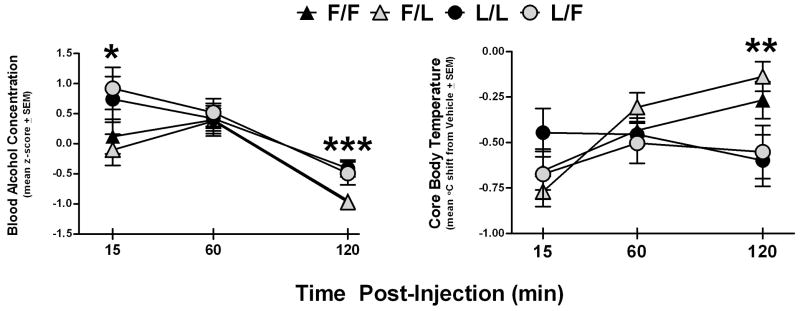
The samples yielded raw values ranging from 18–202 mg/dl. Blood was collected from two consecutive batches of eight animals (one rat from each group) per day over the course of three days. A preliminary ANCOVA with a covariate of batch (1st vs. 2nd group tested that day) revealed a significant sequence effect, so for formal analyses, the raw mg/dl data were converted to standardized z-scores within each batch. One sample from the vehicle pretreated F/F group was lost during the assay, so in order to preserve n across the repeated measures factor, that datum was replaced by the group’s mean value at that time point. These data were then analyzed by a 3 × 2 × 2 × 2 mixed ANOVA evaluating the effects of Time since injection (15, 60 and 120 min), genotype, maternal environment and nor-BNI pretreatment. This analysis yielded significant main effects of time (F(2,80) = 39.4, p < 0.001) and genotype (F(1,40) = 7.0, p < 0.05). No other terms approached significance (Fs < 2.2, ps > 0.10) except for a time × genotype trend (F(2,80) = 3.1, p = 0.052). Follow-up t tests collapsed across maternal environments and nor-BNI pretreatment groups confirmed significantly higher BACs in animals of the Lewis genotype at 15 and 120 min post-injection (t(46)s > 2.5, ps < 0.05; 60 min t(46) = 0.4, p > 0.70).
Ethanol-Induced Hypothermia
For the hypothermia assessment, mean body temperature across all three alcohol injection days was calculated for each individual at each post-injection time point, with identical calculations made for the temperature readings following the vehicle injections administered on alternating days. For formal analyses, each animal served as its own vehicle control, and the mean shifts in body temperature (°C) from vehicle were analyzed by a 3 × 2 × 2 × 2 mixed ANOVA evaluating the effects of Time since injection, genotype, maternal environment and nor-BNI pretreatment. The only significant terms were a main effect of time (F(2,80) = 8.5, p < 0.001) and a time × genotype interaction (F(2,80) = 9.1, p < 0.001; all other Fs < 2.9, ps > 0.06). Follow-up t tests collapsed across maternal environments and nor-BNI pretreatment groups confirmed significantly stronger hypothermic responses in animals of the Lewis genotype at 120 min post-injection (t(46) = 3.2, p < 0.01; other t(46)s < 1.3, ps > 0.20). The BAC and hypothermia results are presented together in Figure 2.
Figure 2.
Blood alcohol concentrations (z-scores) and mean shifts in core body temperature from vehicle controls (°C) at 15, 60 and 120 min after injection(s) of 1.25 g/kg IP ethanol (15% v/v) in adult female in-fostered and cross-fostered Fischer and Lewis rats pretreated with vehicle or 1 mg/kg nor-BNI. There were no effects of nor-BNI, so each data point represents the collapsed mean ± SEM within each rearing group. F/F = Fischer rats raised by Fischer dams (n = 16), F/L = Fischer rats raised by Lewis dams (n = 13), L/L = Lewis rats raised by Lewis dams (n = 9), and L/F = Lewis rats raised by Fischer dams (n = 10). Significant strain difference (collapsed across maternal environments) indicated by *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
The primary goal of the present study was to determine the relative contributions of genetic and early environmental factors to ethanol’s conditioned aversive effects in female Fischer and Lewis rats. We also accounted for some peripheral physiological responses to acute ethanol, namely blood alcohol concentrations and hypothermia, which may influence the development of ethanol CTA. Finally, an initial attempt at identifying relevant neurobiological mechanisms was made through antagonism of the kappa opioid system by nor-BNI prior to ethanol exposure. The experiments revealed stronger conditioned aversive responses in the cross-fostered Lewis animals compared to the other groups despite strain-dependent BACs and hypothermic responses that were unaltered by cross-fostering. There were no effects of kappa opioid antagonism on any of the observed phenotypes.
Consistent with previous work from our laboratory (Roma et al., 2007a), the in-fostered Fischer (F/F) and Lewis (L/L) females did not differ in the magnitude of alcohol-induced CTAs during acquisition. However, an interesting effect emerged whereby the Fischer animals were not influenced by cross-fostering, but the Fischer maternal environment produced significantly stronger CTA acquisition in the cross-fostered Lewis rats versus their in-fostered counterparts. Moreover, even though the L/L animals avoided saccharin to a greater extent than both groups of the Fischer genotype on two extinction trials, the cross-fostered L/F animals were sufficiently affected to produce stronger avoidance responses compared to Fischer animals across nine of the twelve extinction trials. This latter finding is interesting because one might expect the strength of acquisition to correlate with the rate of extinction, but the pattern of group differences was not identical across phases of the experiment. Indeed, acquisition and extinction are generally recognized as distinct learning processes (Myers & Davis, 2002; Rescorla, 2001), and although admittedly transient, the differences between the in-fostered Lewis animals compared to those of the Fischer genotype only emerged in extinction. Since CTAs on the first extinction trial were equivalent in all groups and not absolute, it is unlikely that the extinction data are an artifact of a floor effect; however, these data still do not indicate what mechanisms may be responsible for extinction-specific effects on drug-induced CTAs in these strains (see Roma and Riley, 2007 for a thorough discussion).
Despite the somewhat nuanced interpretation of the extinction data, when considered as a whole, the pattern of uniquely strong avoidance responses in the L/F animals suggests an intriguing cross-fostering effect that is both asymmetrical in that only one strain was affected and de novo in that an otherwise nonexistent strain difference was created by a specific combination of genotype and maternal environment. Asymmetrical effects are not uncommon in cross-fostering studies of gene-environment interaction across species and strains; indeed, asymmetry differentiates additive effects from truly interactive ones. Such effects have been observed before in Fischer and Lewis rats, for example, body weights were altered in cross-fostered Lewis but not Fischer pups (Gomez-Serrano et al., 2001; Siviy et al., 2003) and cocaine-induced CTAs were altered in cross-fostered female Fischer but not Lewis adults (Roma et al., 2007b). However, the exclusive creation of strain differences by cross-fostering, while not unheard of (e.g., open-field behavior in female Fischer and Lewis rats; Gomez-Serrano et al., 2001), is much more rare and much more difficult to explain. This is presumably so because a null hypothesis of perfectly equal (additive) contributions of genetic and environmental factors in strains that do not differ should still predict cross-fostered offspring that do not differ. Clearly, this was not the case in the present study, as cross-fostering the F/F and L/L rats with equivalent alcohol CTAs somehow produced L/F animals with stronger avoidance responses than all other groups. The biobehavioral bases for such effects remain unknown, although our data do not indicate modification of the kappa opioid system as a mediating mechanism affecting alcohol CTA. While any cross-fostering effect reinforces the notion that strain differences cannot be taken for granted as purely genetic phenomena, an effect such as that observed in the present study serves as an important reminder that genetic and early environmental factors still interact to shape responses to drugs of abuse in adulthood, and that individuals who appear otherwise identical may still differ in receptivity to epigenetic modulation of systems underlying responses to the motivational effects of drugs. Determining the genotypic and neurophysiological bases of such apparently different susceptibility and resistance in animal models and human populations remains a major goal.
In addition to the primary CTA results, the female Lewis groups sustained higher BACs than the Fischer groups in response to acute ethanol. This basic strain difference is also consistent with previous work (Roma et al., 2007a), and adequately accounts for the inversely related differences in hypothermia (tantamount to a main effect of genotype for both measures at 120 min post-injection); however, since neither of these phenotypes was affected by cross-fostering, their contributions to the observed differences in CTA are likely negligible. Although aversion is recognized as a particularly salient and relevant feature in experimental procedures involving alcohol (Cunningham, 2007; Meisch, 2001), the processes underlying CTAs induced by drugs of abuse are multifaceted and the subject of considerable investigation and debate (Broadbent et al., 2002; Grigson, 1997; Hunt and Amit, 1987; Parker, 1995; Riley and Tuck, 1985; Stolerman and D’Mello, 1981). Given the stronger hypothermic responses in the Lewis versus Fischer animals, one might predict stronger CTAs in the former (Cunningham et al., 1992; Rinker et al., 2007); however, the equivalent CTAs in the F/F and L/L groups and cross-fostering effects in the L/F animals indicate that mechanisms other than just hypothermia may contribute to ethanol CTA, at least in these strains. Indeed, under virtually identical conditions to those in the present study, male Fischers showed significantly stronger CTAs than Lewis to 1.25 and 1.5 g/kg ethanol but did not differ in hypothermia to 1.5 or 3 g/kg (Roma et al., 2006). Overall, these data suggest that central mechanisms may be more susceptible to early environmental modulation and may play a more predominant role in ethanol’s aversive subjective effects than the peripheral physiological mechanisms of absorption and hypothermia.
The present study yielded results relevant to alcohol’s motivational and physiological effects; however, some procedural issues are worthy of consideration both when interpreting the data and for conducting future work. Cardinal among them is the use of female subjects. The study of females in animal models of alcohol abuse is certainly warranted (el-Guebaly, 1995; Grant et al., 2004b; Hommer, 2003), but sex differences in gonadal hormone function can still introduce unwanted variability to studies of drug-induced phenotypes (Roth et al., 2004). Although estrus cycles were not actively monitored in the present study, orderly acquisition and extinction curves were obtained, but future work involving females would likely still benefit from systematically controlling gonadal hormone levels. In addition to hormonal influences, as suggested above, sex-dependent effects are often observed in studies comparing Fischer and Lewis rats (e.g., Roma et al., 2007b), and it would also be interesting to see if and how cross-fostering would influence responses to alcohol in male offspring. Also, the relevance of the kappa opioid system in ethanol CTA was tested via blockade of kappa receptors by nor-BNI. The preferential affinity of nor-BNI for the kappa receptor coupled with the relatively low single dose administered argues for kappa specificity (Spanagel et al., 1994); however, pretreatment had no effect on CTA, BAC or hypothermia. Although these data do not implicate the kappa opioid system in these alcohol-induced phenotypes, more comprehensive pharmacological tests including kappa antagonist dose-response functions as well as assessments of kappa agonists and mu- and delta-specific compounds would certainly strengthen any conclusions regarding the role of the endogenous opioid system as a mediator of gene-environment interaction effects on ethanol CTA. Finally, future work assessing maternal influences via cross-fostering may benefit from the inclusion of handled but non-fostered and/or completely undisturbed control litters for more valid comparisons to the commercially bred Fischer and Lewis animals purchased directly for experimentation.
In summary, the present study explored the interaction between genetic and early environmental influences on alcohol’s aversive and physiological effects within a cross-fostering paradigm. Although CTAs in the in-fostered Fischer and Lewis animals did not differ from each other, the Fischer maternal environment produced stronger acquisition and retarded extinction in the cross-fostered Lewis rats versus their in-fostered counterparts, whereas CTAs in the Fischer animals were not affected by the different rearing environments. Animals of the Lewis genotype exhibited higher BACs and stronger hypothermic responses with no cross-fostering effects in either strain, and none of the phenotypes observed was significantly affected by nor-BNI pretreatment. These data confirm and extend previous findings dissociating the aversive and peripheral physiological effects of ethanol in female Fischer and Lewis rats, and further highlight the importance of accounting for genetic and early environmental factors in animal models relevant to alcohol abuse.
Acknowledgments
We are indebted to Dr. Markus Heilig of the National Institute on Alcohol Abuse and Alcoholism for generously providing access to the gas chromatography system and to Erick Singley for his expert technical assistance therein. This research was supported by a grant from the Mellon Foundation to A.L.R. and by intramural funds from the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism, both of the National Institutes of Health/Public Health Service/US Department of Health and Human Services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adinoff B, Bone GH, Linnoila M. Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol Adverse Drug Exp. 1988;3:172–196. doi: 10.1007/BF03259881. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carr GD, White NM. Anatomical disassociation of amphetamine’s rewarding and aversive effects: an intracranial microinjection study. Psychopharmacology. 1986;89:340–346. doi: 10.1007/BF00174372. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. If you want to understand alcohol abuse, you must also understand taste aversion conditioning. Retrieved March 13, 2007, from American University, Conditioned Taste Aversion: An Annotated Bibliography Web site: http://www.american.edu/academic.depts/cas/psych/Cunningham_Highlight.pdf.
- Cunningham CL, Niehus JS, Bachtold JF. Ambient temperature effects on taste aversion conditioned by ethanol: contribution of ethanol-induced hypothermia. Alcohol Clin Exp Res. 1992;16:1117–1124. doi: 10.1111/j.1530-0277.1992.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Davis VE, Walsh MJ. Alcohol, amines, and alkaloids: a possible biochemical basis for alcohol addiction. Science. 1970;167:1005–1007. doi: 10.1126/science.167.3920.1005. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Schuckit MA, Bierut L, Hinrichs A, Fox L, Mullaney J, Cloninger CR, Hesselbrock V, Nurnberger JI, Jr, Almasy L, Foroud T, Porjesz B, Edenberg H, Begleiter H. Marital status, alcohol dependence, and GABRA2: evidence for gene-environment correlation and interaction. J Stud Alcohol. 2006;67:185–194. doi: 10.15288/jsa.2006.67.185. [DOI] [PubMed] [Google Scholar]
- el-Guebaly N. Alcohol and polysubstance abuse among women. Can J Psychiatry. 1995;40:73–79. doi: 10.1177/070674379504000204. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, van der Kam EL, van der Elst MCJ, Cools AR. Eur J Pharmacol. 2005:251–258. doi: 10.1016/j.ejphar.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. The genetics of alcoholism and alcohol abuse. Curr Psychiatry Rep. 2001;3:144–151. doi: 10.1007/s11920-001-0012-3. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR. Differential response to alcohol in light and moderate female social drinkers. Behav Pharmacol. 2004;15:167–181. [PubMed] [Google Scholar]
- Foltin RW, Preston KL, Wagner GC, Schuster CR. The aversive stimulus properties of repeated infusions of cocaine. Pharmacol Biochem Behav. 1981;15:71–74. doi: 10.1016/0091-3057(81)90341-5. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- Gomez-Serrano M, Tonelli L, Listwak S, Sternberg E, Riley AL. Effects of cross fostering on open-field behavior, acoustic startle, lipopolysaccharide-induced corticosterone release, and body weight in Lewis and Fischer rats. Behav Genet. 2001;31:427–436. doi: 10.1023/a:1012742405141. [DOI] [PubMed] [Google Scholar]
- Gomez-Serrano MA. The effects of cross-fostering on morphine-induced conditioned taste aversion in fischer and lewis rats. Dissertation Abstracts International: Section B: The Sciences and Engineering. 2005 [Google Scholar]
- Gomez-Serrano MA, Sternberg EM, Riley AL. Maternal behavior in F344/N and LEW/N rats. Effects on carrageenan-induced inflammatory reactivity and body weight. Physiol Behav. 2002;75:493–505. doi: 10.1016/s0031-9384(02)00649-2. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 2003;70:S41–54. doi: 10.1016/s0376-8716(03)00098-x. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: a reinterpretation. Behav Neurosci. 1997;111:129–136. [PubMed] [Google Scholar]
- Gunzerath L, Goldman D. G x E: A NIAAA workshop on gene-environment interactions. Alcohol Clin Exp Res. 2003;27:540–562. doi: 10.1097/01.ALC.0000057944.57330.65. [DOI] [PubMed] [Google Scholar]
- Hommer DW. Male and female sensitivity to alcohol-induced brain damage. Alcohol Res Health. 2003;27:181–185. [PMC free article] [PubMed] [Google Scholar]
- Hunt T, Amit Z. Conditioned taste aversion induced by self-administered drugs: paradox revisited. Neurosci Biobehav Rev. 1987;11:107–130. doi: 10.1016/s0149-7634(87)80005-2. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: Insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clinical Psychopharmacol. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2006;30:982–990. doi: 10.1111/j.1530-0277.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2005;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisch RA. Oral drug self-administration: an overview of laboratory animal studies. Alcohol. 2001;24:117–128. doi: 10.1016/s0741-8329(01)00149-5. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology. 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–84. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev. 1995;19:143–157. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Experimental extinction. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Mahwah, NJ: Erlbaum; 2001. pp. 119–54. [Google Scholar]
- Riley AL, Davis CM, Roma PG. Strain differences in taste aversion learning: Implications for animal models of drug abuse. In: Reilly S, Schachtman TR, editors. Conditioned taste aversion: Behavioral and neural processes. New York: Oxford University Press; in press. [Google Scholar]
- Riley AL, Simpson GR. The attenuating effects of drug preexposure on taste aversion conditioning: Generality, experimental parameters, underlying mechanisms and implications for drug use and abuse. In: Mowrer RR, Klein SB, editors. Contemporary Learning Theory. Hillsdale, NJ: Lawrence Erlbaum Associates; 2001. pp. 505–559. [Google Scholar]
- Riley AL, Tuck DL. Conditioned taste aversions: a behavioral index of toxicity. Ann N Y Acad Sci. 1985;443:272–292. doi: 10.1111/j.1749-6632.1985.tb27079.x. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Busse GD, Roma PG, Chen SA, Barr CS, Riley AL. The effects of nicotine on ethanol-induced conditioned taste aversions in Long–Evans rats. Psychopharmacology. 2008;197:409–419. doi: 10.1007/s00213-007-1050-2. [DOI] [PubMed] [Google Scholar]
- Roma PG, Chen SA, Barr CS, Riley AL. Dissociation between the aversive and pharmacokinetic effects of ethanol in female Fischer and Lewis rats. Behav Brain Res. 2007a;182:51–56. doi: 10.1016/j.bbr.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roma PG, Davis CM, Riley AL. Effects of cross-fostering on cocaine-induced conditioned taste aversions in Fischer and Lewis rats. Dev Psychobiol. 2007b;49:172–179. doi: 10.1002/dev.20168. [DOI] [PubMed] [Google Scholar]
- Roma PG, Flint WW, Higley JD, Riley AL. Assessment of the aversive and rewarding effects of alcohol in Fischer and Lewis rats. Psychopharmacology. 2006;189:187–199. doi: 10.1007/s00213-006-0553-6. [DOI] [PubMed] [Google Scholar]
- Roma PG, Riley AL. Cross-fostering and the extinction of cocaine’s conditioned aversive effects: evidence for gene-environment interaction. Pharmacol Biochem Behav. 2007;88:1–8. doi: 10.1016/j.pbb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Berry RG. Effects of ethyl alcohol on phasic and tonic contractions of the proximal stomach. J Pharmacol Exp Ther. 1985;235:858–863. [PubMed] [Google Scholar]
- Siviy SM, Love NJ, DeCicco BM, Giordano SB, Seifert TL. The relative playfulness of juvenile Lewis and Fischer-344 rats. Physiol Behav. 2003;80:385–394. doi: 10.1016/j.physbeh.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Almeida OF, Shippenberg TS. Evidence that nor-binaltorphimine can function as an antagonist at multiple opioid receptor subtypes. Eur J Pharmacol. 1994;264:157–162. doi: 10.1016/0014-2999(94)00449-8. [DOI] [PubMed] [Google Scholar]
- Steinmiller CL, Young AM. Pharmacological selectivity of CTAP in a warm water tail-withdrawal antinociception assay in rats. Psychopharmacology. 2008;195:497–507. doi: 10.1007/s00213-007-0898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, D’Mello GD. Oral self-administration and the relevance of conditioned taste aversions. In: Thompson T, Dews PB, McKim WA, editors. Advances in behavioral pharmacology. Hillsdale, NJ: L. Erlbaum; 1981. pp. 169–214. [Google Scholar]
- Taylor AN, Tio DL, Bando JK, Romeo HE, Prolo P. Differential effects of alcohol consumption and withdrawal on circadian temperature and activity rhythms in sprague-dawley, lewis, and fischer male and female rats. Alcohol Clin Exp Res. 2006;30:438–447. doi: 10.1111/j.1530-0277.2006.00048.x. [DOI] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Lile JA, Stoops WW, Rush CR. Similar discriminative-stimulus effects of D-amphetamine in women and men. Pharmacol Biochem Behav. 2007;87:289–296. doi: 10.1016/j.pbb.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Gutierrez S. Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacology. 2003;170:242–254. doi: 10.1007/s00213-003-1540-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Blomeyer D, Laucht M, Mann KF. How gene-stress-behavior interactions can promote adolescent alcohol use: The roles of predrinking allostatic load and childhood behavior disorders. Pharmacol Biochem Behav. 2007;86:246–262. doi: 10.1016/j.pbb.2006.09.024. [DOI] [PubMed] [Google Scholar]