Abstract
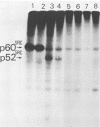
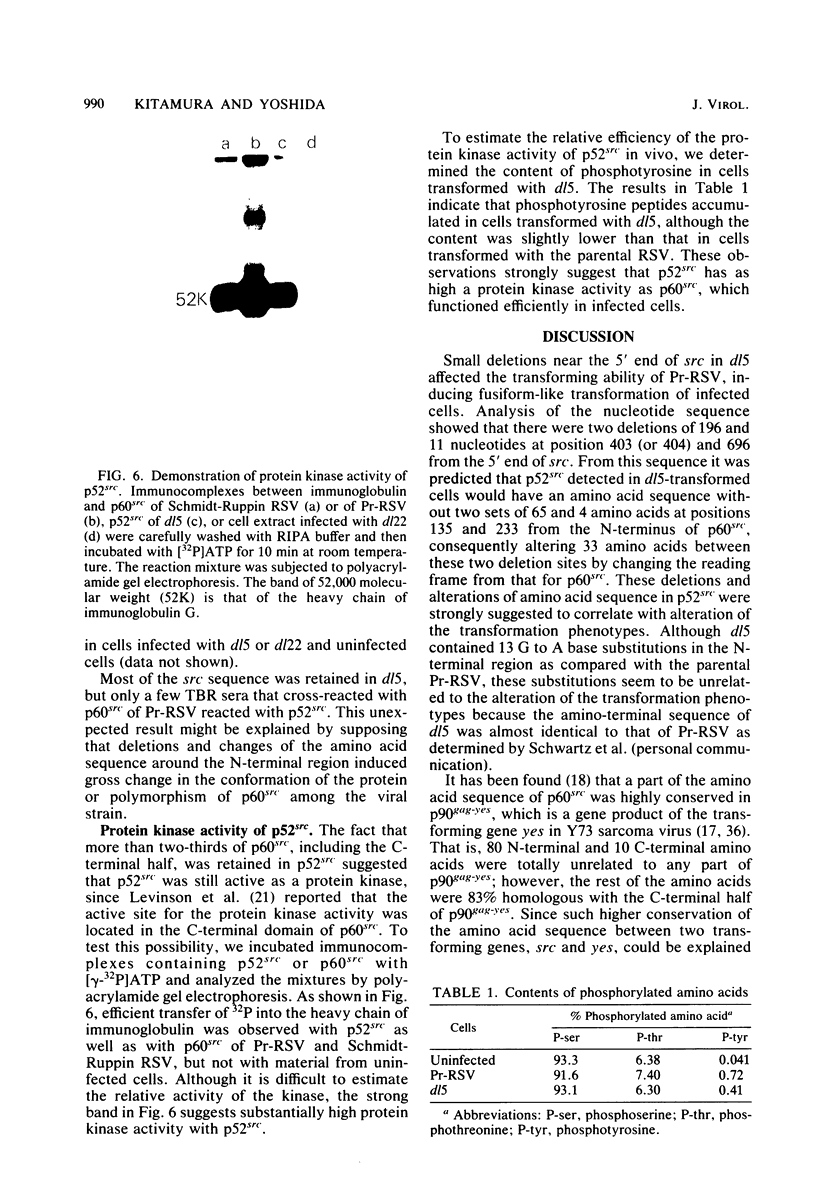
Partial deletion in the src gene and the gene product were characterized in a deletion mutant, dl5, isolated from the Prague strain of Rous sarcoma virus. The mutant induced fusiform-like transformed cells, unlike the parental Prague strain, which induced round transformed cells. Determination of the total nucleotide sequences of src in dl5 and the Prague strain of Rous sarcoma virus demonstrated that in the former two deletions of 196 and 11 nucleotides had occurred at positions 403 and 696, respectively, from the 5' end of src. A protein with a molecular weight of 52,000 (p52src) was detected in cells infected with dl5, as predicted from the deletion size in src. From the nucleotide sequence, it was predicted that p52src had two deletions of 65 and 4 amino acids at positions 135 and 232, respectively, from the N-terminal methionine of p60src and also had 33 amino acid changes between these two deletion sites due to alteration of the reading frame. p52src, which contained deletions and alterations of amino acids near the N-terminus, showed protein kinase activity similar to that of p60src and functioned in the infected cells. These results strongly suggest that changes in the N-terminal region of p60src modified its transforming ability, causing induction of the fusiform-like transformation phenotype.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. D., Beckmann R. P., Harms E. H., Nakamura K., Weber M. J. Biological properties of "partial" transformation mutants of Rous sarcoma virus and characterization of their pp60src kinase. J Virol. 1981 Jan;37(1):445–458. doi: 10.1128/jvi.37.1.445-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T., Sefton B. M. Polymorphism of avian sarcoma virus src proteins. J Virol. 1979 Apr;30(1):190–200. doi: 10.1128/jvi.30.1.190-200.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Steinbaugh P. J., Erikson R. L. Characterization of the avian sarcoma virus protein p60src. Virology. 1978 Nov;91(1):130–140. doi: 10.1016/0042-6822(78)90361-6. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernilofsky A. P., Levinson A. D., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. Nucleotide sequence of an avian sarcoma virus oncogene (src) and proposed amino acid sequence for gene product. Nature. 1980 Sep 18;287(5779):198–203. doi: 10.1038/287198a0. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Fincham V. J., Neiman P. E., Wyke J. A. Novel nonconditional mutants in the src gene of Rous sarcoma virus: isolation and preliminary characterization. Virology. 1980 May;103(1):99–111. doi: 10.1016/0042-6822(80)90129-4. [DOI] [PubMed] [Google Scholar]
- Fujita D. J., Bechberger J., Nedic I. Four Rous sarcoma virus mutants which affect transformed cell morphology exhibit altered src gene products. Virology. 1981 Oct 15;114(1):256–260. doi: 10.1016/0042-6822(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein phosphorylated by the RSV transforming function. Cell. 1980 Dec;22(3):647–648. doi: 10.1016/0092-8674(80)90539-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Duesberg P. H., Hanafusa H. Transformation-defective mutants of Rous sarcoma virus with src gene deletions of varying length. J Virol. 1977 Dec;24(3):910–914. doi: 10.1128/jvi.24.3.910-914.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- Krueger J. G., Garber E. A., Goldberg A. R., Hanafusa H. Changes in amino-terminal sequences of pp60src lead to decreased membrane association and decreased in vivo tumorigenicity. Cell. 1982 Apr;28(4):889–896. doi: 10.1016/0092-8674(82)90068-x. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Courtneidge S. A., Bishop J. M. Structural and functional domains of the Rous sarcoma virus transforming protein (pp60src). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1624–1628. doi: 10.1073/pnas.78.3.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Ghysdael J., Vogt P. K. Tyrosine-specific protein kinase activity associated with p105 of avian sarcoma virus PRCII. Virology. 1981 Feb;109(1):223–228. doi: 10.1016/0042-6822(81)90493-1. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Varmus H. E. The structure and protein kinase activity of proteins encoded by nonconditional mutants and back mutants in the sec gene of avian sarcoma virus. Virology. 1981 Jan 15;108(1):47–70. doi: 10.1016/0042-6822(81)90526-2. [DOI] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Temperature-sensitive transformation by Rous sarcoma virus and temperature-sensitive protein kinase activity. J Virol. 1980 Jan;33(1):220–229. doi: 10.1128/jvi.33.1.220-229.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- TEMIN H. M. Separation of morphological conversion and virus production in Rous sarcoma virus infection. Cold Spring Harb Symp Quant Biol. 1962;27:407–414. doi: 10.1101/sqb.1962.027.001.038. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemer D., Enquist L., Leder P. Improved derivative of a phage lambda EK2 vector for cloning recombinant DNA. Nature. 1976 Oct 7;263(5577):526–527. doi: 10.1038/263526a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Ikawa Y. Induction of some transformation-related properties by a transformation-defective mutant of avian sarcoma virus. Virology. 1977 Dec;83(2):444–448. doi: 10.1016/0042-6822(77)90192-1. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kawai S., Toyoshima K. Genome structure of avian sarcoma virus Y73 and unique sequence coding for polyprotein p90. J Virol. 1981 May;38(2):430–437. doi: 10.1128/jvi.38.2.430-437.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Yamashita M., Nomoto A. Transformation-defective mutants of Rous sarcoma virus with longer sizes of genome RNA and their highly frequent occurrences. J Virol. 1979 May;30(2):453–461. doi: 10.1128/jvi.30.2.453-461.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]