Abstract
Fallen leaves of Ficus altissima, F. virens, F. benjamina, F. fistulosa and F. semicordata, were collected in Chiang Mai Province in northern Thailand and examined for fungi. Eighty taxa were identified, comprising 56 anamorphic taxa, 23 ascomycetes and 1 basidiomycete. Common fungal species occurring on five host species with high frequency of occurrence were Beltraniella nilgirica, Lasiodiplodia theobromae, Ophioceras leptosporum, Periconia byssoides and Septonema harknessi. Colletotrichum and Stachybotrys were also common genera. The leaves of different Ficus species supported diverse fungal taxa, and the fungal assemblages on the different hosts showed varying overlap. The fungal diversity of saprobes at the host species level is discussed.
Keywords: Ficus, Fallen leaves, Saprobes, Fungal diversity
INTRODUCTION
Fungi are chemoheterotrophic organisms that play an important role as decomposers in natural ecosystems (Duarte et al., 2006; Gadd, 2007). Fungi have additional economic importance as biocontrol agents, chemical producers of bioactive compounds used in the pharmaceutical and many other industries (Yuen et al., 1999; Bucher et al., 2004; Duarte et al., 2006). The economic impact of fungi in the environment is likely related to the total fungal species pool (Hyde, 2001; Piepenbring, 2007). Research on fungal diversity therefore provides a basis for estimating the functional role of fungi in ecosystems, exploiting these economic fungi and estimating the total fungal species richness in a region or globally (Arnold et al., 2001; Mueller and Schmit, 2007).
Although the 1.5 million species estimated by Hawksworth (2001) are commonly accepted (Hyde, 2001; Hyde et al., 2007), the actual quantity of fungal species is unclear (Arnold et al., 2001; Hyde, 2001; Hyde et al., 2007; Schmit and Mueller, 2007). Schmit and Mueller (2007) estimated that there is a minimum of 712 000 fungal species worldwide. This estimate was primarily based on the observed ratio between vascular plant species diversity and fungal diversity in a certain area. Meta-analysis of fungal diversity in small plots (Schmit et al., 2005) demonstrated that the numbers of tree species and macrofungal species diversities are correlated (Mueller et al., 2007). The actual number of fungi is still unknown; however, only 5%~13% of the total estimated global fungal species have been described. The undescribed fungi may occur in poorly studied countries, hosts, habitats, niches, or tissues, and are mostly microfungi (Hyde, 2001; Schmit and Mueller, 2007). To determine the accuracy of fungal estimates, more information is needed concerning species diversity in poorly studied areas and hosts, especially in poorly studied areas (Dulymamode et al., 2001; Hyde, 2001).
There have been several studies on fungi in poorly studied habitats in Thailand (Jones and Hyde, 2004). This has included studies on larger fungi in the forests of northern Thailand (Le et al., 2007a; 2007b), fungi on peat swamp palms (Pinnoi et al., 2006; Pinruan et al., 2007), fungi on other monocotyledons (Photita et al., 2001; Thongkantha et al., 2008), fungi on leaf litter of angiosperms (Promputtha et al., 2004; 2005; Duong et al., 2006; 2008), and fungi on decaying wood (Kodsueb et al., 2008a; 2008b). There has been, however, no study on the litter of Ficus species.
Whether fungi are host-specific or generalists are significant in estimating species numbers (Zhou and Hyde, 2001; Hyde et al., 2007), is particularly important in studying leaf litter. In tropical forests, plant litter comprises a mixture of diverse host leaves, unlike that in many temperate forests where stands contain low tree diversity. Several studies on the saprobic microfungal diversity associated with plants have been carried out (Hyde et al., 2001; Yanna et al., 2001; Dulymamode et al., 2001; Paulus et al., 2006a; 2006b), and to a large extent, fungi tend to be host-specific at the host genus level (Taylor et al., 2001). Paulus et al.(2006a; 2006b) studied 6 tree hosts from 4 families and found very little overlap. The low overlap may be because the hosts studied were from different families. The next question to ask is whether litter of different species of the same host genus has host-specific fungi or generalists? We, therefore, chose to study the saprobes on 5 species of Ficus to establish data on the fungal communities involved in the decay of litter from different species of the same host genus.
The genus Ficus (Moraceae) includes some 750 species of woody plants occurring in most tropical and subtropical forests throughout the world (Weiblen, 2000). Ficus is an evergreen or deciduous tree, frequently growing on other trees (strangling) with smooth pale gray bark and abundant white latex (Gardner et al., 2000). There is little known concerning the fungal diversity on this host genus (Suryanarayanan and Vijaykrishna, 2001; Paulus et al., 2006a). Paulus et al.(2006a; 2006b) reported on the fungal diversity in leaf litter of 2 species of Ficus in Australia, while Suryanarayanan and Vijaykrishna (2001) isolated 28 endophyte fungi from Ficus benghalensis. Ficus is the second largest tree genus in northern Thailand after Syzygium with 30 species of Ficus growing in the region (Gardner et al., 2000).
The purpose of the present study is to assess the diversity of saprobic fungi on 5 species of Ficus in northern Thailand in order to (1) investigate fungal distribution on saprobic fallen leaves of Ficus species; and (2) evaluate the different fungal communities involved in the decay of litter from different species of the same host genus.
MATERIALS AND METHODS
Survey design
To examine the effect of host species on microfungal assemblages, 5 species of Ficus were chosen for the study. Individual trees of each species were located at each of the sites selected for sampling (Table 1). Thirty fallen leaves at various stages of decay were haphazardly collected from each Ficus species from each site within 2 d. All samples were placed in Ziplock plastic bags containing tissue paper moistened with sterile distilled water and sealed and incubated at room temperature. Material was examined in the laboratory on Days 5, 15 and 25 after sampling.
Table 1.
Location of Ficus species surveyed in Chiang Mai Province in northern Thailand
| Plant sepcies | Location |
| Ficus altissima | MRC, T. Pa Pae, A. Mae Taeng, Chiang Mai Province, Thailand, 19°07′12″ N, 98°44′2.64″ E. |
| F. virens | Out door of MRC, T. Pa Pae, A. Mae Taeng, Chiang Mai Province, Thailand, 19°07′12″ N, 98°44′2.64″ E. |
| F. benjamina | About 5 km east of MRC, M. Mae Taeng, Chiang Mai Province, Thailand, 19°05′12″ N, 98°44′2.64″ E. |
| F. fistulosa | Near Mae Malai, M. Mae Taeng, Chiang Mai Province, Thailand, 19°05′0″ N, 98°5′42″ E. |
| F. semicordata | Tung Cho National Park, forest trail, Tung Joaw Village, Mae Teng Dist., Chiang Mai Province, Thailand, 19°08′4.2″ N, 98°38′ 54″ E. |
Assessment of microfungal diversity
For the assessment of fungal presence, all leaves were cut into 5 cm×5 cm quadrates for observation. Fruiting bodies of microfungi were observed under a stereoscope. A slide was then prepared for one representative fruiting structure of each taxon and placed in water and observed under a compound microscope. Micrographs were taken and stains were added as appropriate. Slides were made semipermanent by the addition of 90% (v/v) lactic acid. Species identifications were made using morphological characters. Herbarium specimens and slides of all taxa are maintained at the Mushroom Research Foundation Herbarium with some duplicates in Zhejiang University Herbarium.
Definitions and statistical analyses
Taxa were recorded as either present or absent from each leaf. The number of leaves, on which a particular fungal species was observed, designated the ‘occurrence of a fungus’ and was used to calculate the ‘percentage occurrence’ of a taxon on leaves of one tree species using the following formula (Tsui et al., 2001; Yanna et al., 2002):
Percent occurrence of taxon A (%)=occurrence of taxon A/occurrence of all taxa in one tree species×100.
Fungal species diversity on each Ficus species was calculated using Shannon-Wiener’s index H:
| H=−∑PilnPi, |
where Pi is the frequency of fungal species i occurring on specific host leaves (Begon et al., 1993; Wong and Hyde, 2001).
Sørensen’s index of similarity (S) was applied (Magurran, 1988; Tsui et al., 2001) to compare the similarity of the species on leaves of different Ficus spp.: S=2c/(a+b), where a is the total number of species on host A leaves, b is the total number of species on host B leaves, and c is the number of species on both host leaves. Similarity is expressed with values between 0 (no similarity) and 1 (absolute similarity).
RESULTS
Species richness and dominant fungi on Ficus spp.
Examination of decaying leaves of 5 species of Ficus from northern Thailand yielded 80 fungal taxa, comprising 56 anamorphic taxa, 23 ascomycetes and 1 basidiomycete. We have identified many taxa to species level, although in several cases the morphology of the taxa in our study was not identified to species (unidentified species, Table 2). Five species, Beltraniella nilgirica, Lasiodiplodia theobromae, Ophioceras leptosporum, Periconia byssoides and Septonema harknessi were common species occurring on all five host species (>3% occurrence, Table 2). In addition, Colletotrichum and Stachybotrys were common genera, with several species occurring on the Ficus leaves (Table 2). Figs.1~5 also show some rare fungal taxa on the different host species.
Table 2.
Percent occurrence (%) of fungal taxa on leaves of 5 species of Ficus in northern Thailand
| Fungus name | FV | FF | FA | FS | FB |
| Ascomycetes | |||||
| Amphisphaeria fallax | 2.3 | ||||
| Anthostomella limitata | 2.2 | 2.4 | |||
| Anthostomelle nannorrhopis | 2.3 | 3.4 | 5.8 | ||
| Chaetomium sulphureum | 4.7 | ||||
| Diaporthe adunca | 4.7 | ||||
| Diaporthe perjuncta | 2.4 | 3.4 | |||
| Eutypella scoparia | 2.4 | 3.4 | |||
| Glomerella acutata | |||||
| Glomerella cingulata | 1.1 | 1.2 | |||
| Guignardia spp. | 1.1 | 2.4 | 1.2 | 2.9 | |
| Hyponectria populi | 3.4 | ||||
| Kirschsteiniothelia spp. | 1.7 | ||||
| Lasiosphaeria breviseta | 4.4 | ||||
| Lophiostoma minosporum | 6.8 | ||||
| Massarina hepaticarum | 4.7 | ||||
| Melanospora brevirostris | 3.4 | ||||
| Munkovalsaria appendenta | 1.1 | 1.7 | 1.4 | ||
| Mycoenterolobium platysporium | 2.2 | 2.3 | |||
| Mycosphaerella dianthi | 4.8 | 2.9 | |||
| Mycosphaerella freycineitae | 4.4 | 3.4 | |||
| Nectria sinopica | 1.2 | ||||
| Ophioceras leptosporum | 4.4 | 4.8 | 4.7 | 6.8 | 5.8 |
| Rhytisma acerinum | 3.4 | ||||
| Mitosporic fungi | |||||
| Acremonium charticola | 2.3 | ||||
| Ajrekarella polychaetriae | 2.2 | ||||
| Alternaria alternata | 4.8 | 4.3 | |||
| Beltraina querna | 1.2 | ||||
| Beltraniella nilgirica | 4.4 | 4.8 | 3.6 | 6.8 | 5.8 |
| Cercospora apii | 2.2 | 2.9 | |||
| Chaetospermum artocarpi | 4.8 | ||||
| Chaetospermum chaetosporum | 5.8 | ||||
| Cladosporium cladosporioides | 3.3 | 3.6 | 6.8 | ||
| Colletotrichum dematium | 2.2 | 4.8 | 3.6 | 2.9 | |
| Colletotrichum gloeosporioides | 3.6 | ||||
| Colletotrichum graminicola | 4.7 | ||||
| Cylindrocolla urticae | 5.8 | ||||
| Dicyma state of Ascotricha chartarum | 2.2 | 2.3 | |||
| Dinemasporium strigosum | 2.2 | 4.8 | 2.9 | ||
| Discosia artocreas | 2.4 | ||||
| Discosia brasiliensis | 1.4 | ||||
| Ellisiopsis gallesiae | 2.3 | ||||
| Fusariella hughesii | 4.4 | 4.7 | |||
| Fusariella obstipa | 2.3 | ||||
| Fusarium chlamydosporum | 4.8 | ||||
| Fusarium oxysporum | 5.5 | ||||
| Fusarium solani | 4.4 | ||||
| Giulia tenuis | 1.7 | ||||
| Kellermania yuccifolia | 2.4 | ||||
| Kontospora halophila | 1.7 | ||||
| Koorchaloma jamaicensis | 2.4 | ||||
| Lasiodiplodia theobromae | 3.3 | 4.8 | 3.6 | 6.8 | 5.8 |
| Menispora ciliata | 3.4 | ||||
| Menispora glauca | 3.4 | ||||
| Microdochium phragmitis | 4.8 | 4.7 | |||
| Mycoenterolobium platysporum | 2.2 | 2.3 | |||
| Periconia byssoides | 4.4 | 4.8 | 4.7 | 6.8 | 4.3 |
| Pestalotiopsis gigas | 2.2 | 2.9 | |||
| Pestalotiopsis owenii | 4.4 | 2.9 | |||
| Phaeoisaria clematidis | 2.3 | ||||
| Phoma eupyrena | 5.8 | ||||
| Phoma valerianae | 2.2 | 2.4 | |||
| Phomopsis asparagi | 2.2 | 1.2 | 1.4 | ||
| Plectophomella nypae | 2.2 | ||||
| Sagenomella striatispora | 2.4 | ||||
| Schwarzmannia goebeliae | 1.1 | ||||
| Septonema harknessi | 4.4 | 4.8 | 4.7 | 5.1 | 5.8 |
| Sphaeridium candidum | 2.4 | 2.9 | |||
| Stachybotrys bisbyi | 2.9 | ||||
| Stachybotrys nephrospora | 2.9 | ||||
| Stachybotrys oenanthes | 4.4 | 2.3 | 2.9 | ||
| Stachybotrys renispora | 3.3 | ||||
| Stachybotrys sansevieriae | 2.3 | 3.4 | |||
| Torula graminis | 5.8 | ||||
| Torula herbarum | 4.4 | ||||
| Trichothecium roseum | 2.4 | 3.6 | 3.4 | ||
| Volutella ciliata | 4.4 | 3.6 | 6.8 | ||
| Wiesneriomyces javanicus | 2.2 | 4.8 | 3.4 | ||
| Xylohypha nigrescens | 4.7 | ||||
| Zygosporium chartarum | 2.9 | ||||
| Basidiomycete | |||||
| Halocyphina spp. | 1.2 |
FV: Ficus virens; FF: F. fistulosa; FA: F. altissima; FS: F. semicordata; FB: F. benjamina
Fig. 1.
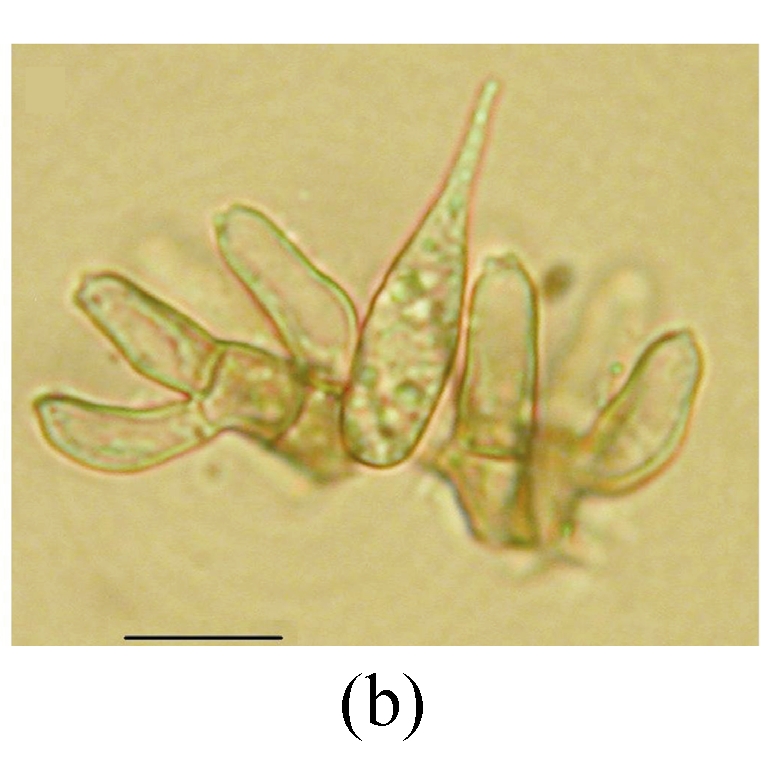
Ellisiopsis gallesiae on fallen leaves of Ficus altissima. (a) Morphology of conidia; (b) and (c) Morphology of conidiophores. Bar=10 µm
Fig. 5.

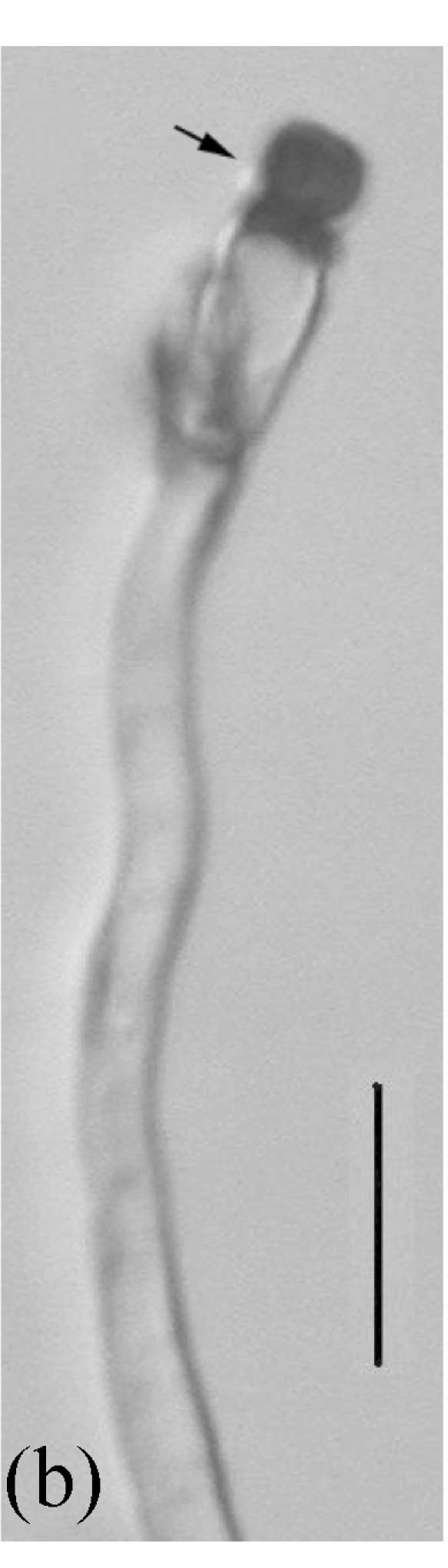
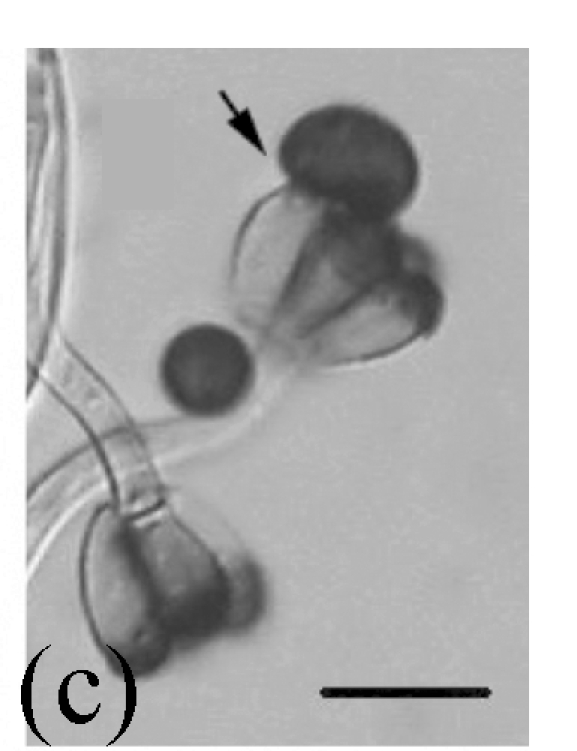
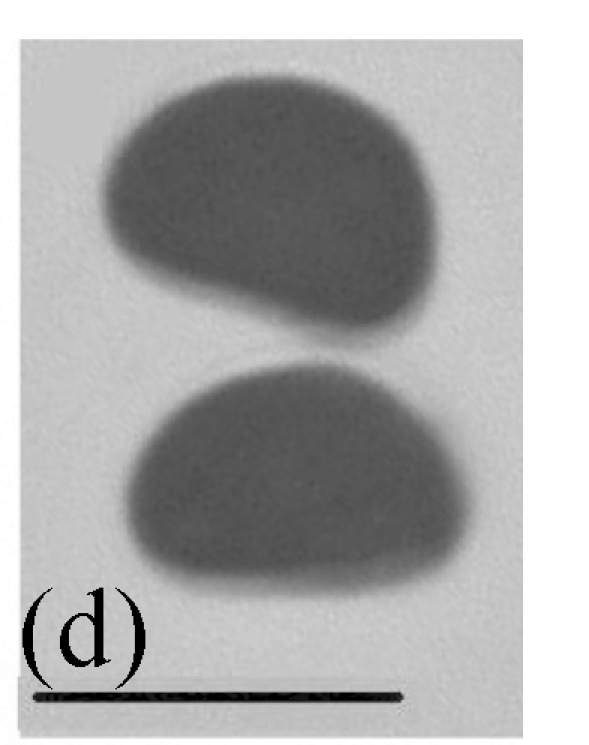
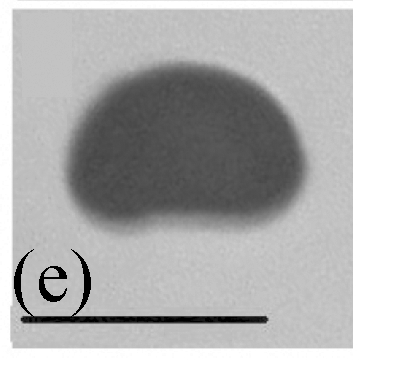
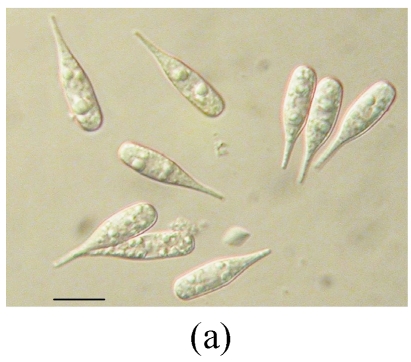
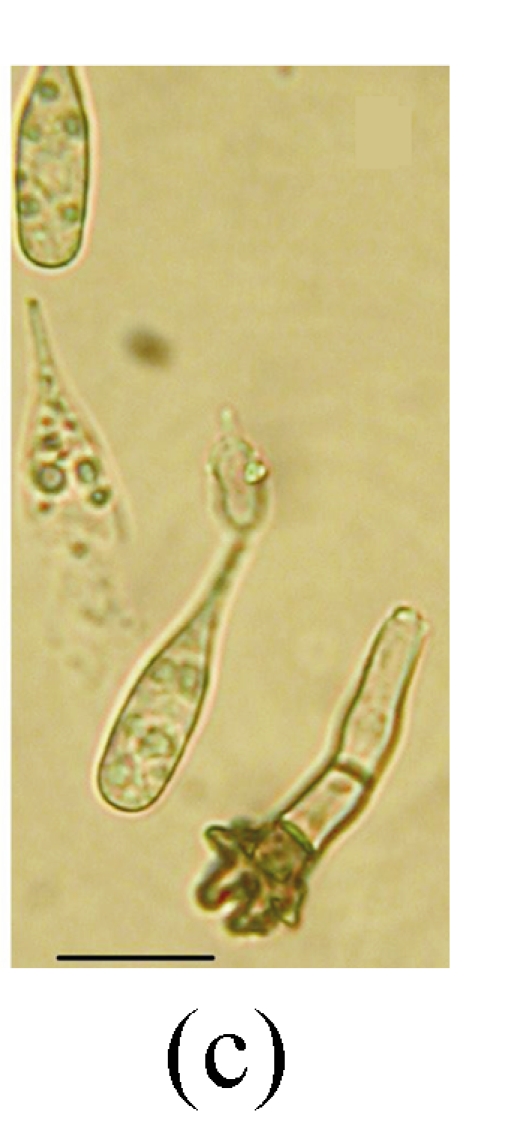
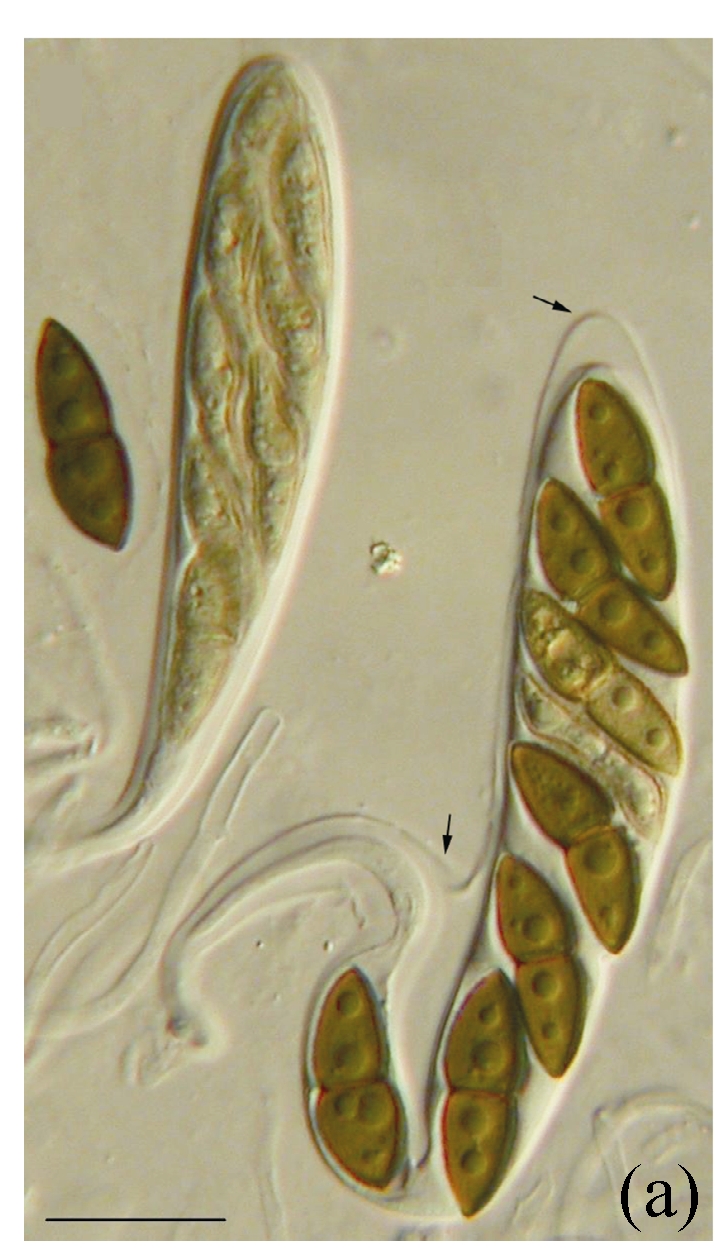
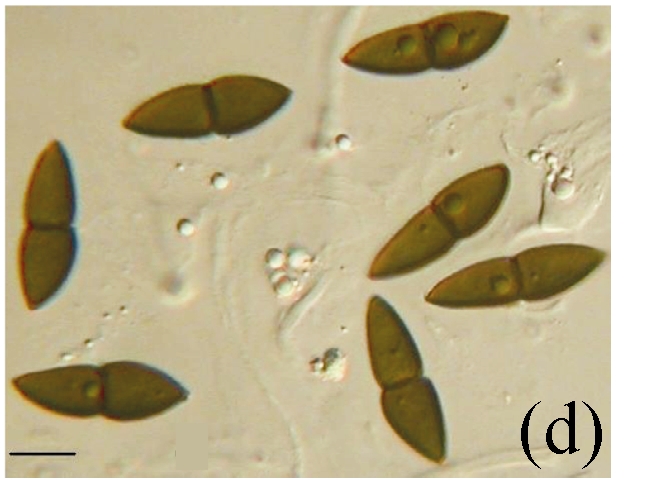
Morphology of Stachybotrys renispora on fallen leaves of Ficus virens. (a) Characters of conidiophore; (b) and (c) Characters of conidia and conidiophores. Arrow indicates the succession of conidium; (d) and (e) Characters of conidia. Bar=10 µm
Fig. 2.
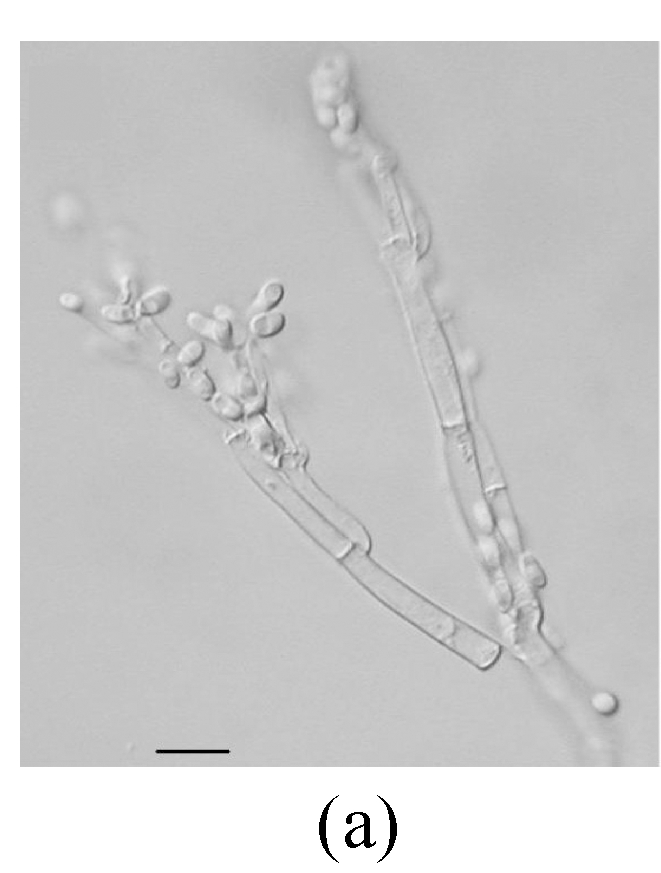
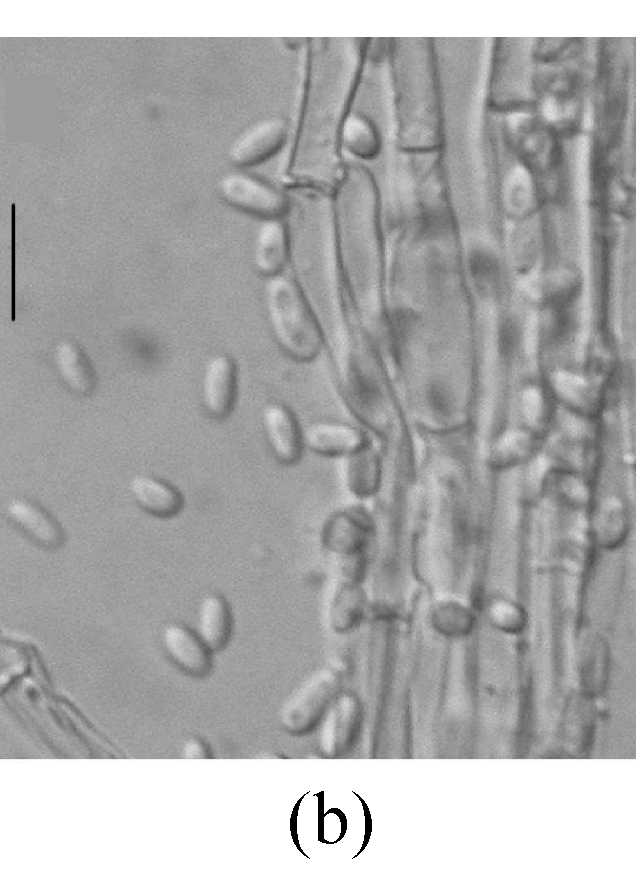
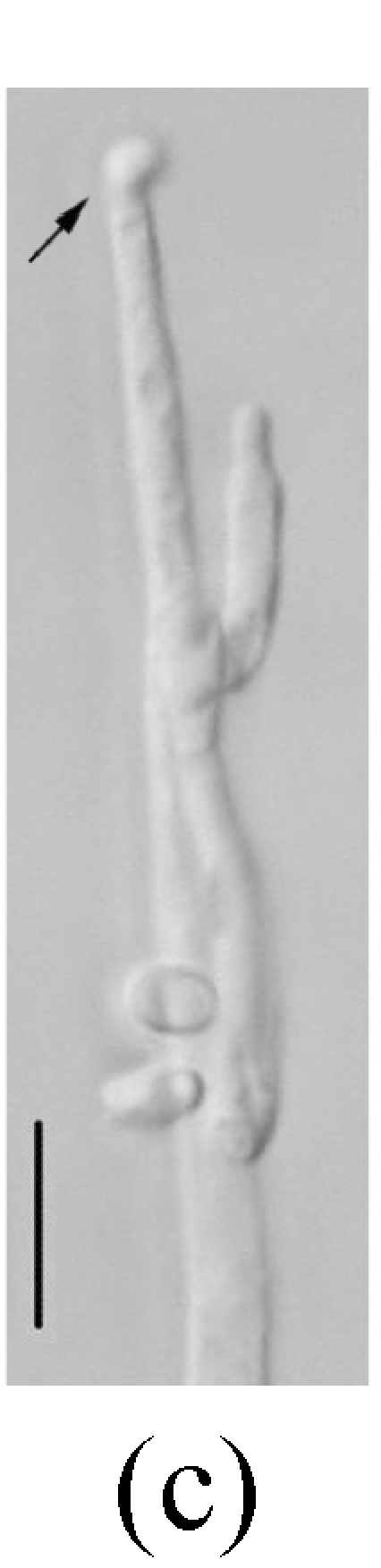
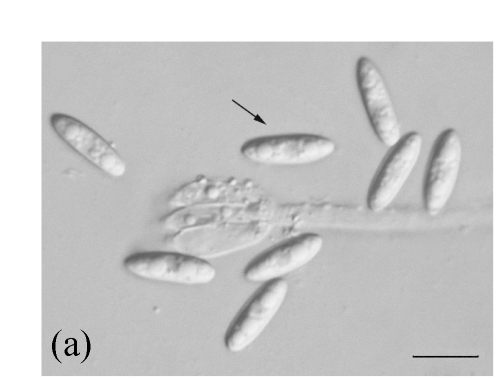
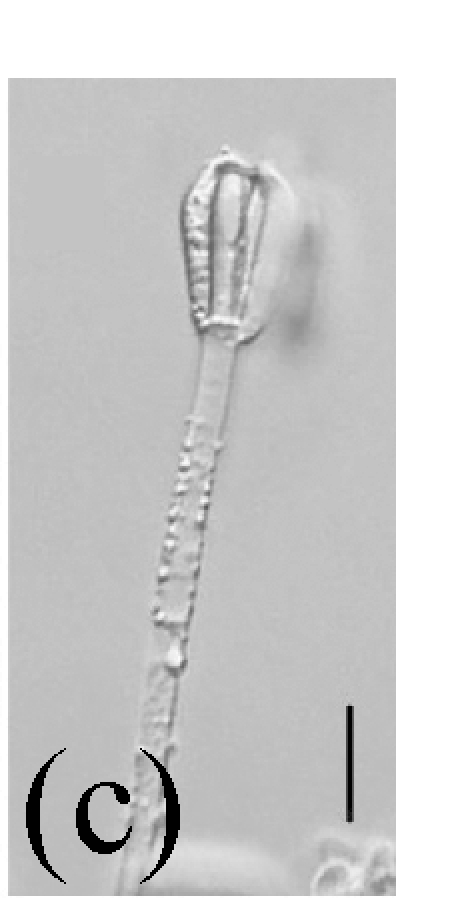
Morphology of Sagenomella striatispora on fallen leaves of Ficus fistulosa. (a) Characters of conidiophore; (b) Characters of conidia and conidiophore; (c) and (d) developing conidia from conidiophores. Arrow indicates the young conidium. Bar=10 µm
Fig. 3.


Morphology of Kirschsteiniothelia spp. on fallen leaves of Ficus semicordata. (a) Characters of asci. Arrows indicate the outer layer of the ascus wall; (b) Characters of ascus; (c) Characters of pseudoparaphyses; (d) Characters of ascospores. (a) Bar=20 µm; (b)~(d) Bar=10 µm
Fig. 4.
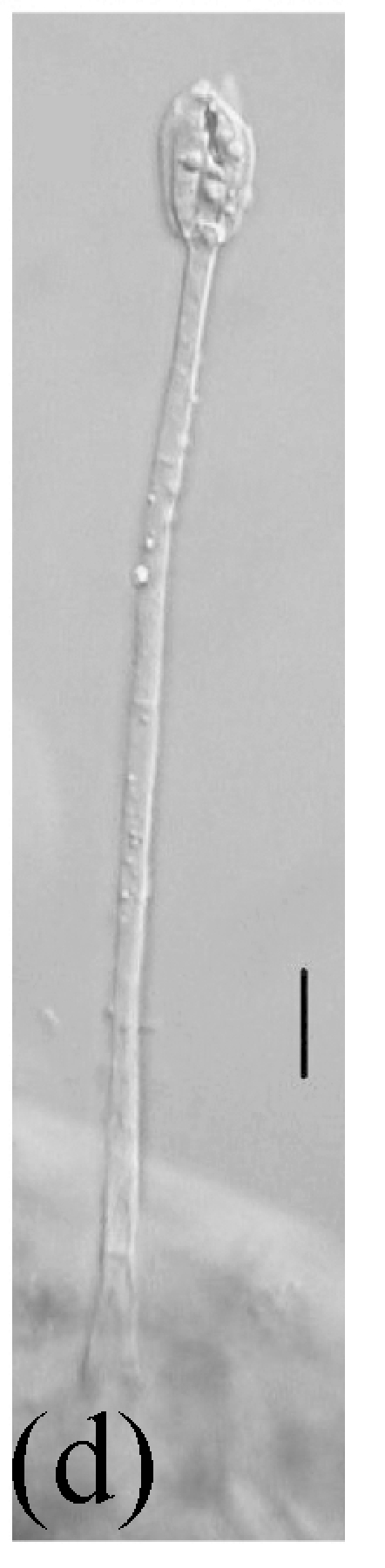
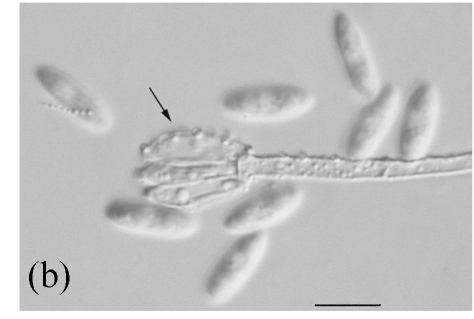
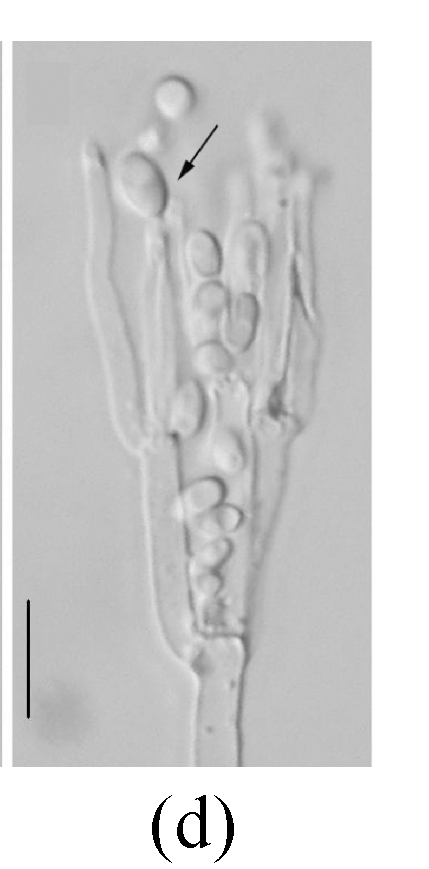
Morphology of Stachybotrys bisbyi on fallen leaves of Ficus benjamina. (a) Characters conidia. Arrow indicates the smooth wall of the conidium; (b) Characters of conidiophore. Arrow indicates the rough wall of conidiophore cell; (c) and (d) Characters of conidiophores. Bar=10 µm
Fungal diversity
Communities of fungal taxa on different Ficus species leaf litter were relatively distinct. The number of taxa varied from 24 (F. semicordata) to 33 (F. virens and F. altissima) (Table 3). The fungal communities on F. virens and F. altissima were the most similar but were different from those on F. semicordata (Table 4). The percentages of ascomycetes on F. virens and F. altissima were similar (24% and 30%, respectively). F. semicordata had the highest percentage of ascomycetes (46%) among the host species. The percentages of anamorphic taxa on F. virens and F. altissima were also similar.
Table 3.
Diversity of fungi on different Ficus species
| Ficus species | Number of species |
Total | Index H | ||
| Ascomycetes | Mitosporic fungi | Basidomycetes | |||
| Ficus virens | 8 | 25 | 33 | 3.3525 | |
| F. fistulosa | 6 | 19 | 25 | 3.0887 | |
| F. altissima | 10 | 22 | 1 | 33 | 3.3196 |
| F. semicordata | 11 | 13 | 24 | 3.0881 | |
| F. benjamina | 5 | 21 | 26 | 3.1496 | |
Table 4.
Index of similarity among different Ficus species
| Ficus virens | F. fistulosa | F. altissima | F. semicordata | F. benjamina | |
| Ficus virens | 0.41 | 0.45 | 0.35 | 0.44 | |
| F. fistulosa | 0.31 | 0.41 | 0.43 | ||
| F. altissima | 0.35 | 0.31 | |||
| F. semicordata | 0.28 | ||||
| F. benjamina |
DISCUSSION
Studies of fungal diversity on Ficus have not been carried out until recently, although this genus is very common in forests throughout the world (Weiblen, 2000; Paulus et al., 2006a; 2006b). Suryanarayanan and Vijaykrishna (2001) studied fungal endophyte diversity on different host tissues of F. benghalensis in India. They found little overlap between fungal endophyte composition on the leaves and aerial roots. Paulus et al.(2006a; 2006b) studied saprobic microfungi in leaf litter of Australia tropical rainforests, including two Ficus species. They found that fungal assemblages of F. destruens and F. pleurocarpa grouped closely together. The overlapping species were Beltraniella portoricensis, Beltrania cf. concurvispora, Cylindrocladium coulhounii, Dictypchaeta cf. novae-guineensis, Cylindrosympodium cryptocaryae, Idriella acerosa, Idriella lunata, Menisporopsis theobromae, Ophiognomonia elasticae, Parasypodiella elongate and Trichoderma viride. In the present study, we investigated fungal diversity on leaves of five Ficus species. Our results show that the overlap of fungal species was much higher than that in previous studies on fallen leaves from different hosts in different families. Similarity ranged from 28% to 45%. Some species, such as Ophioceras leptosporum, Beltraniella nilgirica, Lasiodiplodia theobromae, Periconia byssoides and Septonema harknessi, were shared by all hosts with high occurrence, indicating that a ‘core fungal group’ might be responsible for the decay of Ficus leaves (Wong and Hyde, 2001). In this study some fungi are new records on Ficus. Among these, many species are widespread, such as Diaporthe adunca, Lasiosphaeria breviseta, Colletotrichum gloeosporioides and Torula herbarum. At least one species, Munkovalsaria appendenta, is a new record for Thailand. Further studies are needed to find more fungi on the leaves of Ficus, for our investigation was limited, with one tree of each host being sampled once.
Some previous research results showed that the hosts or tissues are important factors that influence the fungal communities (Ho et al., 2001; Paulus et al., 2003; Rambelli et al., 2004) and that some saprobes might be host-specific (Hyde et al., 1997). Wong and Hyde (2001) investigated fungal diversity on Gramineae, and their results showed that fungal composition on host species level varied. Studies on microfungi of tree leaves in tropical regions suggested that the fungal assemblages on different host species were variable, and some other terms, such as ‘host exclusivity’, ‘host fidelity’, ‘host affinity’, ‘host affinity’, ‘host preference’ and ‘host recurrence’, were used to describe plant-fungal associations with definitive host (Zhou and Hyde, 2001). Taylor et al.(2001) noted that saprobic fungi were specific at a host genus level. In the present study, our results indicated that saprobic fungal diversity was high at the host species level, with some rare fungal taxa occurring on each of the different host species.
Acknowledgments
We are grateful to Dr. Dhanasekaran Vijaykrishna, University of Hong Kong, China and Dr. Ruilin Zhao, Southwest Forestry University, China for technical help.
Footnotes
Project supported by the Mushroom Research Centre, Chiang Mai, Thailand and the National Natural Science Foundation of China (No. 30670072) and the Natural Science Foundation of Zhejiang Province of China (No. Y350568)
Selected Papers of 2008 International Symposium on Fungal Diversity, Oct. 16~19, Hangzhou, China. The symposium is supported by the National Natural Science Foundation of China (No. 30671351)
References
- 1.Arnold AE, Zuleyka MZ, Gilbert GS. Fungal endophytes in dicotyledonous neotropical trees: patterns of abundance and diversity. Mycol Res. 2001;105(12):1502–1507. doi: 10.1017/S0953756201004956. [DOI] [Google Scholar]
- 2.Begon M, Harper JL, Townsend CR. Ecology: Individuals, Population and Communities. 3rd Ed. Boston: Blackwell Science; 1993. [Google Scholar]
- 3.Bucher VVC, Pointing SB, Hyde KD, Reddy CA. Production of wood decay enzymes, loss of mass, and lignin solubilization in wood by diverse tropical freshwater fungi. Microbial Ecology. 2004;48(3):331–337. doi: 10.1007/s00248-003-0132-x. [DOI] [PubMed] [Google Scholar]
- 4.Duarte S, Pascoal C, Cássio F, Bärlocher F. Aquatic hyphomycete diversity and identity affect leaf litter decomposition in microcosms. Oecologia. 2006;147(4):658–666. doi: 10.1007/s00442-005-0300-4. [DOI] [PubMed] [Google Scholar]
- 5.Dulymamode R, Cannon PF, Peerally A. Fungi on endemic plants of Mauritius. Mycol Res. 2001;105(12):1472–1479. doi: 10.1017/S0953756201004701. [DOI] [Google Scholar]
- 6.Duong LM, Jeewon R, Lumyong S, Hyde KD. DGGE coupled with ribosomal DNA gene phylogenies reveal uncharacterized fungal phylotypes. Fungal Diversity. 2006;23(1):121–138. [Google Scholar]
- 7.Duong LM, McKenzie EHC, Lumyong S, Hyde KD. Fungal succession on senescent leaves of Castanopsis diversifolia in Doi Suthep-Pui National Park, Thailand. Fungal Diversity. 2008;30(1):23–36. [Google Scholar]
- 8.Gadd GM. Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol Res. 2007;111(1):3–49. doi: 10.1016/j.mycres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Gardner S, Sidisunthorn P, Anusarnsunthorn. V. A Field Guied to Forest Trees of Northern Thailand. Bangkok: Kobfai Publishing Project; 2000. [Google Scholar]
- 10.Hawksworth DL. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res. 2001;105(12):1422–1432. doi: 10.1017/S0953756201004725. [DOI] [Google Scholar]
- 11.Ho WH, Hyde KD, Hodgkiss IJ, Yanna Fungal communities on submerged wood from streams in Brunei, Hong Kong, and Malaysia. Mycol Res. 2001;105(12):1492–1501. doi: 10.1017/S095375620100507X. [DOI] [Google Scholar]
- 12.Hyde KD. Where are the missing fungi? Does Hong Kong have any answers? Mycol Res. 2001;105(12):1514–1518. doi: 10.1017/S0953756201004889. [DOI] [Google Scholar]
- 13.Hyde KD, Fröhlich J, Taylor JE. Diversity of Ascomycetes on Palms in the Tropics. In: Hyde KD, editor. Biodiversity of Tropical Microfungi. Hong Kong: The Hong Kong University Press; 1997. pp. 141–156. [Google Scholar]
- 14.Hyde KD, Ho WH, Mckenzie EHC, Dalisay T. Saprobic fungi on bamboo culms. Fungal Diversity. 2001;7(1):35–48. [Google Scholar]
- 15.Hyde KD, Bussaban B, Paulus B, Crous PW, Lee S, Mckenzie EHC, Photita W, Lumyong S. Diversity of saprobic microfungi. Biodivers Conserv. 2007;16(1):7–35. doi: 10.1007/s10531-006-9119-5. [DOI] [Google Scholar]
- 16.Jones EBG, Hyde KD. Introduction to Thai Fungal Diversity. In: Jones EBG, Tanticharoen M, Hyde KD, editors. Thai Fungal Diversity. Thailand: BIOTEC; 2004. [Google Scholar]
- 17.Kodsueb R, McKenzie EHC, Lumyong S, Hyde KD. Diversity of saprobic fungi on Magnoliaceae . Fungal Diversity. 2008;30(1):37–53. [Google Scholar]
- 18.Kodsueb R, McKenzie EHC, Lumyong S, Hyde KD. Fungal succession on woody litter of Magnolia liliifera (Magnoliaceae) Fungal Diversity. 2008;30(1):55–72. [Google Scholar]
- 19.Le HT, Nuytinck J, Verbeken A, Lumyong S, Desjardin DE. Lactarius in Northern Thailand: 1. Lactarius subgenus Piperites . Fungal Diversity. 2007;24(1):173–224. [Google Scholar]
- 20.Le HT, Stubbe D, Verbeken A, Nuytinck J, Lumyong S, Desjardin DE. Lactarius in Northern Thailand: 2. Lactarius subgenus Plinthogali . Fungal Diversity. 2007;27(1):61–94. [Google Scholar]
- 21.Magurran AE. Ecological Diversity and its Measurement. London: Croom Helm; 1988. [Google Scholar]
- 22.Mueller GM, Schmit JP. Fungal biodiversity: What do we know? What can we predict? Biodivers Conserv. 2007;16(1):1–5. doi: 10.1007/s10531-006-9117-7. [DOI] [Google Scholar]
- 23.Mueller GM, Schmit JP, Leacock PR, Buyck B, Cifuentes J, Desjardin DE. Global diversity and distribution of macrofungi. Biodivers Conserv. 2007;16(1):37–48. doi: 10.1007/s10531-006-9108-8. [DOI] [Google Scholar]
- 24.Paulus B, Gadek P, Hyde KD. Estimation of microfungal diversity in tropical rain forest leaf litter using particle filtration: the effects of leaf storage and surface treatment. Mycol Res. 2003;107(6):748–756. doi: 10.1017/S0953756203007913. [DOI] [PubMed] [Google Scholar]
- 25.Paulus B, Gadek P, Hyde KD. Successional patterns of microfungi in fallen leaves of Ficus pleurocarpa (Moraceae) in an Australian tropical rainforest. Biotropica. 2006;38(1):42–51. [Google Scholar]
- 26.Paulus B, Kanowski J, Gadek P, Hyde KD. Diversity and distribution of saprobic microfungi in leaf litter of an Australian tropical rainforest. Mycol Res. 2006;110(12):1441–1454. doi: 10.1016/j.mycres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Photita W, Lumyong S, Lumyong P, Hyde KD. Endophytic fungi of wild banana (Musa acuminata) at Doi Suthep Pui National Park, Thailand. Mycol Res. 2001;105(12):1508–1513. doi: 10.1017/S0953756201004968. [DOI] [Google Scholar]
- 28.Piepenbring M. Inventoring the fungi of Panama. Biodivers Conserv. 2007;16(1):73–84. doi: 10.1007/s10531-006-9051-8. [DOI] [Google Scholar]
- 29.Pinnoi A, Lumyong S, Hyde KD, Jones EBG. Biodiversity of fungi on the palm Eleiodoxa conferta in Sirindhorn peat swamp forest, Narathiwat, Thailand. Fungal Diversity. 2006;22(2):205–218. [Google Scholar]
- 30.Pinruan U, Hyde KD, Lumyong S, McKenzie EHC, Jones EBG. Occurrence of fungi on tissues of the peat swamp palm Licuala longicalycata . Fungal Diversity. 2007;25(1):157–173. [Google Scholar]
- 31.Promputtha I, Lumyong S, Lumyong P, McKenzie EHC, Hyde KD. Fungal saprobes on dead leaves of Magnolia lillifera (Magnoliaceae) in Thailand. Cryptogamie Mycologie. 2004;25(1):43–47. [Google Scholar]
- 32.Promputtha I, Jeewon R, Lumyong S, McKenzie EHC, Hyde KD. Ribosomal DNA fingerprinting in the identification of non sporulating endophytes from Magnolia liliifera (Magnoliaceae) Fungal Diversity. 2005;20(1):167–186. [Google Scholar]
- 33.Rambelli A, Mulas B, Pasqualetti M. Comparative studies on microfungi in tropical ecosystems in Ivory Coast forest litter: behaviour on different substrata. Mycol Res. 2004;108(3):325–336. doi: 10.1017/S0953756204009396. [DOI] [PubMed] [Google Scholar]
- 34.Schmit JP, Mueller GM. An estimate of the lower limit of global fungal diversity. Biodivers Conserv. 2007;16(1):99–111. doi: 10.1007/s10531-006-9129-3. [DOI] [Google Scholar]
- 35.Schmit JP, Mueller GM, Leacock PR, Mata JL, Wu QX, Huang YQ. Assessment of tree species richness as a surrogate for macrofungal species richness. Biol Conserv. 2005;121(1):99–110. doi: 10.1016/j.biocon.2004.04.013. [DOI] [Google Scholar]
- 36.Suryanarayanan TS, Vijaykrishna D. Fungal endophytes of aerial roots of Ficus benghalensis. Fungal Diversity. 2001;8(1):155–161. [Google Scholar]
- 37.Taylor JE, Lee S, Pedro CW. Biodiversity in the Cape Floral Kingdom: fungi occurring on Proteaceae . Mycol Res. 2001;105(12):1480–1484. doi: 10.1017/S0953756201004944. [DOI] [Google Scholar]
- 38.Thongkantha S, Lumyong S, McKenzie EHC, Hyde KD. Fungal saprobes and pathogens occurrence on tissues of Dracaena loureiri and Pandanus spp. Fungal Diversity. 2008;30(1):149–179. [Google Scholar]
- 39.Tsui CKM, Hyde KD, Hodgkiss IJ. Colonization patterns of wood-inhabiting fungi on baits in Hong Kong rivers, with reference to the effects of organic pollution. Antonie van Leeuwenhoek. 2001;79(1):33–38. doi: 10.1023/A:1010210631215. [DOI] [PubMed] [Google Scholar]
- 40.Weiblen GD. Phylogenetic relationship of functionally dioecious Ficus (Moraceae) based on ribosomal DNA sequences and morphology. American Journal of Botany. 2000;87(9):1342–1357. doi: 10.2307/2656726. [DOI] [PubMed] [Google Scholar]
- 41.Wong MKM, Hyde KD. Diversity of fungi on six species of Gramineae and one species of Cyperaceae in Hong Kong. Mycol Res. 2001;105(12):1485–1491. doi: 10.1017/S0953756201004695. [DOI] [Google Scholar]
- 42.Yanna , Ho WH, Hyde KD. Fungal communities on decaying palm fronds in Australia, Brunei, and Hong Kong. Mycol Res. 2001;105(12):1458–1471. doi: 10.1017/S0953756201005214. [DOI] [Google Scholar]
- 43.Yanna , Ho WH, Hyde KD. Fungal succession on fronds of Phoenix hanceana in Hong Kong. Fungal Diversity. 2002;10(1):185–211. [Google Scholar]
- 44.Yuen TK, Hyde KD, Hodgkiss IJ. Interspecific interactions among tropical and subtropical freshwater fungi. Microbial Ecology. 1999;37(4):257–262. doi: 10.1007/s002489900151. [DOI] [PubMed] [Google Scholar]
- 45.Zhou D, Hyde KD. Host-specificity, host-exclusivity and host-recurrence in saprobic fungi. Mycol Res. 2001;105(12):1449–1457. doi: 10.1017/S0953756201004713. [DOI] [Google Scholar]