Abstract
Perioperative suppression of NK activity has been suggested to compromise host resistance to tumor progression. Here we sought to develop a clinically applicable pre-operative regimen to prevent immunosuppression and promotion of metastasis by stress or surgery. The synthetic ds-RNA, poly I-C, was used in vivo in F344 rats, based on its alleged in vitro ability to protect immunocytes from suppression by cAMP elevating agents. Different regimens of poly I-C were studied in controls and in rats subjected to a pharmacological stressor, swim stress, or surgical stress. Resistance to lung experimental metastasis of the syngeneic non-immunogenic MADB106 mammary adenocarcinoma was assessed. Numbers of circulating and marginating-pulmonary NK cells and their cytotoxicity against the MADB106 and YAC-1 target lines were also studied. Our findings established a regimen of repeated low-dose poly I-C administration with minimal side effects (0.2 mg/kg i.p. 5, 3, & 1 day before tumor inoculation). This regimen, while hardly affecting resistance levels in non-stressed animals, prevented all stressors from promoting metastases. These beneficial effects occurred in the presence of a primary tumor and in both sexes. Poly I-C increased the numbers of NK cells, and, on a per NK cell basis, while not increasing cytotoxicity, profoundly protected marginating-pulmonary NK cells from suppression by surgery. This study suggests a non-toxic clinically-translatable prophylactic use of poly I-C to target the critical perioperative period. By increasing the number of marginating-pulmonary NK cells, and by transforming them into a mode of resistance to immunosuppression, this approach may reduce postoperative metastasis in cancer patients.
Keywords: poly I-C, metastasis, surgery, immunity, natural killer, stress
INTRODUCTION
Metastasis is the leading cause of death in cancer patients. Excision of the primary tumor is a mainstay of therapy in many tumor types, but may promote spread and seeding of malignant cells and growth of preexisting micrometastases in some cancers (Sugarbaker, 2005; Thompson et al., 2005). Mechanisms that may mediate these adverse effects of surgery, independently or synergistically, include dissemination of malignant cells due to the mechanical manipulation of the primary tumor or its vascularization (Weitz and Herfarth, 2001), a drop in the systemic levels of tumor-related anti-angiogenic factors (Zetter, 1998), excess release of growth factors and pro angiogenic factors (Hofer et al., 1999; Lutgendorf et al., 2003), and perioperative suppression of cell mediated immunity (Little et al., 1993). Thus the immediate postoperative period may contribute substantially to the risk of subsequent emergence of metastases, and perioperative interventions that reduce this risk may improve long-term outcome (Shakhar and Ben-Eliyahu, 2003).
Our studies in this domain focus mainly on the role of NK cells in protection from surgery-induced metastasis. Animal studies suggest that NK cells are important for cancer control, especially with respect to dissemination and growth of metastases (Wu and Lanier, 2003). Surgical and psychological stress have been reported to suppress NK activity in animals and humans (Andersen et al., 1998; Koga et al., 2001), and this suppression was shown to compromise animal resistance to tumor progression (Ben-Eliyahu et al., 1999a; Shakhar and Ben-Eliyahu, 1998). Importantly, several clinical studies have reported that levels of NK activity at the time of surgery predict long-term survival rates (Taketomi et al., 1998; Takeuchi et al., 2001; Tsutsui et al., 1996).
Several of the factors that are involved in the stress response to surgery have been shown to suppress NK activity in vitro. These include catecholamines, prostaglandins, and corticosteroids (Di Lorenzo et al., 2001; Dokur et al., 2004). Using the MADB106 tumor model in rats, we have implicated sympathetic responses in suppressing NK activity and promoting metastasis during stress and surgery. Specifically, we showed that administration of β1 and β2 adrenoceptor antagonists, or interventions that blunt sympathetic responses, reduced the suppression of NK activity and inhibited the increased susceptibility to MADB106 experimental metastasis that follow surgical stress, swim stress, hypothermia, and social confrontation (Ben-Eliyahu et al., 2000; Ben-Eliyahu et al., 1999b; Melamed et al., 2005). Conversely, administration of physiologically relevant doses of adrenaline or of the β-adrenergic agonist, metaproterenol, induced NK-suppressing and metastasis-promoting effects similar to those induced by surgery and stress (Ben-Eliyahu et al., 2000; Shakhar and Ben-Eliyahu, 1998). Additionally, we recently reported the involvement of prostaglandins in the NK-suppressive and metastasis-promoting effects of surgery (Melamed et al., 2005; Yakar et al., 2003). Both catecholamines and prostaglandins were shown in vitro to suppress NK activity by elevating intracellular cyclic adenosine monophosphate (cAMP) levels (Ellis et al., 1990; Whalen and Bankhurst, 1990).
Studies employing human leukocytes suggested that in vitro exposure of human NK cells to interferons (IFNs) or to the synthetic ds-RNA, polyriboinosinic acid-polyribocytidylic acid (poly I-C), rendered NK cells partially resistant to suppression by cAMP-elevating agents, including β-adrenergic agonists and prostaglandins (Leung and Koren, 1982, 1984). Thus, a possible in vivo approach would be to treat animals with poly I-C before stressful conditions, hence protecting their NK cells from potential suppression. In vivo administration of poly I-C induces immune responses resembling those elicited by viral infections (Manetti et al., 1995; Whitmore et al., 2004), and it would seem evolutionary adaptive to have a mechanism that renders immunity resistant to immunosuppression when the organism is fighting a viral infection.
In the current work, we aimed at developing a prophylactic regimen based on low doses of poly I-C, in order to prevent the NK-suppressive and metastasis-promoting effects of stress and surgery. As early as three decades ago, high doses of poly I-C or type-1 cytokines (e.g. IL-2 and recently IL-12) have been tested in clinical trials (Eklund and Kuzel, 2004; Leonard et al., 1997; Levine and Levy, 1978; Wadler et al., 2004). However, these high doses caused significant toxicity and commonly yielded limited success in therapy of existing tumors (Levine and Levy, 1978). Interestingly, when used at a lower dose for prolonged periods, stabilized poly I-C did show significant efficacy against inoperable malignant gliomas with only minimal toxicity (Salazar et al., 1996). This low-dose approach was not assessed for its efficacy in preventing suppression of immune function or preventing promotion of metastasis following surgery.
MATERIALS AND METHODS
Animals
Fisher 344 male and female rats were purchased from Harlan laboratories, Jerusalem, Israel, to be used at the age of 13 to 16 weeks. Animals were housed four in a cage with free access to food and water on a 12:12 h light:dark cycle at 22±1 C°. Housing conditions are regularly monitored by the Institutional Animal Care and Use Committee (IAUC) of Tel Aviv University. All studies were approved by the IAUC. Each study reported was conducted using a separate group of animals. In three studies we used both females and males, in order to test whether the in vivo protective effects of poly I-C occur in both sexes.
Pharmaceuticals
Metaproterenol (Sigma, Israel), a nonselective β-adrenergic agonist with a higher affinity to β2 than to β1 receptors, was dissolved in phosphate buffered saline (PBS). Poly I-C (polyriboinosinic acid-polyribocytidylic acid; Sigma, Israel), a synthetic compound that resembles viral double-stranded RNA and triggers an anti-viral immune response, was dissolved in PBS.
Swim Stress
A weight of 25 g/kg body weight was attached to the tails of the rats. Each rat was then placed in a tank containing water 35 cm deep at 23 °C for 3 min alternating 5 times with 3 min rest periods.
Experimental Laparotomy
The procedure has been described elsewhere (Melamed et al., 2005; Page et al., 1993). Rats were anesthetized with 2.5% halothane and a 4 cm midline abdominal incision was performed. The small intestine was externalized, rubbed in four places with a PBS-soaked gauze pad, and covered with PBS-soaked gauze for 50 min. Finally the intestine was internalized and the abdomen was closed in one layer with 3 - 0 nylon sutures. Total procedure time from incision to the last stitch was set to one hour.
Radio-labeling of MADB106 tumor cells and assessment of lung tumor retention
To radiolabel MADB106 tumor cells we cultured them in 0.5 μCi/ml of 125iodeoxyyuridine for 1 day. We then lightly anesthetized the rats with halothane, and injected 4×105/kg labeled MADB106 cells in 0.5 ml of PBS containing 0.1% bovine serum albumin (BSA) into the tail vein. For more details see (Melamed et al., 2005).
Twenty one hours following tumor inoculation, we removed the lungs and placed them in a gamma counter for assessment of radioactive content. Lung tumor retention was calculated as the ratio between lung radioactivity and total radioactive content of the injected tumor cells. The MADB106 tumor cells are syngeneic to the F344 rat used herein, originate from a lung metastasis of a mammary adenocarcinoma, and metastasize only to the lungs (Barlozzari et al., 1983). The number of tumor cells retained in the lung 24 h following i.v. inoculation is highly dependent on NK activity (see discussion).
Assessment of MADB106 lung tumor metastases
Non labeled MADB106 tumor cells were injected as above. Three weeks later, lungs were removed and placed for 24 h in Bouin's solution (72% saturated picric acid solution, 23% formaldehyde [37% solution], and 5% acetic acid glacial). Lungs were then washed in ethanol, and two researchers unaware of the lungs' origin counted visible surface metastases independently.
Preparation of blood and lung effector cells
As described elsewhere (Melamed et al., 2005), after rats were killed with an overdose of halothane and their thoracic cavities were opened, blood was drawn from the right cardiac ventricle into a heparinized syringe (30 μl per 1 ml blood). One ml of blood was washed and used in the assay. Marginating pulmonary leucocytes were collected by injecting heparinized PBS (30 units per 1 ml PBS) into the right cardiac ventricle and collecting 50 ml of perfusate from the left ventricle to be concentrated to 1 ml in complete medium.
In vitro assessment of NK cytotoxicity
We used a 4 h chromium (51Cr) release assay to assess NK-mediated lysis of YAC-1 and of syngeneic MADB106 cells, as described elsewhere (Melamed et al., 2005; Shakhar et al., 2001). Effector cells were serially diluted and co-incubated with 5000 radiolabeled target cells, yielding different effector to target (E:T) ratios. Our previous studies indicate that cytotoxicity in this assay depends on NK cells, since their selective depletion nullified all specific killing (Page et al., 1994).
Flow cytometry
Flow cytometry was used to assess the number of NK cells in the blood and lungs (Melamed et al., 2005). NK cells were identified as CD161bright. To assess the absolute number of NK cells per μl of effector cells, we supplemented each sample with 600 polystyrene microbeads (20 μm Ø, Duke Scientific, Palo Alto, CA) per μl. Following cytometry, we used the formula 600×CD161bright/microbeads to calculate the number of NK cells per μl. In our laboratory, the coefficient of variation for this method was 6% for identical samples (Ben-Eliyahu et al., 1999a).
Marginating pulmonary cytotoxicity per NK cell
To standardize the NK:target ratios in all samples, lung perfusates were diluted based on the concentration of NK cells determined using FACS analysis. The NK concentration in each sample was brought to 200 NK/μl, yielding an E:T ratio of 6:1 NK:MADB106 target cells. Following this procedure, NK cytotoxicity was assessed as described above.
Statistical analysis
One- or two-way ANOVA was used to analyze lung tumor retention, numbers of lung metastases, and numbers of NK cells. Provided ANOVA indicated significant group differences, we used Fisher's PLSD contrasts to conduct pair-wise comparisons testing our hypothesized effects: metaproterenol, stress, or surgery worsens the outcome, and poly I-C treatment reduces these effects. NK cell activity was analyzed using repeated measures two-way ANOVA (the 4 highest E:T ratios serving as repeated measures), followed by PLSD pair-wise comparisons (as above). P < 0.05 was considered significant in all studies. Because in several experiments the variance in some experimental groups was markedly and significantly different from other groups, violating an assumption of ANOVA, we conducted t-tests to compare pairs of groups, using Bonferroni α correction for multiple comparisons. In the first study, three outliers (each from a different group) were removed, as each deviated more than 4 SD from its respective group's mean.
RESULTS
Repeated low-dose poly I-C protects against the tumor-promoting effects of metaproterenol
In the first experiments we started to optimize the poly I-C regimen, aiming at protecting rats from the metastasis-promoting effects of metaproterenol (a pharmacological stressor) while minimizing side effects. To this end, we compared different doses of poly I-C, in single vs. repeated (x 3) administration. Rats were randomly assigned to receive vehicle injections or poly I-C injections, and each group was further subdivided to receive metaproterenol or vehicle. All rats received i.p. injections on days 1, 4, and 7. Injection schedules' consisted of: poly I-C provided on days 1, 4, and 7; a single dose of poly I-C on day 7 only; or saline injections. On the 8th day all rats were inoculated with radiolabeled MADB106 tumor cells. Simultaneously with tumor cells, rats received a subcutaneous injection of either metaproterenol (1 mg/kg) or vehicle (saline). Twenty-one hours later, rats were sacrificed to assess MADB106 lung tumor retention.
Both males and females were used in this study, and the results are presented and analyzed separately, as not all groups had both sexes. In males (Figs 1A) (n = 155), one way ANOVA indicated significant group differences (F(11, 143) = 64.5, p < 0.05), and PLSD contrasts indicated that without poly I-C treatment metaproterenol caused a significant 26-fold increase in MADB106 lung tumor retention (LTR). All groups treated with poly I-C, either by one or three injections, exhibited significantly reduced effect of metaproterenol, as indicated by significant pair-wise PLSD contrasts to the no-poly I-C metaproterenol group (p < 0.05 for each of the five comparisons). To compare the efficacy of one injection to three injections in the two doses common to both schedules (0.1 and 0.3 mg/kg), we conducted a 2 × 2 ANOVA on the four groups that received metaproterenol in these conditions. Significant main effects for both factors were revealed (F(1, 30) = 13.0, p < 0.05, for schedule; and F(1, 30) = 4.6, p < 0.05, for dose) without significant interaction, indicating that three injection exert greater protection, and that 0.3 mg/kg is more effective than 0.1 mg/kg. In females (Figs 1B) (n = 64) only a single poly I-C administration was studied, and the same pattern of results was observed, indicated by the same statistical analyses (F(7, 56) = 23.8, p < 0.05; PLSDs p < 0.05 for the above contrasts).
Figure 1.

Repeated dosing of low-dose poly I-C is highly effective against the promotion of MADB106 metastasis by a pharmacological stressor, metaproterenol. Poly I-C was administered at the indicated dose either once, or three times (three days apart). Twenty four hours after the last poly I-C injection, all rats were intravenously inoculated with radiolabeled tumor cells, and simultaneously injected with metaproterenol (MP) or saline (Saline). MADB106 lung tumor retention (LTR) was assessed 21 h later. In both males (A) and females (B), MP significantly increased MADB106 LTR (**) in rats not treated with poly I-C, and all doses of poly I-C significantly reduced this effect (*). In males, three injections of poly I-C were more effective than one injection (#), and 0.3 mg/kg was more effective than 0.1 mg/kg.
A single administration of poly I-C is protective for up to 72 hours
In order to rationally plan the timing of poly I-C injections in subsequent experiments, we first determined the duration of the protective effect of a single dose of poly I-C. F344 males and females were randomly assigned to saline (no poly I-C) or poly I-C treated groups. Poly I-C (0.2 mg/kg) was administered only once to each rat in the poly I-C groups, at either 96, 72, 48, 24, or 12, hours before inoculation with MADB106 tumor cells. Saline was injected to all rats at all time points at which they did not receive poly I-C. Each group was further subdivided to receive metaproterenol (1 mg/kg) or vehicle (no metaproterenol) simultaneously with radiolabeled MADB106. LTR was assessed as in the first experiment (n = 10-18 rats per group). Males and females showed very similar effects, and are therefore presented together. As seen in Fig 2, in rats not treated with poly I-C, metaproterenol significantly increased lung tumor retention (t(34) = 44.3, p < 0.01 Bonferroni corrected). We conducted separate ANOVAs for rats treated with metaproterenol and those not treated with metaproterenol, as different variance characterize each category. ANOVA indicated significant group differences in rats receiving metaproterenol (F(5, 72) = 5.1, p < 0.05), and PLSD contrasts indicated that poly I-C reduced LTR in these groups when administered 12, 24, 48, or 72 h prior to metaproterenol (p < 0.05 for all comparisons to the no ploy I-C group), but not 96 h prior to it. ANOVA revealed no significant effects for poly I-C in rats not receiving metaproterenol.
Figure 2.
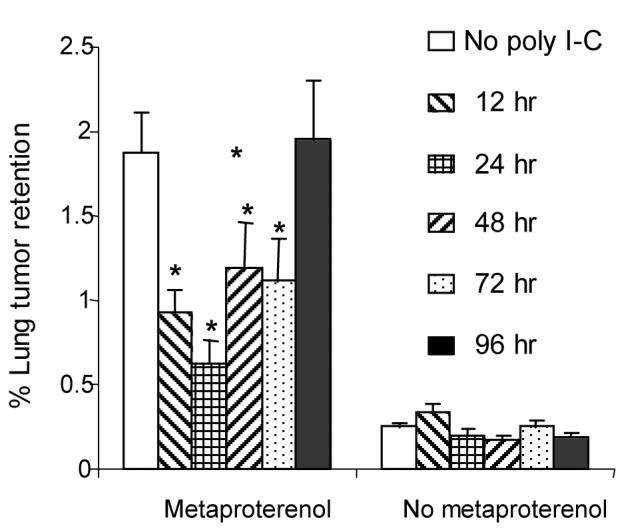
A single administration of poly I-C protects against the metastasis-promoting effect of metaproterenol for up to 72 hrs. Male and female F344 rats were treated with a single dose of poly I-C or with saline (no poly I-C). Poly I-C (0.2 mg/kg) was administered once at either 96, 72, 48, 24, or 12 h prior to inoculation with radiolabeled MADB106 tumor cells. Each group was further subdivided to receive metaproterenol or vehicle, simultaneously with MADB106 cells, and lung tumor retention (LTR) was assessed 21 hr later. Poly I-C significantly reduced the metastasis-promoting effects of metaproterenol (*) when administered up to 72 h before tumor inoculation.
No direct in vitro effects of poly I-C on MADB106 proliferation
To test whether there is a direct influence of poly I-C on tumor proliferation we co-incubated dividing MADB106 cells with the drug. Poly I-C did not affect the proliferation of MADB106 cells during a 48 h (i.e., 2.5 cells divisions) incubation with 2 mg/l, 0.66 mg/l, 0.2 mg/l, 0.066 mg/l, and 0.02 mg/l poly I-C, concentrations which span 10-fold lower to 10-fold higher than the estimated in vivo concentration following the administration of 0.2 mg/kg (also 0.2 mg/l) poly I-C in vivo (data not shown due to space limitation).
The presence of a primary tumor does not alter the protective effects of poly I-C
In the clinical setting, patients harbor primary tumors during the preoperative period, for which we propose the prophylactic poly I-C treatment. The presence of a primary tumor and the host response to it could potentially alter the host response to poly I-C (e.g., the cytokines secreted). Thus, we examined whether presence or absence of a primary tumor would alter the efficacy of poly I-C in reducing stress-induced metastasis. F344 male and female rats were randomly assigned to receive a s.c. injection of 106 MADB106 cells that give rise to a local tumor, or sham injection. Two weeks later, when the subcutaneous tumors reached a diameter of approximately 1 cm, each of these two groups was subdivided into four subgroups that were treated with either poly I-C (0.1 mg/kg on days 1, 3, 5) or saline, and, on the sixth day, received i.v. radiolabeled MADB106 cells with either metaproterenol (1 mg/kg) or vehicle, forming a 2×2×2 factorial design (n = 85 in these 8 groups; 30 females and 55 male, evenly distributed in all groups) (Figure 3). Twenty one hours later rats were sacrificed to assess lung tumor retention.
Figure 3.

A subcutaneous tumor does not alter the protective effect of poly I-C. Rats were inoculated subcutaneously with a million MADB106 tumor cells, or with vehicle. Two weeks later, when a local MADB106 tumor (1 cm, s.c.) formed, each group was subdivided into 4 conditions: treated with repeated poly I-C (on days 1, 3, & 5, 0.1 mg/kg/injection) or with saline, and on the sixth day receiving metaproterenol (MP) or saline. MADB106 lung tumor retention (LTR) was studied. In rats bearing a local tumor and in naïve rats, metaproterenol significantly increased lung tumor retention, and poly I-C markedly and significantly reduced this increase. Bearing a local tumor did not influence tumor retention and did not interact with any other factor.
Female and male rats showed similar LTR in all experimental conditions and are therefore presented together. A 2×2×2 ANOVA yielded a significant interaction between MP and poly I-C (F(1, 77) = 14.7, p < 0.05), but no main effects for primary tumor or interaction of primary tumor with any other factor. PLSD revealed that metaproterenol increased lung tumor retention (p < 0.05), and poly I-C markedly and significantly reduced this increase (p < 0.05). These effects were similar in the groups with the local subcutaneous tumor and in the group without it (Fig 3). These results indicate that bearing a primary tumor does not impair the effectiveness of poly I-C in protecting against the metastasis-promoting effects of metaproterenol.
Low-dose poly I-C has minimal toxicity
Administration of poly I-C is associated with significant toxicity (Levine and Levy, 1978). We therefore went on to compare the toxicity of poly I-C at a dose commonly used in vivo in rats (4m g/kg) to the low dose we intended to use in our future study (0.2 mg/kg). In rodents, toxic doses of poly I-C are known to reduce body weight in a dose dependent manner (Hartmann et al., 1987). In the current study, F344 males were randomly assigned to receive either saline, 4 mg/kg poly I-C (high dose), or 0.2 mg/kg poly I-C (low dose) i.p. on days 1, 3 and 5 (n = 9-10 males per group). Rats were weighed before and 8, 11 and 24 hours after each injection. Between days 6 and 16 rats were weighed daily (Figure 4). Body temperature was measured before and 4, 8, 11 and 24 hours after each injection, until day 6.
Figure 4.
Low repeated doses of poly I-C have relatively small adverse effects on body weight and on core body temperature. All rats received three i.p. injections (every other day, one hour after the beginning of the dark phase). Rats were subjected to either saline injections (no poly I-C), a low poly I-C dose (0.2 mg/kg), or a high poly I-C dose (4 mg/kg). Arrows indicate injection times. The high dose poly I-C regimen caused significantly 4-fold greater body weight loss than the low-dose regimen (A). The high, but not the low poly I-C dose significantly disrupted circadian rhythm of body temperature (B).
One way repeated measure (time points) ANOVA indicated that poly I-C affected body weight (F(2, 576) = 25.0, p < 0.05). Importantly, in the 4 mg/kg group, weight loss was markedly and significantly greater than in the 0.2 mg/kg group (approximately 4-fold greater, averaging 26 g vs. 7 g during 107-240 h following the first injection) (PLSD, p < 0.05) (Figure 4A). Body temperature was also affected by poly I-C. The circadian rhythm (Fig 4B) of body temperature was disrupted in the 4 mg/kg group, as indicated by a significant interaction between time and body temperature (F(14, 238) = 3.5, p < 0.05), but was not altered in the 0.2 mg/kg group. Thus the data demonstrate that compared to traditional dosing, repeated low doses of poly I-C only slightly reduced body weight and did not affect body temperature, indicating minimal toxicity.
Choosing a regimen of poly I-C to be used in the following studies
Based on the above four experiments, we chose to use a regimen of three doses of 0.2 mg/kg poly I-C, administered every other day. The lower dose of 0.1 mg/kg was overall less effective. Three rather than two day intervals between injections seemed too long, as the effect of the first injection was optimal after one day and completely dissipated by four days. This low dose poly I-C regimen has minimal toxicity, while retaining the protective effect against tumor-promotion induced by metaproterenol, a non-specific β-adrenergic agonist shown previously to be a potent NK-suppressive drug (Shakhar and Ben-Eliyahu, 1998).
Poly I-C prevents the increase in lung tumor retention induced by swim stress
To test the protective effects of our poly I-C regimen against an endogenous response to stressful conditions, we employed the swim stress paradigm and compared its effects to those of metaproterenol. Rats were either pretreated i.p. with poly I-C (0.2 mg/kg) or with saline on days 1, 3, & 5, and on the sixth day were either subjected to swim stress, injected with metaproterenol, or injected with vehicle. Rats were inoculated with radiolabeled MADB106 tumor cells one hour after the end of the swim stress, or simultaneously with the metaproterenol or vehicle, as in previous studies (n = 34 males in these six groups). All rats housed in a cage were either exposed to swim stress or not.
In groups not receiving poly I-C, metaproterenol and swim stress each significantly increased LTR (t(12) = 5.3, t(10) = 7.2, respectively, p < 0.008 Bonferroni α correction to 0.008) (Fig 5A). Poly I-C dramatically and significantly reduced the tumor promoting effect of both metaproterenol and swim stress (t(10) = 4.0, t(9) = 6.3, respectively, p < 0.008). Poly I-C alone did not significantly reduce baseline levels of LTR in non-stressed rats. These data demonstrate that the poly I-C regimen was efficient in negating the adverse effects of an endogenous stress response on LTR.
Fig 5.

Poly I-C protects from metaproterenol- and stress-induced increases in lung tumor retention (LTR) and lung metastases. Rats were treated with poly I-C (0.2 mg/kg) or saline (no poly I-C) on days 1, 3, & 5, and on the sixth day were either subjected to swim stress, injected with metaproterenol (MP) (1 mg/kg), or injected with saline (No stress). All rats were then inoculated with MADB106 tumor cells. (A) LTR was assessed 21 hr after tumor inoculation. Poly I-C treatment abrogated the tumor promoting effects of both swim stress and metaproterenol, while having no effect on LTR levels in non-stressed rats. (B) Rats were sacrificed three weeks after tumor inoculation and surface lung metastases were counted. In the no-poly I-C groups, MP significantly increased the number of lung metastases. Poly I-C treatment abrogated this tumor promoting effect of MP. Poly I-C also significantly reduced the number of metastases in non-stressed rats.
The development of actual lung metastases: protection by poly I-C against promotion by metaproterenol
LTR assessed in the previous experiments reflects an early step in the metastatic activity in this rat model. It is mainly sensitive to NK activity, and indicates a cumulative effect restricted to the first 24 hr after tumor inoculation (Shakhar and Ben-Eliyahu, 1998) (Ben-Eliyahu and Page, 1992) (Barlozzari et al., 1983). Therefore, to examine whether the protective effect of poly I-C has long-term significance and influence the actual development of macro-metastases, we evaluated the lungs three weeks following tumor inoculation. Rats were treated as in the above study with poly I-C or with saline (no poly I-C) on days 1, 3, & 5. On the sixth day, all rats were inoculated with MADB106 tumor cells, and simultaneously received either metaproterenol (1 mg/kg) or vehicle. Three weeks later, all rats were sacrificed and macroscopic surface metastases on the lungs were counted (n = 59 males in the four groups; Fig 5B). In the no poly I-C groups, metaproterenol caused a significant 3.5-fold increase in the number of lung surface metastases (t(29) = 4.6, p < 0.01 Bonferroni α correction to 0.01). Poly I-C completely abolished the metastasis-promoting effect of metaproterenol (t(29) = 3.9, p < 0.01), reducing lung metastases to baseline levels (no poly I-C no metaproterenol). In rats not subjected to metaproterenol poly I-C also significantly reduced the number of metastases by 5.3-fold (t26) = 6.7, p < 0.01). Thus, the beneficial effects of poly I-C against metaproterenol-induced increases in LTR also translate to a decrease in the number of subsequent lung metastases in this model. In contrast to the findings of LTR described above, poly I-C also markedly and significantly reduced baseline levels of metastases, an index that is most likely also affected by immune functions other than NK activity.
NK cells are crucial for the in vivo control of MADB106 LTR and lung metastases in both normal rats and in poly I-C treated rats
To understand the in vivo role of NK cells in controlling MADB106 metastasis in normal rats and in poly I-C treated rats, we conducted a study based on selective depletion of NK cells. We used the anti-NKR-P1 antibody which recognizes the CD161 marker that is highly expressed on NK cells. We have previously shown that this antibody, but not isotype control antibodies, renders NK cells ineffective in vivo immediately upon administration, and selectively depletes NK cells within a day (Ben-Eliyahu and Page, 1992). In the current study, F344 males were randomly assigned to be treated with poly I-C (0.2 mg/kg on days 1, 3, & 5) or with saline, and on the sixth day each group was further divided to receive anti-NKR-P1 antibody (1.5 mg/kg, i.v.) or vehicle. Tumor cells were administered simultaneously with anti-NKR-P1. In the first study LTR was assessed twenty-one hours after inoculation with radiolabeled MADB106 tumor cells (n = 40 males in these four groups). In the second study metastases were counted three weeks after tumor inoculation (n = 40 males in these four groups).
Selective depletion of NK cells caused a very large increase in LTR (Fig 6A) and in the number of lung metastases (Fig 6B) in control rats (t(16) = 20.5, p < 0.01), (t(18) = 21.3, p < 0.01 respectively; Bonferroni α correction to 0.01 in each study) and in rats treated with poly I-C (t(20) = 9.6, p < 0.01), (t(18) = 5.8, p < 0.01 respectively). With respect to both LTR and number of metastases, poly I-C did not significantly impact the magnitude of the effect of NK-depletion (e.g., 321-fold and 376-fold increase in LTR in control and in poly I-C treated rats, respectively).
Figure 6.

NK cells are required in vivo to control MADB106 LTR and metastasis in normal and in poly I-C treated rats. Rats were repeatedly injected with poly I-C (0.2 mg/kg, i.p. × 3) or saline (no poly I-C). Just before inoculation with tumor cells, half of each group was treated with anti-NKR-P1 to selectively block NK activity (NK-depleted), or with vehicle (naïve). All rats were then inoculated with MADB106 tumor cells. (A) LTR was assessed 21 hr after tumor inoculation. Selective elimination of NK activity markedly increased lung tumor retention in both poly I-C treated and untreated rats (Notice the logarithmic scale). Poly I-C had no significant effect on LTR in naïve rats. (B) Rats were sacrificed three weeks after tumor inoculation and surface lung metastases were counted. Selective elimination of NK activity markedly increased the number of lung metastases in both poly I-C treated and untreated rats (Notice the logarithmic scale). Poly I-C significantly reduced the number of metastases in naïve rats.
These findings indicate that NK cells play a critical role in controlling MADB106 LTR and lung metastases in both normal rats and in poly I-C treated rats. These studies, however, do not indicate whether poly I-C protects NK cells from suppression, which is the aim of the next two studies.
Poly I-C protects against metaproterenol-induced suppression of cytotoxicity in marginating pulmonary NK cells, but not in circulating NK cells
To elucidate the role of NK cells in mediating the protective effects of poly I-C, we examined the effect of poly I-C on number and activity of NK cells following stress. Our recent studies indicate that marginating pulmonary NK cells are unique in their ability to kill MADB106 cells (Melamed et al., 2005), an important finding in the current context since the lungs are the target organ for metastasis in this model system. We therefore assessed NK cell number and NK cytotoxicity in marginating pulmonary leukocytes and compared them with leukocytes from the general circulation. Rats were pretreated with poly I-C (0.2mg/kg) or saline on days 1, 3, and 5, and on the sixth day were injected with metaproterenol (1 mg/kg s.c.) or vehicle (n = 41 males in these 4 groups). One hour after metaproterenol injection, NK cell number was assessed in the blood (circulating NK cells) and in the marginating-pulmonary compartment. NK cytotoxicity of marginating pulmonary leukocytes was tested against the syngeneic MADB106 tumor cells and against the standard YAC-1 target cells. Circulating NK cytotoxicity was tested only against standard xenogeneic YAC-1 target cells, since circulating NK cells do not show NK cytotoxicity against the MADB106 line (Ben-Eliyahu et al., 1991).
Circulating NK cells
A 2 × 2 repeated measure ANOVA of NK cytotoxicity (Fig 7A) yielded main effect for metaproterenol (F(1, 37) = 28.7, p < 0.05), but no main effect for poly I-C and no interaction. These findings indicate that metaproterenol suppressed circulating NK cytotoxicity to a similar degree in control and in poly I-C treated rats. Importantly, this suppression seems unrelated to alterations in the numbers of circulating NK cells, as a 2 × 2 ANOVA on numbers of circulating NK cells (Fig 7C) yielded no main effect for metaproterenol nor an interaction with poly I-C. Thus the suppression of NK cytotoxicity by metaproterenol seems to occur on a per NK cell basis, both in control rats and in poly I-C treated rats. Irrespective of the effects of metaproterenol, poly I-C significantly increased numbers of circulating NK cells (a main effect for poly I-C F(1, 37) = 35.9, p < 0.05) without interaction) (Fig 7C). Taken together the findings indicate that the current poly I-C regimen increases the numbers of circulating NK cells (Fig 7C), but does not protect them from suppression by metaproterenol (Fig 7A).
Figure 7.
Poly I-C protects cytotoxicity of pulmonary NK cells, but not circulating NK cells, against suppression by metaproterenol. Rats were pretreated with poly I-C (0.2 mg/kg, i.p. × 3) or saline (no poly I-C), then injected with metaproterenol (MP) or vehicle (no MP). An hour later, the numbers of circulating and marginating pulmonary NK cells were assessed, and their cytotoxicity was tested against the YAC-1 and MADB106 target cells. In the blood, metaproterenol reduced NK cytotoxicity per ml blood in both poly I-C and no poly I-C treated rats (A). This suppression occurred on a per NK cell basis, as metaproterenol did not reduce the numbers of circulating NK cells in either condition (C). In contrast, in the lungs, poly I-C significantly reduced (or abrogated) the metaproterenol-induced suppression of NK cytotoxicity per lung against the YAC-1 (B) and MADB106 (D) target cells, which was evident in non poly I-C treated rats. Irrespective of the effects of MP, poly I-C significantly increased the numbers of circulating and marginating pulmonary NK cells (C).
Marginating pulmonary NK cells
The killing of YAC-1 (Fig 7B) and MADB106 (Fig 7D) target cells by marginating pulmonary NK cells yielded similar patterns of results, and are therefore described together. A 2 × 2 repeated measure ANOVA yielded a significant interaction between the effects of poly I-C and of metaproterenol (F(1, 37) = 5.5, 3.5, for YAC-1 and MADB106, respectively, p < 0.05 for both). Specifically, whereas metaproterenol significantly suppressed cytotoxicity in control rats (pair-wise PLSD < 0.05 for both YAC-1 and MADB106 target cells), it had no such effect in poly I-C treated rats. Assessing whether these findings are related to the numbers of marginating pulmonary NK cells, we conducted a 2 × 2 ANOVA on this index. Poly I-C significantly increased NK cell numbers (a main effect (F(1, 37) = 137.3, p < 0.05)), and metaproterenol significantly reduced it (a main effect (F(1, 37) = 10.4, p < 0.05)), without interaction.
Because metaproterenol significantly reduced both the numbers and the activity of NK cells in the no poly I-C groups, using the current approach we cannot determine whether metaproterenol suppressed lung NK activity per NK cell. On the other hand, in poly I-C treated rats, in which cytotoxicity was not suppressed, metaproterenol clearly did not suppress NK activity per NK cell. Take together, these findings indicate that poly I-C increased total marginating pulmonary NK activity, and that poly I-C treated rats do not exhibit suppression of individual marginating pulmonary NK cells.
Poly I-C protects against surgery-induced increase in lung tumor retention and against suppression of cytotoxicity in marginating pulmonary NK cells, but not in circulating NK cells
To test the potential protective effects of poly I-C under more clinically-relevant conditions, we employed a surgical stress paradigm and conducted (1) an in vivo study assessing MADB106 LTR, and (2) an ex-vivo study (in different rats) assessing numbers and activity of NK cells. Cytotoxicity of lung NK cells was tested both per the complete marinating pulmonary NK population as well as in samples containing equal numbers of NK cell (see Methods), in order to directly test whether poly I-C protects against suppression by surgery on a per NK-cell level. In both studies, rats were pretreated with poly I-C (0.2 mg/kg) or saline (no poly I-C) on days 1, 3, 5, and were further assigned to serve as controls or to undergo surgery 10 hours after the third poly I-C injection (a 2×2 design). All rats housed in a cage were either subjected to surgery or not (n = 6-8 male rats in each of the 4 groups in each of the 2 studies).
(1) In the in vivo study, rats were inoculated with MADB106 cells at the end of surgery and lung tumor retention was tested 21 h later. A 2 × 2 ANOVA indicated a significant interaction between the effects of surgery and of ploy I-C (F(1,26) = 10.13, p < 0.05). Specifically, whereas surgery increased LTR by more than 8 fold in non-poly I-C treated rats (pair-wise PLSD < 0.05), the prophylactic use of poly I-C completely prevented this increase, reducing LTR to the level seen in no-surgery rats that received poly I-C (Fig. 8A). Thus, poly I-C completely protected the rats from the increase in LTR caused by surgery.
Figure 8.
Poly I-C protects cytotoxicity of pulmonary NK cells, but not circulating NK cells, against suppression by surgery. Rats were pretreated repeatedly with poly I-C (0.2 mg/kg, i.p. × 3) or saline (no poly I-C), then subjected either to surgery, or left intact. In panel A, rats were inoculated with radiolabeled MADB106 cells, and lung tumor retention was assessed. In panels B-E, the number of circulating and marginating pulmonary NK cells was assessed and their cytotoxicity was tested against YAC-1 and MADB106 target cells. (A) Surgery increased MADB106 lung tumor retention, and poly I-C completely abolished this effect. (B) In the NK study, surgery markedly suppressed marginating pulmonary NK activity against MADB106 cells in the no poly I-C groups, but poly I-C completely prevented this suppression. (C) The numbers of pulmonary NK cells were not affected by surgery in either the vehicle or the poly I-C treated rats, suggesting that the suppression of NK activity and its prevention by poly I-C (evident in B) occurred on a per NK cell basis. (D) When equal numbers of lung NK cells were tested for their cytotoxicity, this deduction was demonstrated empirically. (E) In contrast, poly I-C did not protect blood NK cytotoxicity against suppression by surgery. Irrespective of the effects of surgery, poly I-C increased the numbers of NK cells in the lungs and circulation (C).
(2) In the ex-vivo study, rats were sacrificed 12 hr after surgery to study NK number and cytotoxicity in the circulation and in the marginating pulmonary compartment. All statistical analysis of this study (described below) employed 2 × 2 ANOVA with repeated measures when NK cytotoxicity was the dependent index.
Marginating pulmonary NK cells
ANOVA of NK cytotoxicity against MADB106 target cells revealed a significant interaction between the effects of surgery and of poly I-C (F(1,27) = 5.7, p < 0.05) (Fig 8B). Specifically, whereas surgery suppressed cytotoxicity in non-poly I-C treated rats (pair-wise PLSD, p < 0.05), the prophylactic use of poly I-C completely prevented this suppression. These findings do not seem to be related to alterations in the numbers of marginating pulmonary NK cells, as surgery did not reduce numbers of NK cells, nor did it interact with the effects of poly I-C on this index (Fig 8C). Empirically confirming this deduction are the results from the assessment of marginating pulmonary NK cytotoxicity per NK cell (using equal numbers of NK cells) (Fig 8D). ANOVA indicated a significant interaction between the effects of surgery and poly I-C (F(1,24) = 10.4, p < 0.05). That is, whereas surgery suppressed cytotoxicity per NK cell in non-poly I-C treated rats (pair-wise PLSD, p < 0.05), the prophylactic use of poly I-C completely prevented this suppression (Fig 8D). Irrespective of this protection, poly I-C increased the number of marginating pulmonary NK cells by 30-4-% (Fig 8C), but this increase did not reach a significant levels in this study. Overall, these findings indicate that poly I-C protects marginating pulmonary NK them from suppression by surgery.
Circulating NK cells
Surgery significantly suppressed NK activity per ml blood (a main effects of surgery F(1,28) = 42.3, p < 0.05), and poly I-C significantly increased NK cytotoxicity per ml blood (a main effects of surgery (F(1,28) = 7.7, p < 0.05) with no interaction between these factors (Figure 8E). The suppressive effects of surgery are not related to alterations in the numbers of NK cells, as surgery did not affect this index (no main effect of surgery and no interaction with the effects of poly I-C) (Figure 8C). On the other hand, poly I-C increased numbers of NK cells per ml blood in both operated and non operated rats, as indicated by a significant main effects of poly I-C (F(1,28) = 22.6, p < 0.05) without interaction with surgery (Figure 8C). This increase is likely to contribute to the elevation of NK cytotoxicity caused by poly I-C. Overall, these findings indicate that surgery suppressed NK activity per ml blood and per NK cell, and that poly I-C did not protect against this suppression.
DISCUSSION
Our goal in this study was to find a clinically-translatable approach to prevent stressors such as surgery from suppressing cellular immunity and promoting metastasis. We examined and developed different prophylactic regimens of low-dose poly I-C in rats, and chose to use a regimen of 3 doses of 0.2 mg/kg given every other day before applying stress. Compared to the traditionally-used doses (e.g., 4 mg/kg, (Liekens et al., 1999)), this low-dose regimen had minimal side effects in our rats. Importantly, it was more effective than a single administration of poly I-C, and was effective in both males and females, as well as in the presence of a primary tumor. We used three stress paradigms to test the efficacy of this regimen, a pharmacological stressor (metaproterenol), swim stress, and laparotomy, all of which increased in vivo measures of MADB106 metastasis. Our poly I-C regimen effectively eliminated the pro-metastatic effects of all three stress paradigms. In a different study, we now also assessed the efficacy of this exact poly I-C regimen using a different syngeneic tumor model – the CRNK-16 leukemia that constitutes a natural cause of mortality in senescent F344 rats. Poly I-C reduced mortality rates by more than three-fold (Avraham et al., 2006), supporting the generalizability of the beneficial effects of this paradigm to other tumors.
Immunotherapeutic approaches commonly aim at boosting anti-tumor immunity (Eklund and Kuzel, 2004). However, because major surgeries are often immunosuppressive (Mokart et al., 2002), boosting anti-tumor immunity in-and-of-itself may not suffice to prevent postoperative metastasis. Specifically, postoperative immunosuppression (Mokart et al., 2002) may eliminate the beneficial effects of pre-operative immunostimulation, rendering the patient susceptible to metastatic development during this critical period. Thus, while boosting baseline levels of immunity may prove beneficial in the absence of stress, during significant stress periods, including the immediate post-operative phase, prevention of stress-induced immunosuppression is critical. Unlike other approaches of immunotherapy, our poly I-C regimen is effective mainly in preventing immunosuppression. Indeed, in the absence of stress, poly I-C decreased MADB106 lung tumor retention only slightly, and did not increase cytotoxicity per individual NK cell. In contrast, in stressed rats poly I-C was markedly effective. This was demonstrated in our in vivo studies, where the metastasis-promoting effects of stress and surgery were dramatically decreased or completely abolished in poly I-C treated rats. More directly, whereas the ex vivo levels of pulmonary NK cytotoxicity per NK cell were significantly suppressed by surgery, poly I-C treatment prevented suppression of this cytotoxicity (without affecting baseline levels of NK activity) (Figures 7B, 7D, 8B, 8D). Interestingly, circulating NK cells were not protected by poly I-C (Figure 7A and 8E). We recently provided additional evidence supporting the difference between circulating and pulmonary NK cells. The same in vivo regimen of poly I-C protected pulmonary leukocytes, but not circulating leukocytes, from in vitro suppression of NK activity by prostaglandins and corticosterone (Shakhar et al., 2006). In yet another study we found that although IL-12 was more potent than poly I-C in enhancing baseline levels of NK activity, IL-12 did not protect these indices from suppression by surgery (Avraham et al., 2004). Overall, these findings suggest that boosting immune functions and protecting immunity from postoperative suppression act via different cellular mechanisms, and could be complementary treatments in cancer patients. Our herein regimen of low doses poly I-C protects pulmonary NK activity from immunosuppression without boosting NK activity per NK cell.
Several of our findings suggest a key role for pulmonary NK cells in controlling MADB106 metastasis and in mediating the protective effects of poly I-C. Selective depletion of NK cells increased lung tumor retention and lung metastasis of the MADB106 tumor more than 300–fold in both naïve and poly I-C treated rats. Our recent findings indicated that marginating-pulmonary, but not circulating or splenic NK cells, can kill MADB106 tumor cells in vitro, suggesting a key role for marginating-pulmonary NK cells in the in vivo resistance to MADB106 metastasis (Shakhar et al., 2006). Taken together, the similarity between the protective effects of poly I-C on ex-vivo cytotoxicity of marginating-pulmonary NK cells against MADB106 target cells on the one hand, and on the in vivo resistance to MADB106 lung metastasis on the other hand, suggests that marginating-pulmonary NK cells underlie the in vivo effects. This does not exclude a role for other mechanisms in mediating the beneficial effects of poly I-C, possibly via other leukocyte populations (Quan et al., 1999; Shingu et al., 2002).
The literature points to cellular mechanisms that may mediate the protective effects we observed with poly I-C. Poly I-C is recognized by the toll-like receptor-3 (TLR-3), which is expressed on hematopoietic and nonhematopoietic cells (Alexopoulou et al., 2001; Matsumoto et al., 2004). Recently it was demonstrated that TLR3 is constitutively expressed in lung epithelial cells (Guillot et al., 2005), and that TLR3 and its signaling-associated molecule, TRIF, play a key role in the immune response of pulmonary epithelial cells to poly I-C. Poly I-C also triggers ICAM-1 expression and IFN-β secretion by these cells (Guillot et al., 2005). ICAM-1 is an adhesion molecule which is involved in the recruitment and the local accumulation of leukocytes (Doukas et al., 1994; Guillot et al., 2005), which can explain our finding of increased numbers of marginating pulmonary NK cells. With respect to protecting NK activity against suppression by surgery, catecholamines and prostaglandins, both released during surgery (Baxevanis et al., 1994; Koltun et al., 1996), suppress NK activity by increasing intracellular cAMP levels (Malygin et al., 1993; Whalen and Bankhurst, 1990). In vitro studies indicate that interferons, which are secreted following poly I-C challenge, can reduce the intracellular rise in lymphocyte's cAMP, thus reducing the suppression of NK activity by catecholamines and prostaglandins, (Davis et al., 1984). Additionally, ds-RNAs, such as poly I-C, were shown to induce secretion of TNF-α and IL-1β by human lung epithelial cells (Meusel et al., 2002). TNF-α and IL-1β can also reduce intracellular increases in cAMP levels induced by a β-adrenoceptor agonist (Kelsen et al., 1997; Koto et al., 1996; Singh et al., 1993). Thus, the protective effect of poly I-C may be induced locally in the lungs by these proinflammatory cytokines. This provides an attractive explanation for our finding that poly I-C protected pulmonary, but not circulating NK cells, from suppression by surgery.
Surgery, by removing the major tumor mass, opens a window of opportunity for the immune system to eradicate minimal residual disease, and for the herein poly I-C regimen to support such a role for immunity. During the immediate postoperative period, oncological patients are at high risk for initiation and progression of metastasis due to various physiological responses, and competent anti-metastatic immunity is a critical factor determining long-term survival (for review see (Shakhar and Ben-Eliyahu, 2003)). The lung is a target site for many metastatic tumors, and our studies suggest that marginating pulmonary NK cells are unique in their ability to destroy relatively resistant tumor cells such as the MADB106 (Melamed et al., 2005). Thus, the specific effect of our treatment on marginating-pulmonary NK cells may prove especially beneficial in malignancies prone to develop late pulmonary metastases following complete resection of an initially localized cancer.
In summary, these data demonstrate that prophylactic low-dose poly I-C can transform marginating pulmonary immunocytes into a mode of resistance to immunosuppression by stress and surgery, and can protect against impaired resistance to experimental lung metastasis. This suggests a clinically-translatable novel application for poly I-C, to decrease the risk of surgery-induced metastasis in human cancers. Since the safety profile of poly I-C has already been examined in humans (Salazar et al., 1996), a clinical trial to examine the efficacy of a protective regimen of low-dose pre-operative poly I-C in cancer patients undergoing surgery for resection of a clinically-localized primary tumor could be feasible. A surrogate marker in such a clinical trial could be a moderate increase in the number of circulating NK cells following the low-dose poly I-C treatment. If effective, prophylactic pre-operative poly I-C may decrease the incidence of later metastasis in cancers that were initially deemed clinically-localized, and thus improve patient outcome.
Acknowledgment
This study was supported by NIH/NCI CA73056 grant (S. B-E) and by a grant from the Israel Science Foundation (S. B-E).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll- like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Andersen BL, Farrar WB, Golden-Kreutz D, Kutz LA, MacCallum R, Courtney ME, Glaser R. Stress and immune responses after surgical treatment for regional breast cancer. J. Natl. Cancer Inst. 1998;90(1):30–36. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham R, Inbar S, Rosenne E, Ben-Eliyahu S. Autologous control of a highly malignant syngeneic CRNK-16 leukemia in the rat: a role for NK cells. Cancer Immunol. Immunother. 2006;55(11):1348–1357. doi: 10.1007/s00262-006-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham R, Schwartz Y, Rosenne E, Melamed R, Ben-Eliyahu S. IL-12-based immunotherapy reduces post-surgery immunosuppression and metastasis, and increases survival from experimental leukemia; Paper presented at the Psychoneuroimmunology Research Society; Titisee, Germany. 2004. [Google Scholar]
- Barlozzari T, Reynolds CW, Herberman RB. In vivo role of natural killer cells: involvement of large granular lymphocytes in the clearance of tumor cells in anti-asialo GM1-treated rats. J. Immunol. 1983;131(2):1024–1027. [PubMed] [Google Scholar]
- Baxevanis CN, Papilas K, Dedoussis GV, Pavlis T, Papamichail M. Abnormal cytokine serum levels correlate with impaired cellular immune responses after surgery. Clin. Immunol. Immunopathol. 1994;71(1):82–88. doi: 10.1006/clin.1994.1055. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Page GG. In vivo assessment of natural killer cell activity in rats. Prog. Neuroendocrinemmunol. 1992;5:199–214. [Google Scholar]
- Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int. J. Cancer. 1999a;80(6):880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8(3):154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Shakhar G, Rosenne E, Levinson Y, Beilin B. Hypothermia in barbiturate-anesthetized rats suppresses natural killer cell activity and compromises resistance to tumor metastasis: a role for adrenergic mechanisms. Anesthesiology. 1999b;91(3):732–740. doi: 10.1097/00000542-199909000-00026. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Yirmiya R, Liebeskind JC, Taylor AN, Gale RP. Stress increases metastatic spread of a mammary tumor in rats: evidence for mediation by the immune system. Brain. Behav. Immun. 1991;5(2):193–205. doi: 10.1016/0889-1591(91)90016-4. [DOI] [PubMed] [Google Scholar]
- Davis VL, Earp HS, Stempel DA. Interferon inhibits agonist-induced cyclic AMP accumulation in human lymphocytes. Am. Rev. Respir. Dis. 1984;130(2):167–170. doi: 10.1164/arrd.1984.130.2.167. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo G, Esposito Pellitteri M, Drago A, Di Blasi P, Candore G, Balistreri C, Listi F, Caruso C. Effects of in vitro treatment with fluticasone propionate on natural killer and lymphokine-induced killer activity in asthmatic and healthy individuals. Allergy. 2001;56(4):323–327. doi: 10.1034/j.1398-9995.2001.00879.x. [DOI] [PubMed] [Google Scholar]
- Dokur M, Boyadjieva N, Sarkar DK. Catecholaminergic control of NK cell cytolytic activity regulatory factors in the spleen. J. Neuroimmunol. 2004;151(12):148–157. doi: 10.1016/j.jneuroim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Doukas J, Cutler AH, Mordes JP. Polyinosinic:polycytidylic acid is a potent activator of endothelial cells. Am. J. Pathol. 1994;145(1):137–147. [PMC free article] [PubMed] [Google Scholar]
- Eklund JW, Kuzel TM. A review of recent findings involving interleukin-2-based cancer therapy. Curr. Opin. Oncol. 2004;16(6):542–546. doi: 10.1097/01.cco.0000142070.45097.68. [DOI] [PubMed] [Google Scholar]
- Ellis NK, Duffie GP, Young MR, Wepsic HT. The effects of 16,16-dimethyl PGE2 and phosphodiesterase inhibitors on Con A blastogenic responses and NK cytotoxic activity of mouse spleen cells. J. Leukoc. Biol. 1990;47(4):371–377. doi: 10.1002/jlb.47.4.371. [DOI] [PubMed] [Google Scholar]
- Guillot L, Le Goffic R, Bloch S, Escriou N, Akira S, Chignard M, Si-Tahar M. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 2005;280(7):5571–5580. doi: 10.1074/jbc.M410592200. [DOI] [PubMed] [Google Scholar]
- Hartmann D, Schneider MA, Lenz BF, Talmadge JE. Toxicity of polyinosinic-polycytidylic acid admixed with poly-L-lysine and solubilized with carboxymethylcellulose in mice. Pathol. Immunopathol. Res. 1987;6(1):37–50. doi: 10.1159/000157040. [DOI] [PubMed] [Google Scholar]
- Hofer SO, Molema G, Hermens RA, Wanebo HJ, Reichner JS, Hoekstra HJ. The effect of surgical wounding on tumour development. Eur. J. Surg. Oncol. 1999;25(3):231–243. doi: 10.1053/ejso.1998.0634. [DOI] [PubMed] [Google Scholar]
- Kelsen SG, Anakwe O, Aksoy MO, Reddy PJ, Dhanasekaran N. IL-1 beta alters beta-adrenergic receptor adenylyl cyclase system function in human airway epithelial cells. Am. J. Physiol. 1997;273(3 Pt 1):L694–700. doi: 10.1152/ajplung.1997.273.3.L694. [DOI] [PubMed] [Google Scholar]
- Koga C, Itoh K, Aoki M, Suefuji Y, Yoshida M, Asosina S, Esaki K, Kameyama T. Anxiety and pain suppress the natural killer cell activity in oral surgery outpatients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001;91(6):654–658. doi: 10.1067/moe.2001.115465. [DOI] [PubMed] [Google Scholar]
- Koltun WA, Bloomer MM, Tilberg AF, Seaton JF, Ilahi O, Rung G, Gifford RM, Kauffman GL., Jr. Awake epidural anesthesia is associated with improved natural killer cell cytotoxicity and a reduced stress response. Am. J. Surg. 1996;171(1):68–73. doi: 10.1016/S0002-9610(99)80076-2. [DOI] [PubMed] [Google Scholar]
- Koto H, Mak JC, Haddad EB, Xu WB, Salmon M, Barnes PJ, Chung KF. Mechanisms of impaired beta-adrenoceptor-induced airway relaxation by interleukin-1beta in vivo in the rat. J. Clin. Invest. 1996;98(8):1780–1787. doi: 10.1172/JCI118977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90(7):2541–2548. [PubMed] [Google Scholar]
- Leung KH, Koren HS. Regulation of human natural killing. II. Protective effect of interferon on NK cells from suppression by PGE2. J. Immunol. 1982;129(4):1742–1747. [PubMed] [Google Scholar]
- Leung KH, Koren HS. Regulation of human natural killing. III. Mechanism for interferon induction of loss of susceptibility to suppression by cyclic AMP elevating agents. J. Immunol. 1984;132(3):1445–1450. [PubMed] [Google Scholar]
- Levine AS, Levy HB. Phase I-II trials of poly IC stabilized with poly-L-lysine. Cancer Treat. Rep. 1978;62(11):1907–1912. [PubMed] [Google Scholar]
- Liekens S, Verbeken E, Vandeputte M, De Clercq E, Neyts J. A novel animal model for hemangiomas: inhibition of hemangioma development by the angiogenesis inhibitor TNP-470. Cancer Res. 1999;59(10):2376–2383. [PubMed] [Google Scholar]
- Little D, Regan M, Keane RM, Bouchier-Hayes D. Perioperative immune modulation. Surgery. 1993;114(1):87–91. [PubMed] [Google Scholar]
- Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, Rainwater K, Ritchie JM, Yang M, Sood AK. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin. Cancer Res. 2003;9(12):4514–4521. [PubMed] [Google Scholar]
- Malygin AM, Meri S, Timonen T. Regulation of natural killer cell activity by transforming growth factor-beta and prostaglandin E2. Scand. J. Immunol. 1993;37(1):71–76. doi: 10.1111/j.1365-3083.1993.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Manetti R, Annunziato F, Tomasevic L, Gianno V, Parronchi P, Romagnani S, Maggi E. Polyinosinic acid: polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon- alpha and interleukin-12. Eur. J. Immunol. 1995;25(9):2656–2660. doi: 10.1002/eji.1830250938. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Funami K, Oshiumi H, Seya T. Toll-like receptor 3: a link between toll-like receptor, interferon and viruses. Microbiol. Immunol. 2004;48(3):147–154. doi: 10.1111/j.1348-0421.2004.tb03500.x. [DOI] [PubMed] [Google Scholar]
- Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain. Behav. Immun. 2005;19(2):114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Meusel TR, Kehoe KE, Imani F. Protein kinase R regulates double-stranded RNA induction of TNF-alpha but not IL-1 beta mRNA in human epithelial cells. J. Immunol. 2002;168(12):6429–6435. doi: 10.4049/jimmunol.168.12.6429. [DOI] [PubMed] [Google Scholar]
- Mokart D, Capo C, Blache JL, Delpero JR, Houvenaeghel G, Martin C, Mege JL. Early postoperative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with cancer. Br. J. Surg. 2002;89(11):1450–1456. doi: 10.1046/j.1365-2168.2002.02218.x. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben Eliyahu S, Yirmiya R, Liebeskind JC. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993;54(1):21–28. doi: 10.1016/0304-3959(93)90095-7. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S, Liebeskind JC. The role of LGL/NK cells in surgery-induced promotion of metastasis and its attenuation by morphine. Brain. Behav. Immun. 1994;8(3):241–250. doi: 10.1006/brbi.1994.1022. [DOI] [PubMed] [Google Scholar]
- Quan N, Zhang Z, Demetrikopoulos MK, Kitson RP, Chambers WH, Goldfarb RH, Weiss JM. Evidence for involvement of B lymphocytes in the surveillance of lung metastasis in the rat. Cancer Res. 1999;59(5):1080–1089. [PubMed] [Google Scholar]
- Salazar AM, Levy HB, Ondra S, Kende M, Scherokman B, Brown D, Mena H, Martin N, Schwab K, Donovan D, Dougherty D, Pulliam M, Ippolito M, Graves M, Brown H, Ommaya A. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study. Neurosurgery. 1996;38(6):1096–1104. [PubMed] [Google Scholar]
- Shakhar G, Abudarham N, Melamed R, Schwartz Y, Rosenne E, Ben-Eliyahu S. Amelioration of operation-induced suppression of marginating pulmonary NK activity using poly IC: A potential approach to reduce post operative metastasis. Ann. Surg. Oncol. 2006 doi: 10.1245/s10434-006-9078-9. in press. [DOI] [PubMed] [Google Scholar]
- Shakhar G, Bar-Ziv I, Ben-Eliyahu S. Diurnal changes in lung tumor clearance and their relation to NK cell cytotoxicity in the blood and spleen. Int. J. Cancer. 2001;94(3):401–406. doi: 10.1002/ijc.1477. [DOI] [PubMed] [Google Scholar]
- Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J. Immunol. 1998;160(7):3251–3258. [PubMed] [Google Scholar]
- Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients? Ann. Surg. Oncol. 2003;10(8):972–992. doi: 10.1245/aso.2003.02.007. [DOI] [PubMed] [Google Scholar]
- Shingu K, Helfritz A, Kuhlmann S, Zielinska-Skowronek M, Jacobs R, Schmidt RE, Pabst R, von Horsten S. Kinetics of the early recruitment of leukocyte subsets at the sites of tumor cells in the lungs: natural killer (NK) cells rapidly attract monocytes but not lymphocytes in the surveillance of micrometastasis. Int. J. Cancer. 2002;99(1):74–81. doi: 10.1002/ijc.10279. [DOI] [PubMed] [Google Scholar]
- Singh M, Notterman DA, Metakis L. Tumor necrosis factor produces homologous desensitization of lymphocyte beta 2-adrenergic responses. Circ. Shock. 1993;39(4):275–278. [PubMed] [Google Scholar]
- Sugarbaker PH. Strategies for the prevention and treatment of peritoneal carcinomatosis from gastrointestinal cancer. Cancer Invest. 2005;23(2):155–172. [PubMed] [Google Scholar]
- Taketomi A, Shimada M, Shirabe K, Kajiyama K, Gion T, Sugimachi K. Natural killer cell activity in patients with hepatocellular carcinoma: a new prognostic indicator after hepatectomy. Cancer. 1998;83(1):58–63. doi: 10.1002/(sici)1097-0142(19980701)83:1<58::aid-cncr8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Maehara Y, Tokunaga E, Koga T, Kakeji Y, Sugimachi K. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: a multivariate analysis. Am. J. Gastroenterol. 2001;96(2):574–578. doi: 10.1111/j.1572-0241.2001.03535.x. [DOI] [PubMed] [Google Scholar]
- Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365(9460):687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Sonoda K, Sumiyoshi K, Kitamura K, Toh Y, Kitamura M, Kuwano H, Sugimachi K, Okamura S. Prognostic significance of immunological parameters in patients with esophageal cancer. Hepatogastroenterology. 1996;43(9):501–509. [PubMed] [Google Scholar]
- Wadler S, Levy D, Frederickson HL, Falkson CI, Wang Y, Weller E, Burk R, Ho G, Kadish AS. A phase II trial of interleukin-12 in patients with advanced cervical cancer: clinical and immunologic correlates. Eastern Cooperative Oncology Group study E1E96. Gynecol. Oncol. 2004;92(3):957–964. doi: 10.1016/j.ygyno.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Weitz J, Herfarth C. Surgical strategies and minimal residual disease detection. Semin. Surg. Oncol. 2001;20(4):329–333. doi: 10.1002/ssu.1051. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Bankhurst AD. Effects of beta-adrenergic receptor activation, cholera toxin and forskolin on human natural killer cell function. Biochem. J. 1990;272(2):327–331. doi: 10.1042/bj2720327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D, Williams BR. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res. 2004;64(16):5850–5860. doi: 10.1158/0008-5472.CAN-04-0063. [DOI] [PubMed] [Google Scholar]
- Wu J, Lanier LL. Natural killer cells and cancer. Adv. Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, Page GG, Ben-Eliyahu S. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann. Surg. Oncol. 2003;10(4):469–479. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Zetter BR. Angiogenesis and tumor metastasis. Annu. Rev. Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]





