Abstract
Background
Quantitative endophenotypes are needed to better understand the pathogenesis of schizophrenia. The psychobiological model of temperament and character suggests that personality traits are heritable and regulated by brain systems influencing schizophrenia susceptibility. Thus, measures of temperament and character may serve as schizophrenia-related endophenotypes in individuals with schizophrenia and their non-psychotic siblings.
Methods
Individuals with schizophrenia (n=35), their non-psychotic siblings (n=34), controls (n=63), and their siblings (n=56) participated in a study of the clinical, cognitive and neuromorphological characteristics of schizophrenia. A mixed-model approach assessed group differences on the Temperament and Character Inventory (TCI). Neurocognitive deficits and psychopathology were correlated with the TCI. Configurations of TCI domains were examined using a generalized linear model.
Results
Individuals with schizophrenia and their siblings had higher harm avoidance than controls and their siblings. Individuals with schizophrenia had lower self-directedness and cooperativeness, and higher self-transcendence than their non-psychotic siblings, controls, and the siblings of controls. Neurocognition was not related to temperament and character in individuals with schizophrenia or either control group. In non-psychotic siblings, self-directedness and cooperativeness were correlated with working memory and crystallized IQ.
Conclusion
Evidence supports harm avoidance as a schizophrenia-related endophenotype. An increased risk of schizophrenia may be associated with asociality (configured as high harm avoidance and low reward dependence), schizotypy (configured as low self-directedness, low cooperativeness, and high self-transcendence), and neurocognitive deficits (poor executive functioning, working/episodic memory, attention, and low IQ). The non-psychotic siblings demonstrated features of a mature character profile including strong crystallized IQ, which may confer protection against psychopathology.
1. Introduction
Although several studies have examined schizotypal personality in first-degree relatives of schizophrenia patients to better understand familial transmission of the illness (Fogelson et al., 2007; Fogelson et al., 1999; Battaglia et al., 1995; Kendler et al. 1993), much less is known about the profile of temperament and character in schizophrenia (Guillem et al.,. 2002; Szöke et al., 2002). The psychobiological model of personality suggests an individual’s temperament is heritable and regulated by neurotransmitters linked to the pathophysiology of schizophrenia (Cloninger, 1987). More recently, evidence has appeared that dimensions of character are also heritable and may influence the risk for schizotypy (Bora & Veznedaroglu, 2007; Gillespie et al., 2003).
Research on the average values of personality dimensions suggests that schizophrenia patients and their first-degree relatives have a profile of temperament and character that is unique from the general population (Bora & Veznedaroglu, 2007; Glatt et al., 2006; Kurs et al., 2005; Guillem et al., 2002; Szöke et al., 2002). The Temperament and Character Inventory (TCI: Cloninger et al., 1993) was used to assess domains of personality for these individuals. The TCI segments temperament into four dimensions – novelty seeking (NS), harm avoidance (HA), reward dependence (RD), and persistence (PS) – and character into three dimensions – self-directedness (SD), cooperativeness (CO), and self-transcendence (ST).
In regards to temperament, individuals with schizophrenia have high average values of HA, while having levels of NS similar to controls (Kurs et al., 2005; Guillem et al., 2002; Szöke et al., 2002). Findings on other temperament dimensions remain mixed (Kurs et al., 2005; Guillem et al., 2002). With respect to character, schizophrenia patients have lower average values of SD and CO, but higher ST when compared to controls (Guillem et al., 2002), which supports Cloninger’s theory that schizotypy is characterized by low SD and CO, and high ST (Cloninger et al., 1993).
In addition to contrasting average levels of personality traits, analyzing configurations of traits may be informative due to potential nonlinear interactions between individual traits. In previous work, Cloninger reported that configurations of TCI domains marked by high and low average values were associated with subtypes of temperament and character (Cloninger et al., 1997). Accordingly, we will examine configurations of personality traits to assess potential influence on susceptibility to schizophrenia.
Although measures of psychopathology and neurocognition have emerged in the literature as endophenotypes (Gottesman & Gould, 2003) for the genetic liability to schizophrenia (Delawalla et al., 2006; Nuechterlein et al., 2002; Egan et al., 2001), research assessing temperament and character as schizophrenia-related endophenotypes in first-degree relatives is limited, with mixed findings (Bora & Veznedaroglu, 2007; Glatt et al., 2006). Some research did not find differences in temperament between first-degree relatives and controls (Bora & Veznedaroglu, 2007), while others found that sibling temperament may be intermediate between controls and schizophrenia patients (Calvo de Padilla et a., 2006). In regards to character, some studies found first-degree relatives of schizophrenia patients with lower CO and SD, and higher ST than controls (Calvo de Padilla et a., 2006; Glatt et al., 2006), while others found relatives scored higher than controls on SD and CO (Bora & Veznedaroglu, 2007).
Few studies have examined the relationship between personality and psychopathology or neurocognition in schizophrenia patients and their relatives (Bora & Veznedaroglu, 2007). Research suggests that higher levels of cognition and fewer positive and negative symptoms were related to greater openness, agreeableness, and conscientiousness, and lower neuroticism in schizophrenia patients (Lysaker & Davis, 2004). Thus, studying the relationship between psychopathology and individual personality traits, and the interaction of personality trait configurations with neurocognitive deficits relevant to schizophrenia may help to improve our understanding of the illness (Harvey et al., 2006; Snitz et al., 2006). To our knowledge, the present study will be the first to explore configurations of temperament and character in schizophrenia and examine associations between neurocognition and temperament and character among individuals with schizophrenia and their non-psychotic siblings.
Our objectives were to [1] compare individual personality traits between individuals with schizophrenia, non-psychotic siblings of individuals with schizophrenia, controls, and the siblings of controls, [2] explore if there were particular configurations of temperament and character that were more prevalent in individuals with schizophrenia than controls, [3] examine whether temperament and character traits or particular configurations of temperament and character occurred in non-psychotic siblings in a manner that was intermediate between the ill relatives and the siblings of controls, [4] examine how the susceptibility to develop schizophrenia could be configured via the interaction of temperament, character, and neurocognitive deficits, [5] examine whether temperament and character were correlated with specific clinical and neurocognitive features that were supported as schizophrenia-related endophenotypes.
2. Methods
2.1 Participants and inclusion criteria
Participants were recruited through the Conte Center for the Neuroscience of Mental Disorders at Washington University in St. Louis and included 35 individuals with DSM-IV schizophrenia (SCZ), 34 of their non-psychotic siblings (SCZ-SIB), 63 controls (CON), and 56 of their siblings (CON-SIB). Participants provided informed consent for study participation. All participants were assessed for the presence of DSM-IV Axis I disorders using the Structured Clinical Interview for DSM-IV-TR (First et al., 2002). Individuals with schizophrenia were stable and recruited from local inpatient (upon discharge) and outpatient treatment centers. With the consent of the individuals with schizophrenia (or guardian if the participant was a minor), their non-psychotic siblings were asked to participate in the study. Community controls and their siblings were recruited through local advertising. CON were excluded if they had a lifetime history of an Axis I psychotic or major mood disorder, or if they had a first-degree relative with a psychotic disorder. SCZ-SIB and CON-SIB were excluded if they had a lifetime history of any Axis I psychotic disorder, but not for other Axis I disorders. CON-SIB were included because they are less subject to selection effects and represent an additional comparison group more closely matched to SCZ-SIB in terms of their history of non-psychotic Axis I disorders. All subjects were excluded if [1] they met DSM-IV criteria for current substance abuse or dependence within the previous month, [2] had a severe medical disorder, [3] had a head injury with neurological sequelae, or [4] met DSM-IV criteria for mental retardation.
2.2 Measures
The Temperament and Character Inventory (TCI) is a self-report measure with 240 true/false items measuring four domains of temperament (i.e., NS, HA, RD, PS) and three domains of character (i.e., SD, CO, ST). Novelty seeking (NS) is the tendency to explore novel stimuli or pursue potential rewards. Harm avoidance (HA) is the inclination to avoid punishment. Reward dependence (RD) is social attachment based on approval and warmth. Persistence (PS) is perseverance in the face of adversity. Self-directedness (SD) is the will power to adapt changes to one’s environment. Cooperativeness (CO) is the degree to which a person is agreeable. Self-transcendence (ST) is the extent that you identify yourself as an essential part of the universe. A detailed review of the TCI, including an analysis of its reliability and validity, is available elsewhere (Cloninger et al., 1993).
Negative, positive, and disorganized symptoms of schizophrenia were assessed by calculating a standardized score using the Scale for the Assessment of Negative Symptoms (SANS: Andreasen, 1983a), the Scale for the Assessment of Positive Symptoms (SAPS: Andreasen, 1983b), the Structured Interview for Prodromal Symptoms (SIPS: McGlashan et al., 2000), and the Chapman Psychosis Proneness Scales (Chapman et al., 1995).
Based on prior research (Nuechterlein et al., 2004), we converted raw scores from a battery of neuropsychological tests into five domains of standardized scores: executive functioning, working memory, episodic memory, attention, and crystallized IQ. A detailed description of all measures and the individual scores used for each psychopathological and cognitive domain can be found elsewhere (Harms et al., 2008; Delawalla et al., 2006) and in appendix A.
2.3 Statistical Analysis
Group differences for the domains of psychopathology, neurocognition, temperament and character were assessed using a mixed-model with group, gender, and age as fixed effect predictors. The mixed model (PROC MIXED, SAS 9.1) estimated the covariance in the residuals due to the sibling relationships, allowing for a heterogeneous covariance structure for the two sets of sibling pairs. Post hoc comparisons between groups were examined when a significant main effect was present (based on Type III statistics). Age and gender were included as covariates due to between-group differences of those variables. Education was not included as a covariate as it may be influenced by factors related to the development of schizophrenia.
In order to examine the configurations of temperament and character, each domain was dichotomized using the overall sample median, with high levels defined as scores at or above the median. Based on prior research (Cloninger et al., 1997), persistence was not used in TCI configurations. Capital letters from the first word of the domain reflect scores at/above the median while lower-case letters reflect scores below the median (e.g., scT = low self-directedness, low cooperativeness, high self-transcendence; “t”indicates low self-transcendence). Dichotomous variables were created to indicate the presence/absence of each configuration in the study subjects.
The temperament and character configuration were analyzed with a generalized linear model having a binomial distribution and logit link function, and using generalized estimating equations to handle the possible correlation in the dichotomous dependent variable due to the sibling pairs (SPSS 16.0). Group, age and gender were included as effects to estimate whether group status had a main effect on the presence of each configuration. Estimated marginal means for each group were reported based on the original scale of the dependent variable (i.e., the marginal means range between 0 and 1, and can be interpreted as estimated proportions). Pairwise comparisons were examined when group status had a significant main effect (based on Type III statistics).
To assess the influence of temperament, character, and neurocognitive deficits on familial susceptibility to schizophrenia, we first created a dichotomous variable to estimate the presence of neurocognitive deficits representative of schizophrenia, also termed “schizophrenia-like.” This was done by using a discriminant analysis applied to the five neurocognitive domains (using a leave-one-out approach with prior probabilities adjusted to reflect the proportion of individuals with schizophrenia and controls) to classify subjects as having “schizophrenia-like” neurocognitive deficits. Predictive probabilities greater than .50 were coded “1” for presence of “schizophrenia-like” neurocognitive deficits. Then we cross tabulated the most prevalent temperament and character configurations in individuals with schizophrenia with the dichotomized neurocognitive variable.
To examine the relationship between the TCI and domains of psychopathology and neurocognition, Pearson correlations were computed for all four groups. To partially correct for multiple comparisons among the correlations, we only highlight correlations significant at p≤.01.
3. Results
3.1 Demographic and Clinical Characteristics
Table 1 presents the demographic characteristics of the study sample. There was a significant main effect on age, gender, and education. Sixty percent (n=21) of individuals with schizophrenia reported currently taking atypical antipsychotic medication only, while thirty-six percent (n=12) reported currently taking both typical and atypical antipsychotic medications. Two individuals with schizophrenia reported that they were not currently taking antipsychotic medication. Raw TCI scores are reported in Table 2. Standardized means for psychopathology and neurocognition are reported in Appendix A.
Table 1.
Demographic Variables
| SCZ N=35 | SCZ-SIB N=34 | CON-SIB N=56 | CON N=63 | |
|---|---|---|---|---|
| Age, mean (SD)a | 22.9 (3.3) | 21.9 (3.7) | 20.7 (3.6) | 21.0 (3.6) |
| Gender (% male)b | 82.9 | 47.1 | 19.6 | 46.0 |
| Years of education, mean (SD) c | 11.6 (1.9) | 12.5 (2.9) | 12.9 (2.6) | 13.2 (2.8) |
| Race (% white) | 60.0 | 61.8 | 78.6 | 77.8 |
| Anti-psychotic medication | ||||
| Atypical only (%) | 60.0 | 0.0 | 0.0 | 0.0 |
| Atypical and typical (%) | 34.3 | 0.0 | 0.0 | 0.0 |
| No antipsychotic | ||||
| medications (%) | 5.7 | 0.0 | 0.0 | 0.0 |
Differences in age and education were evaluated using an ANOVA; differences in gender and race were evaluated using a chi-square analysis.
F3,184=3.4, p=.02; SCZ>CON-SIB (p=.005), CON (p=.01).
χ2(3) = 34.9, p<.001;
F3,184=3.0, p=.03; SCZ<CON-SIB (p=.026), CON (p=.004).
Table 2.
Mean Scores on the Dimensions of the TCI
| SCZ N=35 | SCZ-SIB N=34 | CON-SIB N=56 | CON N=63 | |
|---|---|---|---|---|
| Temperament | ||||
| NS | 18.5 (1.4) | 19.7 (1.2) | 18.7 (1.3) | 18.4 (1.1) |
| HAa | 17.8 (1.5) | 13.9 (1.1) | 9.1 (1.0) | 8.2 (0.8) |
| RDb | 11.4 (0.9) | 16.1 (0.7) | 14.9 (1.0) | 14.5 (0.9) |
| PS | 4.1 (0.4) | 5.4 (0.3) | 4.8 (0.3) | 5.1 (0.3) |
| Character | ||||
| SDc | 20.0 (1.8) | 31.8 (2.0) | 28.3 (1.9) | 29.8 (1.7) |
| COd | 24.5 (1.9) | 33.0 (1.7) | 30.5 (1.9) | 30.7 (1.7) |
| STe | 16.5 (1.6) | 14.7 (1.2) | 11.1 (0.9) | 10.1 (0.8) |
Values are least square means (standard errors) as assessed in a mixed model with age and gender as covariates.
F3,73=12.7, p<.001; SCZ>SCZ-SIB (p=.05), CON-SIB (p<.001), CON (p<.001). SCZ>CON-SIB (p=.002), CON (p<.001).
F3,67=7.9, p<.001; SCZ<SCZ-SIB (p<.001), CON-SIB (p=.02), CON (p=.02).
F3,76=7.5, p<.001; SCZ<SCZ-SIB (p<.001), CON-SIB (p=.004), CON (p<.001).
F3,72=3.8, p=.01; SCZ<SCZ-SIB (p=.002), CON-SIB (p=.04), CON (p=.02).
F3,67=5.7, p<.01; SCZ>CON-SIB (p=.006), CON (p<.001); SCZ-SIB>CON-SIB (p=.02), CON (p=.004).
3.2 Temperament
We found a significant main effect of group status on harm avoidance and reward dependence (Table 1). Upon examining the pairwise comparisons, SCZ had higher HA than SCZ-SIB (MD=4.0, SE=2.0; p=.05), CON-SIB (MD=8.7, SE=1.9; p<.001), and CON (MD=9.6, SE=1.8; p<.001). SCZ-SIB had higher HA than CON-SIB (MD=4.7, SE=1.5; p=.002) and CON (MD=5.6, SE=1.4; p<.001). SCZ had lower RD than SCZ-SIB (MD=−4.7, SE=0.9; p<.001), CON-SIB (MD=−3.5, SE=1.4; p=.02), and CON (MD=−3.1, SE=1.3; p=.02). There was no main effect of group on NS (p=.86) or PS (p=.06).
Novelty seeking was not used in the analysis of temperament configurations due to the lack of a main effect of group status. Thus, there were four potential configurations of temperament (i.e., HR, Hr, hR, hr). We found that the configuration of high harm avoidance and low reward dependence (Hr) was more often present in SCZ than in CON (MD=.52, SE=.10; p<.001) (Table 2). The “Hr” configuration typically describes someone as socially anxious and isolated, which is summarized as “asociality” in later analyses.
3.3 Character
We found significant main effects of group status on all three character domains– self-directedness, cooperativeness, and self-transcendence (Table 1). Upon examining the pairwise comparisons, SCZ had lower SD than SCZ-SIB (MD=−11.7, SE=2.7; p<.001), CON-SIB (MD=−8.2, SE=2.7; p=.004), and CON (MD=−9.7, SE=2.5; p<.001). SCZ also had lower CO than SCZ-SIB (MD=−8.4, SE=2.5; p=.002), CON-SIB (MD=−6.0, SE=2.9; p=.04), and CON (MD=−6.1, SE=2.6; p=.02). SCZ had higher ST than CON-SIB (MD=5.5, SE=1.9; p=.006) and CON (MD=6.4, SE=1.8; p<.001). Also, SCZ-SIB had higher ST than CON-SIB (MD=3.6, SE=1.6; p=.02) and CON (MD=4.6, SE=1.5; p=.004) (Table 1).
There were eight potential configurations of character (i.e., SCT, SCt, ScT, sCT, Sct, sCt, scT, sct). We found that SCZ were more likely than CON (MD=.44, SE=.11; p<.001) to be configured as having low self-directedness, low cooperativeness, and high self-transcendence (scT) (Table 3). The “scT” configuration typically describes someone as irresponsible, aimless, suspicious, and highly imaginative, which is summarized as “schizotypy” in later analyses.
Table 3.
Analysis of Temperament and Character Configurations
| SCZ N=35 | SCZ-SIB N=34 | CON-SIB N=56 | CON N=63 | |
|---|---|---|---|---|
| Temperament | ||||
| HR | .15 (.07) | .37 (.09) | .26 (.06) | .16 (.05) |
| Hra | .65 (.10) | .33 (.08) | .18 (.06) | .13 (.04) |
| hRb | .08 (.05) | .17 (.07) | .38 (.07) | .39 (.06) |
| hrc | .11 (.06) | .12 (.06) | .16 (.05) | .32 (.06) |
| Character | ||||
| SCT | .06 (.04) | .24 (.08) | .23 (.06) | .20 (.05) |
| SCtd | .03 (.03) | .23 (.07) | .22 (.06) | .33 (.06) |
| ScT | .03 (.03) | .12 (.05) | .01 (.02) | .03 (.02) |
| SCT | .08 (.06) | .07 (.04) | .07 (.04) | .01 (.01) |
| Scte | - | - | - | - |
| sCte | - | - | - | - |
| scTf | .53 (.10) | .18 (.07) | .12 (.05) | .09 (.03) |
| Sct | .20 (.08) | .06 (.04) | .20 (.05) | .23 (.06) |
Generalized linear model estimated marginal means (standard errors); these means can be interpreted as an expected fraction after controlling for age, gender, and sibling correlation.
χ2(3) = 24.9, p<.001; SCZ>SCZ-SIB (p=.017), CON-SIB (p<.001), CON (p<.001).
χ2(3) = 12.3, p=.006; SCZ<CON-SIB (p=.001), CON (p<.001); SCZ-SIB<CONSIB (p=.027).
χ2(3) = 7.9, p=.047; SCZ<CON (p=.02).
χ2(3) = 16.3, p=.001; SCZ<SCZ-SIB (p=.013), CON-SIB (p=.004), CON (p<.001).
The model does not converge due to n=0 in one or more cells.
χ2(3) = 15.7, p=.001; SCZ<SCZ-SIB (p=.001), CON-SIB (p<.001), CON (p<.001).
3.4 Interactions among Neurocognition and Personality Configurations
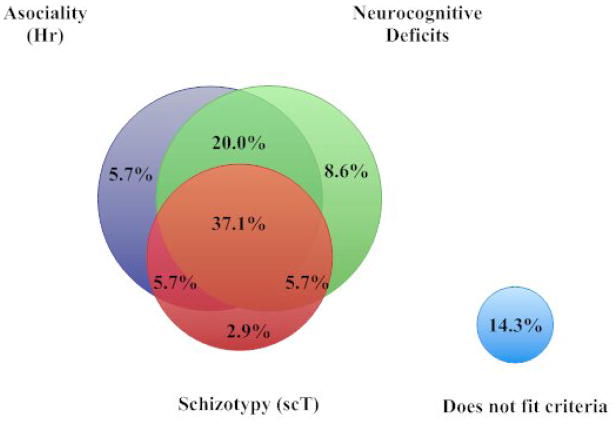
Discriminant analysis showed that 67.5% of individuals with schizophrenia had neurocognitive deficits typical of having schizophrenia (“schizophrenia-like”) while only 11.5% of controls were characterized by such a “schizophrenia-like” neurocognitive profile (Wilks λ=.58; χ2=43.1, df=5, p<.001). In our examination of the interaction of “asociality” (Hr), “schizotypy” (scT), and “schizophrenia-like” neurocognitive deficits we found that all three of these traits co-occurred in 37.1% of individuals with schizophrenia, 20% were configured by asociality and a “schizophrenia-like” neurocognitive profile, and 14.3% of the individuals were not categorized by any of these traits (Figure 1).
Figure 1.
Dimensional Configuration of Asociality, Schizotypy, and Cognitive Deficits in Individuals with Schizophrenia
3.4 Correlations of Clinical Variables
Correlations between the TCI, psychopathology and neurocognition were measured in the study groups. We found that HA was strongly correlated to negative symptoms in both SCZ (r=.42, p=.01) and SCZ-SIB (r=.52, p=.002), while ST was strongly correlated to positive symptoms in SCZ (r=.44, p=.008) and SCZ-SIB (r=.61, p<.001). In non-psychotic siblings, SD was inversely correlated with negative and disorganized symptoms (r=−.44, p=.01; r=−.49, p=.003, respectively), while cooperativeness was inversely correlated with negative symptoms (r=−.48, p=.004). Neurocognition was not correlated with temperament or character in SCZ, CON, and CON-SIB. However, in non-psychotic siblings, crystallized IQ and working memory were strongly correlated with SD (r=.60, p<.001; r=.53, p=.002; respectively) and CO (r=.68, p<.001; r=.59, p<.001; respectively).
4. Discussion
We found that harm avoidance (HA) was higher in SCZ than SCZ, CON, and CON-SIB, while SCZ-SIB was also significantly higher than the control groups. The intermediate level of harm avoidance in non-psychotic siblings provides evidence that HA may be an endophenotype for schizophrenia. These findings are consistent with prior studies examining individuals with schizophrenia (Kurs et al., 2005) and their first-degree relatives (Calvo de Padilla et al., 2006).
When examining the association between HA and psychopathology and neurocognition, we found that negative symptoms and HA were highly correlated in SCZ and SCZ-SIB. This is not surprising given that HA is characterized by inhibition and fatigability, both of which may be subclinical manifestations of negative symptoms. This lends support to the pervasive role of negative symptoms in individuals with schizophrenia (Fenton & McGlashan, 1991) and elevated negative symptomatology in first-degree relatives (Glatt et al., 2006). Also, we found a higher prevalence of SCZ configured as having high harm avoidance and low reward dependence or “Hr,” when compared to SCZ-SIB, CON, and CON-SIB. This indicates that individuals with schizophrenia have a higher prevalence of personalities characterized as socially detached and amotivated. Although the value of “Hr” in SCZ-SIB was intermediate between SCZ and CON-SIB, the difference between SCZ-SIB and CON-SIB did not reach statistical significance (p=.13). Thus, the evidence that the “Hr” configuration may be a schizophrenia endophenotype is unclear.
Our findings support previous research on character, which found that individuals with schizophrenia had lower SD and lower CO than controls (Guillem et al., 2002). Also, individuals with schizophrenia were frequently configured as having low SD, low CO, and high ST or “scT,” which is indicative of a struggle with identity, lack of empathy, and greater magical ideation. These results support prior research indicating that- low average values of self-directedness and cooperativeness, and high average values of self-transcendence represent “schizotypy” (Cloninger et al., 1993).
The Venn diagram (Figure 1) illustrates that individuals with schizophrenia were most frequently configured as having asociality (Hr), schizotypy (scT), and neurocognitive deficits. Notably, 20% of the individuals with schizophrenia had a combination of asociality and neurocognitive deficits, but not schizotypy. This suggests that asociality may drive or actuate the deficits in neurocognition, or vice versa. Thus, treatments that decrease HA, such as cognitive behavioral therapy and antidepressants (Abrams et al., 2004), could potentially reduce risk of schizophrenia. Figure 1 indicates that 85.7% of individuals with schizophrenia met at least one of our three sets of risk factors, which suggests an excellent sensitivity for detecting vulnerability for schizophrenia. The results also suggest that non-psychotic siblings did not receive a discrete “transmission” of schizotypy, but rather individually inherited personality traits, so that configurations like Hr (e.g., social anxiety, isolation) impacted neurocognition and psychobiological character traits in a nonlinear fashion: that is, particular configurations of these variables are critical for risk, not the sum of their average effects.
The profile of character in SCZ-SIB was distinguishable from the profile in SCZ. SCZ-SIB scored higher than SCZ on the measures of SD and CO, while they were similar on ST. Furthermore, the configuration of high SD, high CO, and low ST (SCt) was significantly more prevalent in SCZ-SIB, CON, and CON-SIB when compared to SCZ (Table 3). A similar pattern was present when the character configuration was SCT. High SD and CO in non-psychotic siblings reflects a highly goal-directed, responsible, and empathetic character.
These results are consistent with Bora and Veznedaroglu (2007) who reported high levels of self-directedness and cooperativeness in non-psychotic siblings. They suggested that relatives may develop greater responsibility, goal-orientation, or cooperativeness by stepping into a caregiving role. This is consistent with research indicating that siblings develop personal gains from coping with the challenges of schizophrenia (Smith & Greenberg, 2008). An alternative explanation is that self-directedness is a heritable personality trait that protects individuals at risk for schizophrenia from developing psychosis (Gillespie et al., 2003).
The hypothesis that specific domains of character are potentially protective against the risk of schizophrenia is supported by our findings that SD and CO were highly correlated with crystallized IQ and working memory in SCZ-SIB, and were largely uncorrelated with psychopathology and neurocognition in SCZ, CON, and CON-SIB. This suggests that SD and CO could be protective of crystallized intelligence and other neurocognitive functioning specifically in the non-psychotic siblings. Thus, higher levels of neurocognition, a more maturely developed character, and a positive correlation between them may act as mechanisms of resilience or protection against schizophrenia liability. For instance, high levels of SD and CO could be protective against high levels of ST, which are highly correlated with positive symptoms in SCZ and SCZ-SIB. Additionally, we found that SD and CO were inversely correlated with disorganized and negative symptoms in non-psychotic siblings, which is consistent with research suggesting that a mature character profile in siblings might be protective against their own heritability to psychopathology (Bora & Veznedaroglu, 2007).
There were some limitations to this study. First, the sample was highly selective as all participants agreed to participate in a larger study that required completion of clinical and cognitive testing and brain imaging. Second, the majority of individuals with schizophrenia were male. Thus, our results may have poorly reflected the temperament and character of siblings of females with schizophrenia. Lastly, we may not have sufficient power to detect weak relationships between group membership and temperament and character or between the dimensions of personality and psychopathology and neurocognition in this study. Future research should attempt to replicate this study without a gender bias in a larger sample and explore whether particular TCI domains or configurations act as mechanisms of protection against developing schizophrenia in populations at elevated risk for developing the disorder.
Supplementary Material
Acknowledgments
The authors acknowledge the assistance of the staff of the Conte Center for the Neuroscience of Mental Disorders for clinical and neurocognitive assessments, and for database management. We would also like to thank Deanna M. Barch, Ph.D. and Richard A. Grucza, Ph.D. for their comments on an early draft of this paper and Paul A. Thompson, Ph.D. for his assistance with the statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams KY, Yune SK, Kim SJ, Jeon HJ, Han SJ, Hwang J, Sung YH, Lee KJ, Lyoo AIK. Trait and state aspects of harm avoidance and its implications for treatment in major depressive disorder, dysthymic disorder, and depressive personality disorder. Psychiatry Clin Neurosci. 2004;58:240–248. doi: 10.1111/j.1440-1819.2004.01226.x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983a. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1983b. [Google Scholar]
- Barch DM, Mitropoulou V, Harvey PD, New AS, Silverman JM, Siever LJ. Context-processing deficits in schizotypal personality disorder. J Abnorm Psychol. 2004;113:556–568. doi: 10.1037/0021-843X.113.4.556. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Bernardeschi L, Franchini L, Bellodi L, Smeraldi E. A family study of schizotypal disorder. Schizophr Bull. 1995;21:33–45. doi: 10.1093/schbul/21.1.33. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates; 1976. [Google Scholar]
- Bora E, Veznedaroglu B. Temperament and character dimensions of the relatives of schizophrenia patients and controls: the relationship between schizotypal features and personality. Eur Psychiatry. 2007;22:27–31. doi: 10.1016/j.eurpsy.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Calvo de Padilla M, Padilla E, Alemán GG, Bourdieu M, Guerrero G, Strejilevich S, Escobar JI, Svrakic N, Cloninger CR, de Erausquin GA. Temperament traits associated with risk of schizophrenia in an indigenous population of Argentina. Schizophr Res. 2006;83:299–302. doi: 10.1016/j.schres.2005.12.848. [DOI] [PubMed] [Google Scholar]
- Chapman JP, Chapman LJ, Kwapil TR. Scales for the measurement of schizotypy. In: Rain T, Lencz T, Mednick S, editors. Schizotypal Personality. New York. NY: Cambridge University Press; 1995. [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. Arch Gen Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic D, Przybeck T. A psychobiological model of temperament and character. Arch Gen Psychiatry. 1993;50:975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic NM, Svrakic DM. Role of personality self-organization in development of mental order and disorder. Dev Psychopathol. 1997;9:881–906. doi: 10.1017/s095457949700148x. [DOI] [PubMed] [Google Scholar]
- Delawalla Z, Barch DM, Fisher-Eastep J, Thomason ES, Csernansky JG. Factors mediating cognitive deficits and psychopathology among siblings of individuals with schizophrenia. Schizophr Bull. 2006;32:525–537. doi: 10.1093/schbul/sbj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test. Research. Cleveland: The Psychological Corporation; 1983. [Google Scholar]
- Egan M, Goldberg T, Gscheidle T, Weirich M, Rawlings R, Hyde TM, Bigelow L, Weinberger DR. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50:98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- Fenton WS, McGlashan TH. Natural history of schizophrenia subtypes, II: positive and negative symptoms and long-term course. Arch Gen Psychiatry. 1991;47:978–986. doi: 10.1001/archpsyc.1991.01810350018003. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fogelson DL, Nuechterlein KH, Asarnow RF, Payne DL, Subotnik KL, Jacobson KC, Neale MC, Kendler KS. Avoidant personality disorder is a separable schizophrenia-spectrum personality disorder even when controlling for the presence of paranoid and schizotypal personality disorders* the UCLA family study. Schizophr Res. 2007;91:192–199. doi: 10.1016/j.schres.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson DL, Nuechterlein KH, Asarnow RF, Payne DL, Subotnik KL, Giannini CA. The factor structure of schizophrenia spectrum personality disorders: signs and symptoms in relatives of psychotic patients from the UCLA family members study. Psychiatry Res. 1999;87:137–146. doi: 10.1016/s0165-1781(99)00086-4. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Cloninger CR, Heath AC, Martin NG. The genetic and environmental relationship between Cloninger’s dimensions of temperament and character. Pers Indiv Differ. 2003;35:1931–1946. doi: 10.1016/S0191-8869(03)00042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Stone WS, Faraone SV, Seidman LJ, Tsuang MT. Psychopathology, personality traits and social development of young first-degree relatives of patients with schizophrenia. Brit J Psychiatry. 2006;189:337–345. doi: 10.1192/bjp.bp.105.016998. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould T. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:1–10. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Guillem F, Bicu M, Semkovska M, Debruille J. The dimensional symptom structure of schizophrenia and its association with temperament and character. Schizophr Res. 2002;56:137–147. doi: 10.1016/s0920-9964(01)00257-2. [DOI] [PubMed] [Google Scholar]
- Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their non-psychotic siblings. J Neurosci. 2008;27:13835–13842. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Green MF, Bowie C, Loebel A. The dimensions of clinical and cognitive change in schizophrenia: evidence for independence of improvements. Psychopharmacology (Berl) 2006;187:356–363. doi: 10.1007/s00213-006-0432-1. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, O’Hare A, Spellman M, Walsh D. The Roscommon Family Study. III Schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50:781–788. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- Kurs R, Farkas H, Ritsner M. Quality of life and temperamental factors in schizophrenia: comparative study of patients, their siblings and controls. Qual Life Res. 2005;14:433–440. doi: 10.1007/s11136-004-0799-6. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Davis LW. Social function in schizophrenia and schizoaffective disorder: associations with personality, symptoms and neurocognition. Health Qual Life Outcomes. 2004;2:15. doi: 10.1186/1477-7525-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson L. Structured Interview for Prodromal Syndromes (SIPS) New Haven, Conn: Yale School of Medicine; 2000. [Google Scholar]
- Nuechterlein KH, Asaranow RF, Subotnik KL, Fogelson DL, Payne DL, Kendler KS, Neale MC, Jacobson KC, Minzt J. The structure of schizotypy: relationships between neurocognitive and personality disorder features in relatives of schizophrenic patients in the UCLA family study. Schizophr Res. 2002;54:121–130. doi: 10.1016/s0920-9964(01)00359-0. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Psychological Assessment Resources. Computerized Wisconsin Card Sort Task Version 4 (WCST) Psychological Assessment Resources; 2003. [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsycholoigcal Test Battery: Theory and Clinical Interpretation. Tuscon, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Smith MJ, Greenberg JS. Factors contributing to the quality of sibling relationships for adults with schizophrenia. Psychiatr Serv. 2008;59:57–62. doi: 10.1176/appi.ps.59.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald AW, III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svrakic DM, Whitehead C, Pryzbeck TR, Cloninger CR. Differential diagnosis of personality disorders by the seven-factor model of temperament and character. Arch Gen Psychiatry. 1993;50:991–999. doi: 10.1001/archpsyc.1993.01820240075009. [DOI] [PubMed] [Google Scholar]
- Szöke A, Schürhoff F, Ferhadian N, Bellivier F, Rouillon F, Leboyer M. Temperament in schizophrenia: a study of the tridimensional personality questionnaire (TPQ) Eur Psychiatry. 2002;17:379–383. doi: 10.1016/s0924-9338(02)00700-9. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. San Antonio: The Psychological Corporation; 1997b. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.