Abstract
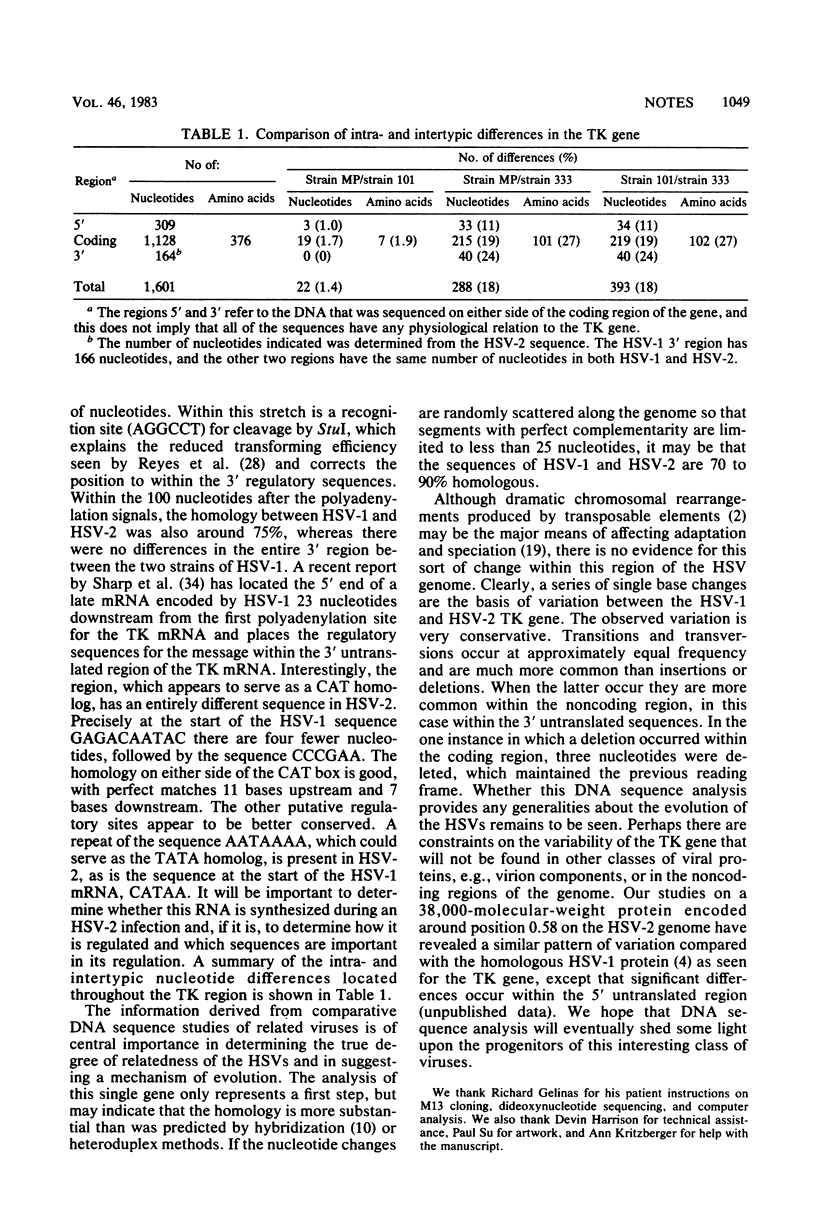
We have determined the complete nucleotide sequence of the thymidine kinase gene of herpes simplex virus (HSV) type 2 strain 333. The sequence of the thymidine kinase gene exhibits an open translational reading frame of 1,128 nucleotides encoding a protein of 376 amino acids. The DNA sequence was compared with that of the HSV type 1 thymidine kinase gene from strain MP (S. L. McKnight, Nucleic Acids Res. 8:5949-5964, 1980) and from strain CL 101 (M. J. Wagner, J. A. Sharp, and W. C. Summers, Proc. Natl. Acad. Sci. U.S.A. 78:1441-1445, 1981) to assess the extent of intra- and intertypic variation for one viral gene. The nucleotides encoding the structural gene varied 1.7% between the two HSV type 1 strains and 19% between HSV type 1 and HSV type 2, which translated to differences in the amino acid sequence of the two proteins of 1.9 and 27%, respectively. The DNA encoding the 5' regulatory sequences appeared to be more conserved than the DNA coding for the structural gene, and the DNA at the 3' end of the gene was the least homologous.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Draper K. G., Frink R. J., Wagner E. K. Detailed characterization of an apparently unspliced beta herpes simplex virus type 1 gene mapping in the interior of another. J Virol. 1982 Sep;43(3):1123–1128. doi: 10.1128/jvi.43.3.1123-1128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A. Differential phosphorylation of (E)-5-(2-bromovinyl)-2'-deoxyuridine monophosphate by thymidylate kinases from herpes simplex viruses types 1 and 2 and varicella zoster virus. Mol Pharmacol. 1982 Mar;21(2):432–437. [PubMed] [Google Scholar]
- Galloway D. A., Swain M. Cloning of Herpes simplex virus 2 DNA fragments in a plasmid vector. Gene. 1980 Nov;11(3-4):253–257. doi: 10.1016/0378-1119(80)90065-7. [DOI] [PubMed] [Google Scholar]
- Gingeras T. R., Milazzo J. P., Sciaky D., Roberts R. J. Computer programs for the assembly of DNA sequences. Nucleic Acids Res. 1979 Sep 25;7(2):529–545. doi: 10.1093/nar/7.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliburton I. W., Morse L. S., Roizman B., Quinn K. E. Mapping of the thymidine kinase genes of type 1 and type 2 herpes simplex viruses using intertypic recombinants. J Gen Virol. 1980 Aug;49(2):235–253. doi: 10.1099/0022-1317-49-2-235. [DOI] [PubMed] [Google Scholar]
- Kieff E., Hoyer B., Bachenheimer S., Roizman B. Genetic relatedness of type 1 and type 2 herpes simplex viruses. J Virol. 1972 May;9(5):738–745. doi: 10.1128/jvi.9.5.738-745.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R. Regulation of herpesvirus thymidine kinase activity in LM(TK) cells transformed by ultraviolet light-irradiated herpes simplex virus. Virology. 1977 Jan;76(1):331–340. doi: 10.1016/0042-6822(77)90306-3. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Woo S. L., Bordelon-Riser M. E., Fraser T. H., O'Malley B. W. Ovalbumin is synthesized in mouse cells transformed with the natural chicken ovalbumin gene. Proc Natl Acad Sci U S A. 1980 Jan;77(1):244–248. doi: 10.1073/pnas.77.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M., Buttyan R., Spear P. G. Herpes simplex virus gene expression in transformed cells. I. Regulation of the viral thymidine kinase gene in transformed L cells by products of superinfecting virus. J Virol. 1976 Nov;20(2):413–424. doi: 10.1128/jvi.20.2.413-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M., Frenkel N., Rapp F. Identification of the herpes simplex virus DNA sequences present in six herpes simplex virus thymidine kinase-transformed mouse cell lines. J Virol. 1980 Jan;33(1):272–285. doi: 10.1128/jvi.33.1.272-285.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCLINTOCK B. Controlling elements and the gene. Cold Spring Harb Symp Quant Biol. 1956;21:197–216. doi: 10.1101/sqb.1956.021.01.017. [DOI] [PubMed] [Google Scholar]
- Maitland N. J., McDougall J. K. Biochemical transformation of mouse cells by fragments of herpes simplex virus DNA. Cell. 1977 May;11(1):233–241. doi: 10.1016/0092-8674(77)90334-8. [DOI] [PubMed] [Google Scholar]
- Mantei N., Boll W., Weissmann C. Rabbit beta-globin mRNA production in mouse L cells transformed with cloned rabbit beta-globin chromosomal DNA. Nature. 1979 Sep 6;281(5726):40–46. doi: 10.1038/281040a0. [DOI] [PubMed] [Google Scholar]
- Matthews D. A. Interpretation of nuclear magnetic resonance spectra for Lactobacillus casei dihydrofolate reductase based on the X-ray structure of the enzyme-methotrexate-NADPH complex. Biochemistry. 1979 Apr 17;18(8):1602–1610. doi: 10.1021/bi00575a035. [DOI] [PubMed] [Google Scholar]
- McDougall J. K., Masse T. H., Galloway D. A. Location and cloning of the herpes simplex virus type 2 thymidine kinase gene. J Virol. 1980 Mar;33(3):1221–1224. doi: 10.1128/jvi.33.3.1221-1224.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R. Expression of the herpes thymidine kinase gene in Xenopus laevis oocytes: an assay for the study of deletion mutants constructed in vitro. Nucleic Acids Res. 1980 Dec 20;8(24):5931–5948. doi: 10.1093/nar/8.24.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Reyes G. R., Jeang K. T., Hayward G. S. Transfection with the isolated herpes simplex virus thymidine kinase genes. I. Minimal size of the active fragments from HSV-1 and HSV-2. J Gen Virol. 1982 Oct;62(Pt 2):191–206. doi: 10.1099/0022-1317-62-2-191. [DOI] [PubMed] [Google Scholar]
- Reyes G. R., LaFemina R., Hayward S. D., Hayward G. S. Morphological transformation by DNA fragments of human herpesviruses: evidence for two distinct transforming regions in herpes simplex virus types 1 and 2 and lack of correlation with biochemical transfer of the thymidine kinase gene. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):629–641. doi: 10.1101/sqb.1980.044.01.066. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Sleigh M., Engler J. A., Broker T. R. The evolution of the adenoviral genome. Ann N Y Acad Sci. 1980;354:426–452. doi: 10.1111/j.1749-6632.1980.tb27983.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier P. H., Cortese R. A fast and simple method for sequencing DNA cloned in the single-stranded bacteriophage M13. J Mol Biol. 1979 Mar 25;129(1):169–172. doi: 10.1016/0022-2836(79)90068-8. [DOI] [PubMed] [Google Scholar]
- Sharp J. A., Wagner M. J., Summers W. C. Transcription of herpes simplex virus genes in vivo: overlap of a late promoter with the 3' end of the early thymidine kinase gene. J Virol. 1983 Jan;45(1):10–17. doi: 10.1128/jvi.45.1.10-17.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Further procedures for sequence analysis by computer. Nucleic Acids Res. 1978 Mar;5(3):1013–1016. doi: 10.1093/nar/5.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E., Skinner G. R. Differences in the properties of thymidine kinase produced in cells infected with type 1 and type 2 herpes virus. J Gen Virol. 1971 Aug;12(2):195–197. doi: 10.1099/0022-1317-12-2-195. [DOI] [PubMed] [Google Scholar]
- Volz K. W., Matthews D. A., Alden R. A., Freer S. T., Hansch C., Kaufman B. T., Kraut J. Crystal structure of avian dihydrofolate reductase containing phenyltriazine and NADPH. J Biol Chem. 1982 Mar 10;257(5):2528–2536. [PubMed] [Google Scholar]
- Wagner M. J., Sharp J. A., Summers W. C. Nucleotide sequence of the thymidine kinase gene of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1441–1445. doi: 10.1073/pnas.78.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]



