Abstract
Background
Hip fractures, common in the elderly population, result in significant morbidity and mortality. A study was undertaken to determine how an evidence based clinical pathway (CP) for treatment of elderly patients with hip fracture affected morbidity, in‐hospital mortality, and health service utilization.
Methods
A pre‐post study design using two population based inception cohorts of hip fracture patients aged ⩾65 years was used. The control group (n = 678) was enrolled between July 1996 and September 1997 before implementation of the pathway and the CP group (n = 663) was enrolled between July 1999 and September 2000 following pathway implementation. Chart reviews were completed during study time frames to determine complications, mortality, and health service utilization.
Results
Only nine patients (1%) in the CP group experienced postoperative congestive heart failure compared with 37 (5%) control patients (p<0.001). Postoperative cardiac arrythmias were significantly lower in the CP group than in the control group (8 (1%) v 36 (5%); p<0.001). Postoperative delirium occurred in 22% of the CP group and 51% of the control group (p<0.001). There was no difference in risk adjusted in‐hospital mortality between the two groups. Overall length of stay (LOS) and costs were unchanged between the groups; however, hospital LOS increased while rehabilitation LOS decreased in the CP group.
Conclusion
Implementation of an evidence based clinical pathway reduced postoperative morbidity and did not affect in‐hospital mortality or overall costs of inpatient care. The effect of changing trends in medical care cannot be ruled out, but the reduction in complications in several clinical areas lends support to the positive impact of the clinical pathway. Perioperative CP is one successful management approach for this fragile patient population as patient morbidity was reduced without negatively affecting resource utilization.
Keywords: clinical pathway, hip fracture, geriatrics, morbidity, health service utilization
Hip fracture is a significant injury requiring increased health services utilization for at least the first year following the fracture.1,2,3 In Canada there are over 35 000 hip fractures annually, a number projected to double by 2040.4 Annual costs of hip fractures are currently estimated at $650 million and are expected to rise by 2041 to $2.4 billion dollars in Canada alone.5 One year post‐fracture mortality varies from 18% to 33% with the highest mortality risk occurring within the first 6 months.6,7 Postoperative complications have been reported to double the mortality risk.8
Standardized evidence based multidisciplinary care implemented through a clinical pathway (CP) may improve post‐fracture outcomes and optimize service utilization.9,10 However, multidisciplinary care for elderly hip fracture patients has not yet shown consistent benefits on morbidity, mortality, or health service utilization.11,12,13,14,15
We implemented an evidence based perioperative CP for elderly hip fracture patients to standardize perioperative care and discharge planning. The study objectives were to evaluate whether the CP implemented within a Canadian health region reduced (1) in‐hospital morbidity, (2) risk adjusted in‐hospital mortality, (3) health service utilization (surgical and rehabilitation hospital length of stay (LOS), discharges to rehabilitation or long term care institutions, readmissions), and (4) costs associated with LOS in all active treatment settings.
Methods
Design
We compared two independent population based inception cohorts in a large urban health region where all hip fracture patients were treated in one of two tertiary hospitals. Prospective data collected from 678 consecutive hip fracture patients between July 1996 and September 1997 served as the control group.16 This group, whose treatment reflected then current practice patterns, was assembled for another population based study just before implementation of the CP. Prospective data were then collected from a consecutive cohort of 663 patients (CP group) who were treated using the CP between July 1999 and September 2000.
Intervention
Development of the perioperative CP was undertaken by a multidisciplinary team of nursing, surgery, rehabilitation, gerontology, and dietary representatives. Although a systematic review was not undertaken to determine the pathway components, published evidence was used to determine treatment choices when possible. Where minimal evidence existed to direct care (for example, rehabilitation and discharge planning), clinical experts used consensus to make treatment decisions. Following implementation of the pathway, our systematic overview of best practices found our pathway was, for the most part, reflective of best care.17 Pre‐printed orders were used to facilitate standardized care delivery. Use of the CP started in March 1998 at both hospitals (see Appendix A available online at http://www.qshc.com/supplemental).
Selection criteria
All patients aged 65 years and over admitted with hip fracture (International Classification of Diseases, 9th Revision, Clinical Modification codes 820.0–820.9, primary/any diagnosis) who lived within the health region's local calling distance during the two study intervals were included. Patients residing in long term care before the hip fracture were eligible. Patients with pathological fractures (other than osteoporosis) and those with a hip re‐fracture within 5 years or requiring further treatment of a previous fracture were ineligible. The research ethics board approved the study.
Measurements
All patients were identified at hospital admission. Important recovery milestones (such as complication rates) and treatment variables (such as time to surgery and postoperative rehabilitation, medication regimens) were measured rather than precise compliance with pathway orders. All pathway patients had the CP on their charts with pre‐printed orders completed at time of data collection. Additional orders were written as necessary for each patient. Overall, there was less practice variation following CP implementation. Mean time to surgical fixation was within 1 day of surgery for both groups with less variation in the CP group. Initial postoperative rehabilitation commenced earlier with the CP and medication regimes became more standardized (see Appendix B available online at http://www.qshc.com/supplemental).
Data were gathered on demographic characteristics, co‐morbidities, pre‐fracture residence, fracture type and fixation, in‐hospital complications, and mortality. Identical standardized data collection forms and assessment time points were used in both cohorts to increase their comparability. Furthermore, the initial data collection team trained the second cohort's data collection team to ensure the same operational definitions were used. Finally, chart reviews were undertaken by experienced research allied health professionals who were blinded to study hypotheses and objectives and who were not involved in direct patient care. Reviews were randomly checked by the research coordinator to ensure accuracy of data collection. A concurrent longitudinal study (results reported elsewhere) was also undertaken with a subgroup of 919 patients who agreed to 6 month post‐fracture follow up.18
The Mini Mental Status Examination (MMSE) was used to evaluate cognition 4–6 days postoperatively in the longitudinal study, with cognitive impairment defined as a score of <22/30.19,20,21 As the MMSE is unable to distinguish between acute confusion, delirium and dementia, patients admitted without documented dementia were examined separately to evaluate the effectiveness of the delirium protocol.
Data on health services provided in acute care hospital or inpatient rehabilitation settings were obtained from regional administrative databases for the 6 month study period commencing the day of the hip fracture. The LOS was calculated separately for initial surgical admission, rehabilitation transfers, and re‐admissions to acute care or rehabilitation facilities. Total LOS was calculated as number of days spent in any of the aforementioned institutions within 6 months of the hip fracture.
Health service costing was undertaken using a case‐mix methodology to estimate the patient‐specific cost of acute care LOS. The Refined Diagnosis Related Grouper (RDRG) was used to classify acute care admissions. Each patient‐specific hospital admission cost was estimated by multiplying the appropriate relative case weight by the corresponding system average cost per weighted case.
Standard costs per day were used to value all services provided by inpatient rehabilitation programs. Because rehabilitation program resource intensity varied, costs per day were estimated for each program. The patient‐specific rehabilitation cost was estimated by the product of the appropriate cost per day and LOS.
A cost minimization analysis was performed, with all costs being expressed in constant 1999/2000 Canadian dollars for both groups without discounting, as the study horizon for costing was only 6 months. The cost analysis was from the health region's perspective and included only direct institutional costs, excluding personal out of pocket, indirect (such as lost work time of patients/caregivers), and physician fee‐for‐service costs.
Statistical methods
Baseline characteristics and in‐hospital complications were compared using standard statistical techniques (t tests for continuous and non‐parametric tests for categorical variables) to identify any systematic cohort differences. Co‐morbidities were conditions present before the fracture whereas complications were defined as new conditions following the fracture and were listed as incidence rates/1000 persons. Odds ratios and 95% confidence intervals were also reported for co‐morbidities and complications.
Two subgroup analyses were planned: (1) cardiac complications in subjects with and without pre‐existing cardiac disease and (2) cognitive status in patients without known dementia on admission to hospital to determine if these subgroups differed between cohorts. Tests of interaction between these subgroups and the study groups were undertaken before subgroup analyses.
In‐hospital mortality was analyzed using conditional step forward logistic regression. Bivariate analysis was initially undertaken with each cohort separately, with variables deemed clinically important or significant at p⩽0.10 included in the multivariate analysis. The Hosmer and Lemeshow goodness of fit test determined final model fit.
Health service utilization was assessed through comparisons of LOS, discharge destination, and readmissions (acute care or rehabilitation) within the first 6 months following the fracture. LOS, which was not normally distributed, was compared using the Mann‐Whitney U test. Discharge location was analyzed using χ2 tests, excluding subjects deceased in hospital. Costs were measured per person for perioperative care, rehabilitation, and re‐admissions. The difference in mean cost per group was analyzed using a bootstrap analysis based on 10 000 repetitions and a 95% confidence level.
The analyses was done as per “intention to treat” as we did not measure strict compliance with the CP. The level of significance was set at p⩽0.01 to reduce the likelihood of a type I error and all analyses were undertaken using SPSS version 12.0.
Results
Demographic data
The two cohorts were similar in age, sex, and co‐morbidity index scores, but significantly more subjects in the CP group had pre‐existing cardiac disease (table 1).
Table 1 Demographic data of all eligible patients sustaining a hip fracture between July 1996 and September 1997 (control) and July 1999 and September 2000 (pathway).
| Variable | Control (n = 678) | CP (n = 663) | p value | Odds ratio* | 95% CI |
|---|---|---|---|---|---|
| Mean (SD) age (years) | 82.0 (7.8) | 82.4 (7.9) | 0.31† | 1.01 | 0.99 to 1.02 |
| Sex (%) | 0.57‡ | 1.08 | 0.84 to 1.38 | ||
| Female | 502 (74) | 500 (75) | |||
| Male | 176 (26) | 163 (25) | |||
| Charlson co‐morbid index (%) | 0.06‡ | ||||
| None | 180 (27) | 140 (21) | 1.0 | 1.019 to 1.75 | |
| Mild (1–2 conditions) | 317 (47) | 329 (50) | 1.33 | 1.06 to 2.01 | |
| Moderate (3–4 conditions) | 133 (19) | 151 (23) | 1.46 | 0.72 to 1.84 | |
| Severe (⩾5 conditions) | 48 (7) | 43 (6) | 1.15 | ||
| Fracture type (%) | 0.96‡ | 0.99 | 0.80 to 1.23 | ||
| FN | 351 (52) | 345 (52) | |||
| IT | 327 (48) | 318 (48) | |||
| Admitted from (%) | 0.29‡ | ||||
| Community | 391 (58) | 390 (59) | 1.0 | 0.57 to 1.10 | |
| Retirement home | 98 (14) | 77 (12) | 0.79 | 0.81 to 1.33 | |
| Institution | 189 (28) | 196 (29) | 1.04 | ||
| Medical conditions on admission | |||||
| Cardiac arrhythmias | 98 (15) | 138 (21) | 0.003‡ | 1.44 | 1.08 to 1.92 |
| Congestive heart failure | 96 (14) | 127 (19) | 0.015‡ | 1.55 | 1.17 to 2.07 |
CP, clinical pathway; SD, standard deviation; FN, femoral neck; IT, intertrochanteric; CI, confidence intervals.
*Control group as reference group.
†Two‐sample independent t test.
‡χ2 test.
§Mann‐Whitney U test.
Morbidity
In patients with/without pre‐existing cardiac disease, significantly more control patients developed postoperative cardiac complications than CP patients (table 2). As there was no interaction between the presence or absence of cardiac diseases, cardiac complications are reported for the whole cohort. The incidence of pressure ulcer was also significantly reduced in the CP cohort, while the incidence of pneumonia and urinary tract infections did not differ between the two groups (table 2).
Table 2 Comparison of incidence rates/1000 persons of in‐hospital complications‡ between July 1996 and September 1997 (control group) and July 1999 and September 2000 (CP group).
| Variable | Control (n = 678) | CP (n = 663) | p value | Odds ratio* | 95% CI |
|---|---|---|---|---|---|
| Congestive heart failure | 54.6 | 13.6 | <0.001† | 0.24 | 0.11 to 0.50 |
| Pulmonary oedema | 66.4 | 34.7 | 0.006† | 0.51 | 0.30 to 0.85 |
| Cardiac arrhythmias | 53.1 | 12.1 | <0.001† | 0.22 | 0.10 to 0.47 |
| Pressure ulcers | 22.1 | 3.0 | 0.002† | 0.20 | 0.06 to 0.70 |
| Urinary tract infections | 212.4 | 180.0 | 0.13† | 0.82 | 0.63 to 1.07 |
| Pneumonia | 78.2 | 87.5 | 0.55† | 1.13 | 0.77 to 1.67 |
*Control group as reference group.
†χ2 test.
‡Conditions not present on hospital admission.
Cognition 4–6 days postoperatively was also significantly different between the overall cohort with 62% of patients in the control group recording <22/30 on the MMSE compared with only 42% of the CP group (p<0.001). In the subgroup analysis of patients without known pre‐existing dementia, the difference between groups was enhanced, with 51% of control patients scoring <22/30 on the MMSE compared with 22% of CP patients (p<0.001).
Risk adjusted mortality
Of 1341 eligible subjects, 52 (8%) control and 48 (7%) CP subjects died in hospital (p = 0.83). After risk adjustment, mortality still did not differ between groups (p>0.05, table 3). Mortality risk estimates for postoperative complications were strongly associated with mortality, but had wide confidence intervals because these events were universally uncommon.
Table 3 Predictors of in‐hospital mortality: multivariate analysis using 1341 subjects†.
| Variable | OR | 95% CI |
|---|---|---|
| Control group (n = 678) | 1.1 | 0.70 to 1.8 |
| Age (per year increase) | 1.1 | 1.05 to 1.12** |
| Male sex (n = 339) | 2.5 | 1.5 to 4.1** |
| Preoperative renal failure (n = 129) | 2.4 | 1.2 to 4.6* |
| Postoperative myocardial infarct (n = 29) | 11.5 | 4.6 to 28.9** |
| Postoperative pneumonia (n = 111) | 6.2 | 3.6 to 10.8** |
| Postoperative respiratory problems (n = 24) | 8.3 | 3.0 to 22.4** |
| Postoperative renal failure (n = 24) | 4.9 | 1.8 to 13.4* |
| Postoperative sepsis (n = 9) | 79.1 | 8.6 to 725.1** |
Homer and Lemeshow goodness of fit: 0.82.
CP, clinical pathway (number with the condition); OR, odds ratio; CI, confidence intervals.
*p<0.05; **p<0.001.
†Including 100 subjects who died in hospital.
Health service utilization
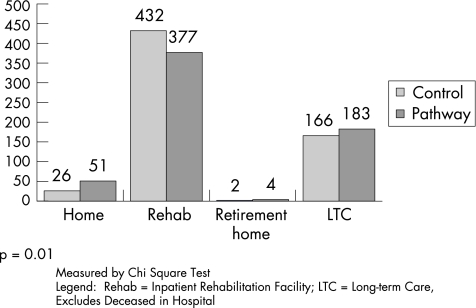
Overall, total LOS did not differ between the groups, with the control cohort staying in hospital a median of 22 (interquartile range (IQR) 11–48) days compared with 24 (9–55) days for the CP group (p = 0.33). Median (IQR) surgical hospital LOS increased to 10 (5–22) days in the CP group compared with 8.5 (6–14) days in the control group (p<0.001), but more CP patients were discharged home or to long term care (p = 0.001, fig 1).
Figure 1 Comparison of discharge location from surgical hospital between July 1996 and September 1997 (control) and from July 1999 to September 2000 (clinical pathway).
For patients transferred to inpatient rehabilitation, the median (IQR) LOS was 28 (14–46) days for the control group and 22 (12–42) days for the CP group (p = 0.04). The number of re‐admissions to hospital or rehabilitation did not differ between the groups, with 94 (16%) control patients and 100 (16%) CP patients being readmitted (p = 0.76). Median (IQR) readmission LOS was 10 (5–22) days for the control group and 13 (5–26) days for the CP group (p = 0.33).
Costs
Overall costs were not different between the two groups. The mean (SD) cost per patient was $19 925 (16 165) for the CP group and $20 466 (17 854) for the control group, while median (IQR) costs/patient were $13 543 (10 226–26 491) for the control group and $13 543 (9392–25 701) for the CP group. A bootstrap analysis of the difference in mean costs (95% CI) confirmed that there was no statistically significant difference between the groups: −$540 (−2464 to 1355).
Discussion
Using an evidence based perioperative CP significantly reduced postoperative morbidity. Although the CP group had slightly more co‐morbidities on admission than the control group, we saw an improvement in outcomes across a number of areas including postoperative cardiac complications, the incidence of pressure ulcers, delirium, and health service utilization. The fact that positive changes occurred in more than one clinical area simultaneously would support the view that the CP, which led to more standardized care (Appendix B), had a positive effect on service delivery and, hence, patient outcomes.
More CP patients were diagnosed with cardiac co‐morbidities on admission, but cardiac complications were markedly reduced in this cohort. These findings would suggest either that the CP cohort was less healthy on admission and standardized care reduced their postoperative complications lower than that experienced by the control cohort, or that each group had a similar burden of co‐morbidities but standardized care improved the preoperative evaluation, thus preventing postoperative complications. Either of these scenarios supports the effectiveness of standardized care in reducing postoperative morbidity.
Furthermore, a significantly higher proportion of control patients showed signs of cognitive impairment compared with the CP group 4–6 days postoperatively. We hypothesize that the new narcotic regimen may account for these findings because fewer CP patients were exposed to meperidine (Appendix B). Opioids, particularly meperidine, in the elderly are associated with increased delirium.22,23 Although the MMSE does not differentiate between acute delirium/confusional states and dementia, the reported results in patients without known pre‐fracture dementia would add support that we were measuring delirium rather than dementia.
Despite reducing complications, in‐hospital mortality was unaffected. Complications associated with mortality tended to be catastrophic events (such as myocardial infarction) which occurred infrequently. Co‐morbidities, particularly cardiac conditions, were associated with increased mortality risk and, although standardized care identified more cardiac co‐morbidities, most were non‐modifiable. Our findings are similar to previous studies which reported no difference in mortality following implementation of standardized multidisciplinary care.11,13 Our study was, however, limited to evaluation of in‐hospital mortality.
Overall, total LOS, including readmissions, did not differ between cohorts. One may speculate that standardized discharge planning resulted in more appropriate discharge placement, which prolonged the acute care stay but discharged more patients to the community rather than to further inpatient care.18
LOS is frequently reported in the evaluation of care following hip fracture, with some studies reporting increased LOS14,15,24 and others reporting decreased LOS.25,26,27,28,29,30 Comparison between studies is difficult as LOS can be influenced by organization of rehabilitation services and/or the healthcare system. In our public healthcare system, patients remain in hospital until appropriate discharge destinations are available, occasionally resulting in prolonged hospital stays beyond that necessary for medical care. Our LOS results may only be generalizable to other similar healthcare systems. However, the perioperative CP is appropriate to most acute care settings as reduced LOS would only result in truncating standardized care earlier.
We focused on inpatient costs which represent, by a wide margin, the largest proportion of total costs. No significant differences were seen in inpatient costs in the various care settings. Although acute care LOS tends to be more expensive than rehabilitation LOS, fewer patients required inpatient rehabilitation, so the net effect of the CP was cost neutral.
One of the strengths of this study was the use of two independent prospective population based cohorts, including patients from institutional settings rather than community dwelling patients only—the typical hip fracture study population reported in the literature.1,6,24,31,32 Furthermore, data collection was prospective despite using a historical control group as we took advantage of a previous prospective study examining hip fracture outcomes. The interim between studies allowed clinical staff to familiarize themselves with the CP before the post‐CP study. Finally, evaluators were not involved in patients' treatment or hospital management, reducing the bias associated with an internal evaluation.
The use of a pre‐post research design rather than a randomized clinical trial (RCT) is, however, the most notable study limitation because we cannot completely rule out secular trends. An RCT would have been challenging after regional CP implementation. Contamination could have occurred as a result of CP and control subjects being treated in the same room by the same staff. Co‐intervention was also possible between hospitals because medical staff work at both sites. Despite minimal changes in resources, either personnel or beds for home care or continuing care settings over the 3 year span between cohorts (D W C Johnston, Medical Director, University of Alberta Hospital, November 2003, personal communication), we cannot state that there were no secular trends in health care that may have had an impact on our reported findings. However, the fact that we saw changes in several clinical areas simultaneously would support the view that the CP was, at least in part, responsible for some of the measured changes in outcomes and service utilization.
In summary, an evidence based standardized CP led to reduced postoperative morbidity but in‐hospital mortality was unchanged. LOS in hospital increased, but LOS in a rehabilitation hospital decreased with use of the CP. Overall, no change in measured costs associated with inpatient care was found. We consider the perioperative CP to be successful for managing this fragile patient population as patient morbidity was reduced without negatively affecting resource utilization.
Details of the care path and changes in care delivery are given in Appendices A and B available online at http://www.qshc.com/supplemental.
Acknowledgements
The authors thank Dr M E Suarez‐Almazar for her assistance with the control cohort and the design of the post‐pathway study.
Abbreviations
CP - clinical pathway
LOS - length of stay
Footnotes
Financial support for this study was provided by the Alberta Heritage Foundation for Medical Research, University Hospital Foundation, the Royal Alexandra Hospital Foundation and the Edmonton Orthopaedic Research Committee.
Competing interests: none.
Details of the care path and changes in care delivery are given in Appendices A and B available online at http://www.qshc.com/supplemental.
References
- 1.Brainsky A, Glick H, Lydick E.et al The economic cost of hip fractures in community‐dwelling older adults: a prospective study. J Am Geriatr Soc 199745281–287. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int 200516S3–S7. [DOI] [PubMed] [Google Scholar]
- 3.Donald I P, Bulpitt C J. The prognosis of falls in elderly people living at home. Age Ageing 199928121–125. [DOI] [PubMed] [Google Scholar]
- 4.Papadimitropoulos E A, Coyte P C, Josse R G.et al Current and projected rates of hip fracture in Canada. Can Med Assoc J 19971571357–1363. [PMC free article] [PubMed] [Google Scholar]
- 5.Wiktorowicz M E, Goeree R, Papaioannou A.et al Economic implications of hip fracture: health service use, institutional care and cost in Canada. Osteopor Int 200112271–278. [DOI] [PubMed] [Google Scholar]
- 6.Magaziner J, Lydick E, Hawkes W.et al Excess mortality attributable to hip fracture in white women aged 70 years and older. Am J Public Health 1997871630–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolinsky F D, Fitzgerald J F, Stump T E. The effect of hip fracture on mortality, hospitalization, and functional status: a prospective study. Am J Public Health 199787398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamlet W P, Lieberman J R, Freedman E L.et al Influence of health status and the timing of surgery on mortality in hip fracture patients. Am J Orthop 199726621–627. [PubMed] [Google Scholar]
- 9.Ogilvie‐Harris D J, Botsford D J, Hawker R W. Elderly patients with hip fractures: improved outcome with the use of care maps with high‐quality medical and nursing protocols. J Orthop Trauma 19937428–437. [DOI] [PubMed] [Google Scholar]
- 10.Zuckerman J D, Sakales S R, Fabian D R.et al Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program. Clin Orthop 1992274213–225. [PubMed] [Google Scholar]
- 11.Cameron I, Handoll H, Finnegan T.et al Co‐ordinated multidisciplinary approaches for inpatient rehabilitation of older patients with proximal femoral fractures. Cochrane Database Syst Rev Issue 3 2001 [DOI] [PubMed]
- 12.Huusko T M, Karppi P, Avikainen V.et al Intensive geriatric rehabilitation of hip fracture patients: a randomized, controlled trial. Acta Orthop Scand 200273425–431. [DOI] [PubMed] [Google Scholar]
- 13.March L M, Cameron I D, Cumming R G.et al Mortality and morbidity after hip fracture: can evidence based clinical pathways make a difference? J Rheumatol 2000272227–2231. [PubMed] [Google Scholar]
- 14.Naglie G, Tansey C, Kirkland J L.et al Interdisciplinary inpatient care for elderly people with hip fracture: a randomized control trial. Can Med Assoc J 200216725–32. [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts H C, Pickering R M, Onslow E.et al The effectiveness of implementing a care pathway for femoral neck fracture in older people: a prospective controlled before and after study. Age Ageing 200433178–184. [DOI] [PubMed] [Google Scholar]
- 16.Cree M, Soskolne C L, Belseck E.et al Mortality and institutionalization following hip fracture. J Am Geriatr Soc 200048283–288. [DOI] [PubMed] [Google Scholar]
- 17.Beaupre L, Jones C A, Saunders L D.et al Best practices for elderly hip fracture patients: a systematic overview of the evidence. J Gen Intern Med 2005201019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaupre L, Cinats J G S A, Scharfenberger A.et al Does standardized rehabilitation and discharge planning improve functional recovery in elderly hip fracture patients? Arch Phys Med Rehabil 2005862231–2239. [DOI] [PubMed] [Google Scholar]
- 19.Folstein M, Anthony J C, Parhad I.et al The meaning of cognitive impairment in the elderly. J Am Geriatr Soc 198533228–235. [DOI] [PubMed] [Google Scholar]
- 20.Folstein M F, Folstein S E, McHugh P R. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 21.Anthony J C, LeResche L, Niaz U.et al Limits of the ‘Mini‐Mental State' as a screening test for dementia and delirium among hospital patients. Psychol Med 198212397–408. [DOI] [PubMed] [Google Scholar]
- 22.Beers M H. Explicit criteria for determining potentially inappropriate medication use by the elderly. An update. Arch Intern Med 19971571531–1536. [PubMed] [Google Scholar]
- 23.Morrison R S, Magaziner J, Gilbert M.et al Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol 200358A76–81. [DOI] [PubMed] [Google Scholar]
- 24.Galvard H, Samuelsson S M. Orthopedic or geriatric rehabilitation of hip fracture patients: a prospective, randomized, clinically controlled study in Malmo, Sweden. Aging 1995711–16. [DOI] [PubMed] [Google Scholar]
- 25.Cameron I D, Lyle D M, Quine S. Accelerated rehabilitation after proximal femoral fracture: a randomized controlled trial. Disabil Rehabil 19931529–34. [DOI] [PubMed] [Google Scholar]
- 26.Choong P F, Langford A K, Dowsey M M.et al Clinical pathway for fractured neck of femur: a prospective, controlled study. Med J Aust 2000172423–426. [DOI] [PubMed] [Google Scholar]
- 27.Gilchrist W J, Newman R J, Hamblen D L.et al Prospective randomised study of an orthopaedic geriatric inpatient service. BMJ 19882971116–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koval K J, Chen A L, Aharonoff G B.et al Clinical pathway for hip fractures in the elderly: the Hospital for Joint Diseases experience. Clin Orthop 200442572–81. [DOI] [PubMed] [Google Scholar]
- 29.Swanson C E, Day G A, Yelland C E.et al The management of elderly patients with femoral fractures. A randomised controlled trial of early intervention versus standard care. Med J Aust 1998169515–518. [PubMed] [Google Scholar]
- 30.Tallis G, Balla J I. Critical path analysis for the management of fractured neck of femur. Austral J Pub Health 199519155–159. [DOI] [PubMed] [Google Scholar]
- 31.Koval K J, Skovron M L, Aharonoff G B.et al Predictors of functional recovery after hip fracture in the elderly. Clin Orthop 199834822–28. [PubMed] [Google Scholar]
- 32.Marottoli R A, Berkman L F, Leo‐Summers L.et al Predictors of mortality and institutionalization after hip fracture: the New Haven EPESE cohort. Established Populations for Epidemiologic Studies of the Elderly. Am J Public Health 1994841807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]