Abstract
OBJECTIVE:
Graft-versus-host disease (GVHD) is a leading cause of morbidity and mortality in patients receiving hematopoietic cell transplant. It is estimated that 40–70% of engrafted patients surviving the initial transplant eventually develop chronic GVHD (cGVHD), which can persist for months to years and require long-term management from multiple disciplines. This review describes the oral component of this transplant complication.
DESIGN:
The search related to GVHD patho-biology, salivary gland disease after hematopoietic cell transplant and treatments for oral GVHD encompassed literature from 1966 through 2008. Searches were limited to the MEDLINE/PubMed database and English language literature in peer-reviewed journals.
RESULTS:
Our understanding of the patho-biology of oral cGVHD is based on studies of other affected tissues. It is difficult to determine the prevalence and incidence of salivary gland disease after transplant because there is no universally accepted case definition. In general, clinical trials for treatment of oral cGVHD have been too small to make strong recommendations for use in clinical practice.
CONCLUSIONS:
Larger well-designed clinical studies are needed to understand the patho-biology of oral cGVHD and determine best treatments for this disease.
Keywords: graft-versus host disease, xerostomia, salivary gland disease, hematopoietic stem cell transplant
Introduction
The increasing success of allogeneic hematopoietic stem cell transplantation (thereafter referred to as HSCT) for treatment of neoplastic and non-neoplastic hematologic diseases has contributed to its steady increase in use (Flowers et al, 2002; Laughlin et al, 2004; Thomas et al, 2004; Appelbaum, 2007; O'Keefe et al, 2007). Worldwide over 40 000 individuals receive HSCT annually, out of which 15 000 are performed with cells from an allogeneic donor (Flowers et al, 2002). After the discovery that grafted allogeneic hematopoietic cells could eliminate residual tumor cells of the recipient via an immune mediated mechanism (graft-versus-tumor effect), HSCT became an experimental treatment for other malignancies. On-going clinical trials are testing HSCT as an adjunctive therapy for solid tumors such as renal cell carcinoma (Ueno and Childs, 2007), and also as a new treatment for severe autoimmune diseases (Pavletic and Illei, 2005). These trends may significantly increase the annual number of transplanted patients in the near future.
Graft-versus-host disease (GVHD) is a principal impediment for broader use of HSCT and is a leading cause of morbidity and mortality in HSCT recipients. It is more likely to occur if the host receives a graft from an unrelated donor, or if the host or donor is older. Both acute and chronic phases of this complication develop, often involving multiple organ systems. Acute GVHD has relatively uniform clinical picture, classically manifested by erythematous rash, diarrhea and/or liver involvement, generally occurs early post-transplant (most commonly within the first 3–4 months) and is the major cause of early lethality. Chronic GVHD (cGVHD) is a distinct syndrome that can affect virtually every major organ system but most commonly involves skin, oral, vaginal and conjunctival mucosa, salivary and lacrimal glands and the liver. It is estimated that 40–70% of engrafted patients surviving the initial transplant eventually develop cGVHD, which can persist for months to years and require long-term management from multiple disciplines (Fraser et al, 2006). This adds significantly to the burden of disease in patients, who often have had prolonged illness before undergoing transplant. cGVHD is the leading cause of death in long-term survivors of transplant, even though it is associated with a decreased risk of relapse in those receiving HSCT for leukemia (Fraser et al, 2006).
Given that the use of HSCT will increase with time, that there will be an undersupply of related donors, and that related donors and transplant recipients will age as the population ages, the incidence of cGVHD will probably increase in coming years. As the oral component of this transplant complication is a source of significant morbidity, the oral health research community is uniquely positioned to help define the pathogenesis and management of this important disease. This review concentrates on the known patho-physiology of oral GVHD, its histopathology and clinical features, GVHD salivary gland disease and clinical trials for treatment of oral GVHD.
Patho-physiology
Acute and cGVHD were traditionally distinguished by time of onset following transplant. However, newer consensus criteria define each by their characteristic clinical and pathologic features rather than chronologically (Filipovich et al, 2005). Acute GVHD often occurs within 100 days of HSCT, but can persist beyond that time or recur (Table 1). Classic features of acute GVHD include erythematous maculo-papular rash, diarrhea and elevation of liver enzymes. The clinical presentation of cGVHD is variable and may include lichen planus-like changes of skin and mucosa, sclerosis of the skin, sicca syndrome secondary to lacrimal and/or salivary gland damage, liver involvement with cholestatis, and decrease in pulmonary function secondary to bronchiolitis obliterans.
Table 1.
Predominant features of acute and chronic graft-versus-host disease (Filipovich et al, 2005)
| Site | More common in Acute | Chronic |
|---|---|---|
| Skin | Erythema | Poikiloderma |
| Maculopapular rash | Lichen planus-like features | |
| Pruritus | Sclerotic features; Morphea-like features, Lichen sclerosus-like features | |
| Often areas of depigmentation, hypopigmentation or hyperpigmentation | ||
| Nails | Dystrophy; longitudinal ridging, splitting, or brittle nails | |
| Onycholysis; pterygium unguis; nail loss | ||
| Hair | Alopecia (after recovery from induction chemotherapy or radiotherapy); scaling, papulosquamous lesions | |
| Oral | Mucositis | Lichen-planus like features |
| Erythema | Xerostomia | |
| Pain | Hyperkeratotic plaques | |
| Mucocele | ||
| Restriction of mouth opening from sclerosis | ||
| Mucosal atrophy | ||
| Pseudomembranes and ulcers | ||
| Eyes | New onset dry, gritty, or painful eyes | |
| Cicatricial conjunctivitis | ||
| Keratoconjunctivitis sicca | ||
| Gastro-intestinal | Anorexia; nausea; vomiting | Esophageal web |
| Diarrhea; weight loss; failure to thrive | Strictures or stenosis in the upper to mid third of the esophagus | |
| Genitalia | Lichen-planus like features with possible scarring | |
| Liver | Elevation of total bilirubin, alkaline phosphatase, alanine aminotransferase or aspartate aminotransferase to >2 times the upper limit of normal with no other cause | Same features as acute |
| Lung | Bronchiolitis obliterans | |
| Muscles, joints | Fasciitis; joint stiffness or contractures secondary to sclerosis, sometimes myositis or polymyositis | |
| Other | Thrombocytopenia; eosinophilia; lymphopenia; hypo- or hyper-gammopathy; autoantibodies |
Billingham first proposed basic conditions necessary for development of GVHD (Billingham, 1966): (i) immunocompetent cells in the graft; (ii) the inability of the host cells to reject the graft (immune compromise); and (iii) the ability of the infused cells to recognize the host as foreign (antigenic mismatch). Each element plays a critical role in the development of both acute and cGVHD.
Acute GVHD
The acute GVHD occurs in three closely overlapping and interrelated stages: conditioning, activation and expansion of alloreactive cells and the effector phase (Ferrara and Reddy, 2006; Shlomchik, 2007; Sun et al, 2007).
Conditioning
Antigenic differences between the donor and the host will lead to graft rejection unless the donor cells in the graft are given advantage over host cells. Such advantage is achieved through depletion of the host immune cells by irradiation and/or chemotherapy with immunosuppressive conditioning. More aggressive myeloablative conditioning is commonly associated with higher risk and increased severity of GVHD in contrast to gentler, non-myeloablative regimens (Couriel et al, 2004; Saliba et al, 2007). Conditioning is accomplished with chemotherapeutic agents and/or total body irradiation, with the goal to deplete the host immune system and allow successful engraftment of donor stem cells. However, increasing degrees of conditioning are associated with progressive cellular damage, particularly in the gastrointestinal (GI) tract and skin, leading to increased permeability of epithelial barriers, leakage of microbial products such as lipopolysaccharide, and release of inflammatory cytokines (Cooke et al, 2001). This ultimately leads to activation of the host antigen presenting cells (APCs), characterized by increased expression of co-stimulatory molecules and migration to the secondary lymphoid organs. The role of microbial products in this stage of GVHD initiation is underscored by the fact that germ-free animals have a much lower incidence of GVHD (Heidt and Vossen, 1992). Similarly, prior gut decontamination was effective in decreasing the risk of GVHD in clinical trials (Beelen et al, 1999).
Activation and expansion of alloreactive cells
As T-cell numbers are primarily limited by the availability of homeostatic cytokines, most notably interleukin (IL)-7, conditioning creates ‘space’ for newly infused donor T cells (Alpdogan et al, 2003). These immuno-competent cells are critical to the development of GVHD, as evidenced by the finding that GVHD can be abrogated effectively or eliminated through T-cell depletion of the graft. However, grafted T cells are vital for transplant success, as they provide adaptive immunity to infections and control the malignancy (the primary reason for allogeneic transplantation). Past clinical studies established that patients who receive T-cell depleted grafts were at a much greater risk for graft rejection, serious infections and relapse of the malignancy (Marmont et al, 1991).
Following migration to secondary lymphoid organs, host APCs encounter donor lymphocytes (Ferrara and Reddy, 2006). Naïve donor T lymphocytes recognize host antigens on the surface of APCs, activate, proliferate and express effector cytokines. The number of T cells that are ultimately activated depends on the prior frequency of the T cells specific for host antigens (precursor frequency). In major histocompatibility antigen (MHC) mismatched transplants, large numbers of donor T cells recognize host HLA antigens as foreign, leading to increased expansion and ultimately more severe GVHD. The degree of antigenic mismatch is one of the most critical factors in development of GVHD, and is the primary limiting factor preventing wider use of allogeneic HSCT (Sasazuki et al, 1998). The incidence and severity of GVHD increase with the degree of MHC mismatch with the best results obtained when matched siblings are used as donors. In MHC-matched transplants (such as those from matched siblings), MHC molecules are identical between the donor and the host, and antigen recognition is dependent on the differences in minor histocompatibility antigens. These are peptides derived from any number of polymorphic genes throughout the genome that differ between the donor and recipient. Peptides generated within the APC through proteosomal processing of the endogenous cell proteins are presented to donor CD8 cells with MHC-I molecules. In addition, antigens that are taken up by APC from the surrounding milieu are processed in the phagolysosomal pathway and are presented to donor CD4 cells in complex with MHC-II molecules (Goulmy, 1996).
Effector phase
Following activation, donor T cells acquire an effector phenotype, exit secondary lymphoid organs, enter the blood stream and migrate to the host target tissues (Ferrara and Reddy, 2006).
Activation of CD8 cells is accompanied by increased production of cytotoxic effector molecules such as perforin and granzymes and secretion of cytokines such as interferon-gamma (IFN-γ). Effector function of CD4 cells is primarily mediated through secretion of cytokines. Depending on the conditions of initial activation and subsequent cytokine profile, CD4 cells can be roughly subdivided into Th1, Th2, and more recently Th17 subtypes characterized by secretion of ‘signature cytokines’ of IFN-γ, IL-4, and IL-17, respectively (Harrington et al, 2005). In addition to acquisition of effector functions, donor T cells acquire specific migratory properties depending on the origin of the APCs that mediated the initial activation. Thus, donor T cells activated by lamina propria APCs in the mesenteric lymph nodes migrate preferentially into the gut and cause GI GVHD, while those activated in the skin draining lymph nodes will migrate to skin. This specificity is determined by the unique pattern of chemokine receptor expression on T cells that direct migration to the tissues expressing corresponding chemokines (Wysocki et al, 2005). Once the effector T cells reach the target organs, they cause tissue damage via direct cytotoxicity against epithelia and release of cytokines such as IFN-γ. This subsequently activates resident macrophages to release proinflammatory molecules such as tumor necrosis factor-alpha (TNF-α), IL-1 and IL-6 leading to further attraction of proinflammatory cells and amplification of the inflammatory cascade (Reddy and Ferrara, 2003).
Chronic GVHD
Graft-versus-host disease occurring or lasting beyond day 100 post-transplant has been defined classically as chronic. Although practical for the epidemiologic purposes, such definition is inadequate for the following reasons:
Symptoms and signs typical of cGVHD frequently develop before day 100.
Signs classically associated with acute GVHD, e.g. diarrhea or diffuse erythematous rash, may occur after day 100, particularly in the setting of donor lymphocyte infusion (DLI).
Therefore, the latest NIH consensus criteria recommend classification based on characteristic symptoms and signs rather than a rigid temporal definition (Table 1), (Filipovich et al, 2005).
Chronic GVHD is the leading long-term complication of allogeneic stem cell transplantation. While there have been significant advances in prevention and treatment of acute GVHD, the pathogenesis of cGVHD is poorly understood. Several factors could be responsible for this:
Most animal studies use major mismatched strain combinations and focus on acute GVHD with short-term mortality and weight loss as the primary outcomes.
Although some animal models of cGVHD have been proposed, none adequately reproduces the complexity of human condition.
Chronic GVHD develops relatively late after transplant, necessitating prolonged follow-up and data collection. This makes prospective studies difficult and expensive to perform.
Almost any organ system can be affected by cGVHD, leading to a wide variety of symptoms and signs.
Until recently, a universally accepted system of diagnosis, staging and response criteria in cGVHD has not been available, preventing interpretation of clinical studies of different centers.
Most human studies focused on describing easily accessible cell populations (i.e. those from the peripheral blood) which may not accurately reflect events occurring in target organs.
In the following section, we will summarize and discuss the evidence available related to patho-biology from animal and human studies to date.
Risk factors
Prior diagnosis of acute GVHD is the risk factor associated most consistently with subsequent cGVHD (Ferrara and Reddy, 2006). If acute GVHD is not fatal, it will either resolve or develop into cGVHD (progressive onset). cGVHD may also develop in patients without clinical acute GVHD (de novo onset) or following complete resolution of acute GVHD (quiescent onset). Other risk factors for cGVHD are increasing donor and recipient age, increasing CD3 (T cell) dose in the graft, female donor and male recipient combination, unrelated donors, mismatched HLA donors, diagnosis of chronic myelogenous leukemia or myelodysplastic syndrome, total body irradiation, and the use of mobilized peripheral blood stem cell transplant (Przepiorka et al, 2001; Pavletic et al, 2005).
Clinical similarity to autoimmunity
In contrast to acute GVHD, cGVHD affects multiple target organs and produces a constellation of clinical manifestations (Filipovich et al, 2005; Ferrara and Reddy, 2006). In many cases, these manifestations closely resemble both clinically and histologically those of common autoimmune disorders including lichen planus, Sjögren's syndrome, scleroderma, systemic lupus erythematosus, dermatomyositis and primary biliary cirrhosis (Filipovich et al, 2005; Baird and Pavletic, 2006). This prompted the suggestion that cGVHD is the disorder of disregulated immunity similar to that in autoimmunity. Unlike classical autoimmune disorders in which tolerance to native antigens is lost and the immune system recognizes and mounts a detrimental immune response to host antigens, donor T cells recognize antigens of the host and perpetuate the disease process in cGVHD. To better understand the potential mechanisms of development and progression of cGVHD, it is important to review basic aspects of mechanisms that ensure tolerance in the healthy individual and contrast them with the setting of allo-HSCT.
Central vs peripheral tolerance
The concept of tolerance is fundamental to the normal immune response (Mak, 2006). The development of T cells occurs in the thymus through the closely linked processes of positive and negative selection. Positive selection ensures that the developing T cell is able to recognize the host MHC molecules, either MHC-I or MHC-II for CD8 and CD4 cells, respectively. As T-cell receptor rearrangement is a random process, it is important to ensure that naïve T cells do not initiate responses to the host antigens. Host proteins are expressed in the thymus on the epithelial and dendritic cells (DCs) and the T cells whose receptors recognize host MHC with high affinity are clonally deleted (negative selection). Only cells which recognize the host MHC with low affinity are allowed to mature and exit to the periphery. This process is fundamental to development of central tolerance. However, despite efficiency of clonal deletion in elimination of the overwhelming majority of autoreactive T cells, some escape the thymus and are thought to be controlled in the periphery, through such mechanisms as anergy (loss of ability to proliferate upon encounter with harmless antigens) and active suppression by regulatory cells (Schwartz, 2003; Kyewski and Klein, 2006).
Regulatory T cells
The concept of regulatory cells has received extraordinary attention in the recent years following the report describing profound autoimmunity developing in animals following neonatal thymectomy. It was later shown that this could be prevented by transfusion of a particular T-cell type expressing high levels of IL-2 receptor α-chain (CD25). These cells were termed natural regulatory T cells and were shown to develop in the thymus along with conventional T cells. Natural regulatory T cells characteristically express transcription factor FoxP3 and recognize a large number of host antigens. Following exit from the thymus, they co-localize with effector T cells in secondary lymphoid organs and target tissues and control immune responses through mechanisms that may involve secretion of immunosuppressive cytokines such as transforming growth factor-beta (TGF-β) and IL-10, contact dependent inhibition, apoptosis of effector T cells, and competition for IL-2. In addition to ‘natural’ regulatory T cells that develop and acquire their function in the thymus, peripheral regulatory T cells developing from conventional T cells under certain conditions (e.g. high TGF-β or IL-10 levels) have also been described (Sakaguchi, 2004; Grazia Roncarolo et al, 2006; Roncarolo and Battaglia, 2007).
T-cell development following allogeneic HSCT
Having reviewed the events of normal T-cell development, we will examine factors that influence the T-cell repertoire in recipients of allo-HSCT. Following successful engraftment, donor stem cells begin producing myeloid and lymphoid progenitors that repopulate the host (Ferrara and Reddy, 2006). Unlike B cells which develop in the bone marrow, T cells require the presence of a functional thymus for development. Most allo-HCST is performed in older adults when thymic function is impaired and the development of naïve T cells is greatly compromised. In addition, the thymus and its epithelial components critical for proper T-cell development are thought to be targets in acute GVHD. Therefore, most of the T cells that eventually repopulate the host have developed in the donor thymus. As both negative selection and generation of regulatory T cells are dependent on the nature of the antigen presentation in the thymus, it is likely that failure of central tolerance contributes to the development of the autoimmune-like manifestations of cGVHD. Empirical evidence in support of this comes from several sources:
The ability of the adult thymus to support renewed thymopoiesis is age dependent, with older recipients having less increase in thymic size and naïve T-cell generation post-transplant.
The incidence of cGVHD increases with increasing age of the recipient. In contrast, young children with functional thymus have a lower incidence of cGVHD.
Therefore, the ultimate T-cell repertoire in the older transplant recipients is primarily determined by the initial T-cell composition of the graft. If the T-cell repertoire contains cells recognizing host antigens but not the natural regulatory T cells that can counteract them, this situation could promote the development of persistent allo-reactivity and cGVHD. Similarly, even if thymopoiesis is preserved post-transplant, the damage inflicted upon the host thymus during acute GVHD may be sufficient to impair mechanisms of central tolerance and thymic generation of regulatory T cells (Fukushi et al, 1990; Hakim and Gress, 2002).
Dendritic cells
Following the initial period of mixed chimerism post-transplant, donor cells replace all hematopoietic compartments (Ferrara and Reddy, 2006). Donor DC precursors emerge from the bone marrow and travel to the peripheral tissues where they mature into fully functional DCs. As both DCs and T cells are now of donor origin, how does the induction of allo-reactivity occur? In the MHC-matched setting, donor CD8 cells should not be able to respond to host antigens as minor histocompatibility antigens recognized by CD8 cells in the context of MHC-I molecules originate from proteins produced within the donor DC itself. In contrast, host molecules can be taken up from the environment by donor MHC, processed in the MHC class II pathway and presented to donor CD4 cells. However, the involvement of CD8 cells in cGVHD is well documented. One possibility is that cells induced at the time of acute GVHD by host APCs persist as memory cells and mediate cGVHD without involvement of donor DCs. Another possibility is explained by the phenomenon of cross-presentation – redirection of exogenous antigens from MHC-II (vacuolar) into the MHC-I (cytoplasmic) pathway with subsequent presentation to CD8 cells (Shlomchik, 2003).
T cells
Several studies have examined the relative roles of various T-cell subsets in development of cGVHD. Depending on the murine model used, both CD4 and CD8 cells have been shown to be critical for the induction of cGVHD. In the human setting, several studies have examined the dynamics of T-cell populations. Yamashita et al (2004) evaluated T-cell subsets in the peripheral blood of patients with cGVHD and found that patients with severe disease had increased numbers of effector-memory CD4 T cells. In a subsequent study, they found that the number of effector-memory cells decreases in those patients who respond to extracorporeal therapy, a treatment for GVHD (Yamashita et al, 2006).
In recent animal studies, Chen et al (2007) demonstrated that Th1 and Th17 cells formed early post-transplant persist and mediate cGVHD immunopathology. They also suggest that the relative lack of regulatory T cells is crucial for the development of this process (Chen et al, 2007). Few human studies have examined the role of regulatory T cells in cGVHD. Clark et al found increased frequency of CD4+CD25+ T cells in the peripheral blood of cGVHD patients and suggested that no numerical deficiency of regulatory cells is responsible for development of cGVHD. However, although CD25 is a relatively reliable marker of regulatory T cells in mice, it is expressed by activated cells and may represent an effector population (Clark et al, 2004). In contrast, a later study found decrease in the regulatory T cells in the cGVHD population (Zorn et al, 2005). The only study published to date that examined levels of regulatory T cells in tissues as well as the peripheral blood found that the ratio of regulatory T cells (defined by FoxP3 expression) relative to CD8 cells similar in cGVHD and normal controls, but decreased compared to patients post-transplant with GI infections (Rieger et al, 2006).
B cells
Good evidence of B-cell involvement in cGVHD comes from the trials using rituximab, a monoclonal antibody (anti-CD20 antibody) that binds subsets of B cells. Following initial reports (Ratanatharathorn et al, 2000, 2003), recent phase II trials demonstrated that rituximab was moderately efficacious in reducing cGVHD, including in steroid-refractory patients (Cutler et al, 2006; Okamoto et al, 2006; Zaja et al, 2007).
Initial evidence for a potential role of autoantibodies in the pathogenesis of cGVHD in humans was provided by a study of antibodies against Y chromosome encoded antigen, H-Y. The presence of H-Y antibodies correlated with cGVHD and maintenance of disease remission (Miklos et al, 2005). Although numerous other autoantibodies have been described in cGVHD, including those against double stranded DNA, other nuclear components, mitochondria and rheumatoid factor, their significance has not been established (Wechalekar et al, 2005; Patriarca et al, 2006). Recently, activating antibodies to platelet derived growth factor (PDGF) receptor have been described both in scleroderma and in chronic sclerodermatous GVHD and may be an important contributor to the development of fibrosis in these conditions (Svegliati et al, 2007).
BAFF (BLyS) is a TNF family cytokine produced by stromal and other cells of non-hematopoietic origin and active B cells. BAFF promotes survival of marginal zone B cells producing low affinity antibodies – the subset that has been linked to autoantibody production. Increased BAFF levels have been shown in several classic autoimmune diseases, in particular systemic lupus erythematosus (Mackay et al, 2007). Recently, elevated BAFF levels have been demonstrated in patients with active cGVHD (Sarantopoulos et al, 2007).
In conclusion, the pathogenesis of cGVHD is complex and poorly understood, but is likely to involve dysfunction of tolerance determining mechanisms similar to classic autoimmune diseases. Further hypothesis driven prospective studies in humans focusing on target tissue events in conjunction with clinical trials are most likely to build our understanding of this challenging condition.
Oral findings in the timeframe of acute GVHD
Mucosal erythema, ulcerations, and painful desquamative oral lesions occur often in patients undergoing conditioning for HSCT. In one study of 150 patients transplanted for acute myelocytic leukemia, stomatitis developed in 26% during the engraftment period (Neudorf et al, 2004). However, a true clinical case definition of oral acute GVHD is lacking, as several factors, including conditioning chemotherapy, concurrent radiation, neutropenia, herpes simplex infection (HSV), treatment with cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), and infusion of donor hematopoietic cells contribute to oral lesion development during the first 28 days following transplant. Oral mucositis induced by chemotherapy in a non-transplanted cancer patient presents clinically as erythema progressing to ulceration of non-keratinized oral tissues. Ulcers often occur within 2 weeks of chemotherapy initiation, healing 2–4 weeks after chemotherapy ceases (Lalla et al, 2008). Palifermin (recombinant human-keratinocyte growth factor), a drug that reduces chemotherapy and radiation induced mucositis, reduces the severity and duration of oral mucositis in patients undergoing autologous HSCT and allo-HSCT patients conditioned with cyclophosphamide and fractionated total-body irradiation (Cy/TBI), suggesting conditioning contributes to early oral lesions (Spielberger et al, 2004; Blazar et al, 2006). Therefore, some investigators feel that oral acute GVHD is not a distinct entity.
While infectious agents can contribute to oral lesions in transplanted patients, most patients receive prophylactic anti-fungal and anti-viral agents. Anti-viral prophylaxis with agents such as acyclovir, given to prevent HSV and cytomegalovirus (CMV) reactivation after transplant, reduces viral shedding and herpetic lesions in most transplanted individuals. Therefore, the contribution of HSV and other herpetic viruses to oral lesions in the first 100 days post-transplant is most likely small (Epstein et al, 1996). However, reactivation of herpetic viruses is still possible in patients receiving this prophylaxis. In a randomized, double-blind study of 618 subjects comparing oral acyclovir and oral valacyclovir to prevent CMV reactivation, seven taking acyclovir and five taking valacyclovir developed herpetic lesions. A clinical diagnosis of herpes zoster was made in four (two per group) (Ljungman et al, 2002).
Oral presentation of cGVHD
Classic features of oral cGVHD include lichenoid changes, ulcerations and mucosal atrophy (Figure 1, panel a), salivary gland dysfunction, and restricted oral opening (Schubert and Sullivan, 1990; Woo et al, 1997; Filipovich et al, 2005). Separately, these presentations mirror several oral autoimmune conditions. The mucosal lesions are very similar to those found in oral lichen planus (OLP, Figure 1, panel b), the salivary gland infiltrates mimic those found in Sjögren's syndrome, and the fibrosis and restricted oral range of motion suggest scleroderma. Clinical findings proposed to be most diagnostic of oral cGVHD are lichenoid changes, white patches, hyperkeratotic plaques, or restricted oral range of motion in patients with sclerotic features of skin GVHD (Filipovich et al, 2005).
Figure 1.
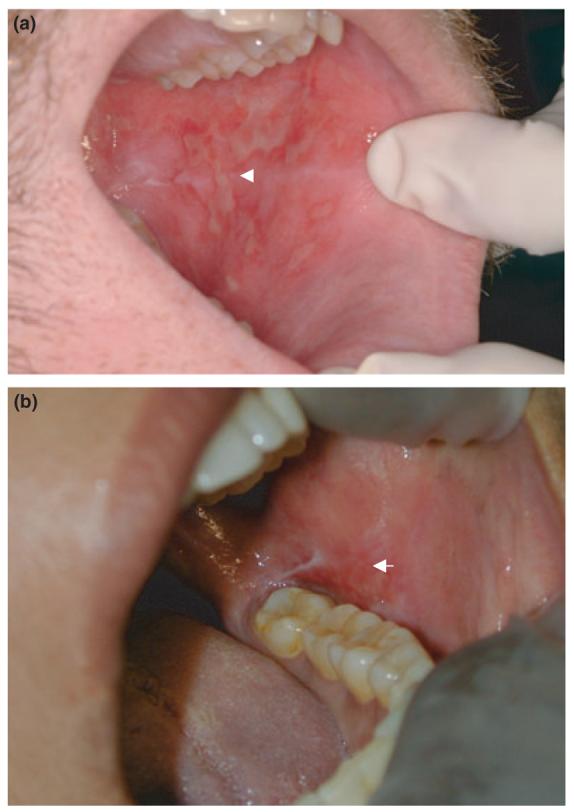
Similarities in clinical picture of oral cGVHD and oral lichen planus. Ulcerative and erythematous lesions of the buccal mucosa in oral cGVHD (a) and lichen planus (b)
In accordance with the new NIH staging criteria, the extent of mucosal lesions can be documented using a scoring system that considers the severity and extent of the most common findings. The score can be totaled to generate a cumulative score of severity for use in clinical studies (Pavletic et al, 2006).
Oral mucosal lesions
Mucosal lesions can be a source of significant pain, and when extensive, can limit nutritional intake and reduce the patient's ability to maintain oral hygiene. Almost any oral site may be involved, with buccal mucosa and tongue among most commonly affected (Treister et al, 2008). Clinically, these lesions exhibit erythema and white striae (hyperkeratotic plaques) and have been described as reticular, erythematous, atrophic and ulcerative (Woo et al, 1997; Filipovich et al, 2005; Imanguli et al, 2006; Sari et al, 2007). Extensive ulcerative lesions may be covered with a pseudomembrane.
Several clinical studies have reported that oral lesions are common in patients with cGVHD, estimated to occur in 45–83% of patients (Treister et al, 2005; Schubert and Correa, 2008). In one longitudinal study, the oral mucosa was the second most commonly affected site in those who developed cGVHD (Flowers et al, 2002). In another study of 101 patients, 51% of surviving patients developed oral cGVHD sometime during the 3 years (Mohty et al, 2002). A similar 3-year cumulative incidence of oral lesions was found in the cohort of 126 patients followed by Flowers et al (2002), with just under 50% of patients developing oral cGVHD sometime during the 3 years.
Mucoceles
Superficial mucoceles are subepithelial extravasations of sialomucin that occur at the epithelial-connective tissue interface and appear to be directly related to minor salivary glands. Clinically, the presentation is a fluid-filled, smooth elevation of the epithelium surrounding the duct of the minor salivary gland. Mucoceles develop when the duct is physically occluded, forcing the saliva into the surrounding tissues. The current belief is that salivary gland inflammation in GVHD, coupled with decreased salivary fluid secretion and viscous saliva, blocks excretory ducts (Garcia et al, 2002; Filipovich et al, 2005).
Fibrosis/limited opening
Continued inflammation and scarring of the buccal mucosa can restrict oral opening to an extent that limits a patient's ability to perform oral hygiene, and increase morbidity by decreasing food intake. This can eventually lead to weight loss (Schubert and Correa, 2008).
Oral squamous cell carcinoma
A rare, but serious complication associated with chronic oral mucosal GVHD is oral squamous cell carcinoma. Squamous cell carcinoma, often in the oral cavity, is one of the most commonly diagnosed secondary cancers in transplanted patients. This has been observed in multiple, multi-national cohorts of persons surviving BMT, (Kolb et al, 1999; Curtis et al, 2005; Shimada et al, 2005). In multivariate analysis, extensive cGVHD and older age at the time of transplantation were associated with a higher risk for squamous cell cancers (Shimada et al, 2005), but it is not clear whether GVHD itself or its treatment is most strongly associated with tumor development.
Histopathology
Most oral mucosal tissues as well as minor salivary glands can be affected by GVHD. Labial (minor) salivary gland involvement occurs more frequently than oral mucosa, possibly due to the higher tissue expression of histocompatibility antigens by salivary tissues and the accessibility of the glands to pathogenic lymphocytes (Soares et al, 2005). Salivary gland histopathology is described in detail below.
It should be noted that large studies examining the histopathologic features of acute GVHD have not been performed in humans, as biopsies are often contraindicated immediately post-HSCT due to the high infectious risk and risk of other complications (Woo et al, 1997). Therefore, most histopathologic knowledge of acute GVHD comes from skin biopsies. Studies that examined the development of acute GVHD skin lesions over time identified the first changes as basal cell layer cytoplasmic vacuolar alterations with perivascular inflammation of the vessels in the superficial plexus (Farmer, 1985). The lymphocytic infiltrate subsequently moves toward the epithelium and is associated with spongiosis. Dyskeratotic cells then appear, subepidermal clefts develop and the epithelium may separate from the connective tissue (Farmer, 1985; Woo et al, 1997).
Biopsy to confirm the clinical diagnosis of active cGVHD is recommended when alternative diagnoses are possible, when no diagnostic clinical features of cGVHD are present, when prior changes might be altering clinical judgment or when only internal organs exhibit clinical signs of active cGVHD. Along with those indications, whenever drug toxicity is suspected and infections with atypical clinical features are present, biopsy is essential to establish the correct diagnosis (Nash et al, 2006).
Histologic interpretation of cGVHD is not always straightforward and can be altered by a large number of factors. Inflammatory activity can be suppressed by immunosuppressive medications (Woo et al, 1997; Nash et al, 2006), drug reactions can mimic GVHD, the tissue sample may not be representative of the involved site, and prior tissue damage can be difficult to separate from new GVHD activity.
Minimal histologic criteria for oral mucosal cGVHD are localized or generalized epithelial changes consisting of lichenoid interface inflammation, exocytosis, and apoptosis (Figure 2) (Shulman et al, 2006). Histopathologic features include hydropic degeneration of the basal cells, interspersed areas of hyperparakeratosis or atrophy, subepithelial clefting, and apoptotic spinal layer cells with pyknotic nuclei (Soares et al, 2005). The connective tissue is characterized by variable amounts of perivascular inflammation and lymphocytic infiltration that is altered by the use of immunosuppressive medications, with occasional separation of the epithelium from the connective tissue (Woo et al, 1997; Soares et al, 2005; Lew and Smith, 2007). Immunohistochemical studies have demonstrated that the infiltrate is predominately lymphocytes and macrophages (Figure 3). The reported ratio of CD8 T cells to CD4 T cells, however, has differed among studies, with some finding a slight predominance of CD8 cells (Soares et al, 2005) and others a predominance of CD4 cells (Nakamura et al, 1996). The increased number of macrophages suggests an important role for this cell type in oral cGVHD (Soares et al, 2005).
Figure 2.
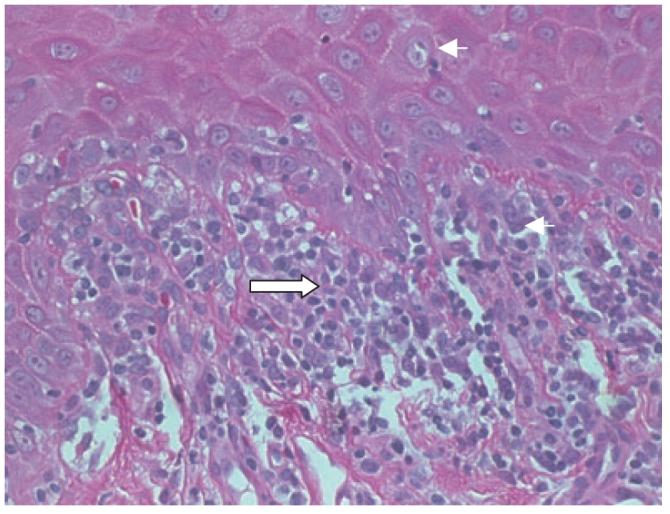
Histopathologic changes of oral cGVHD. Note “dyskeratotic’ (apoptotic) keratinocyte and adjacent lymphocyte (small arrow) and subepithelial mononuclear infiltrate (large arrow).
Figure 3.
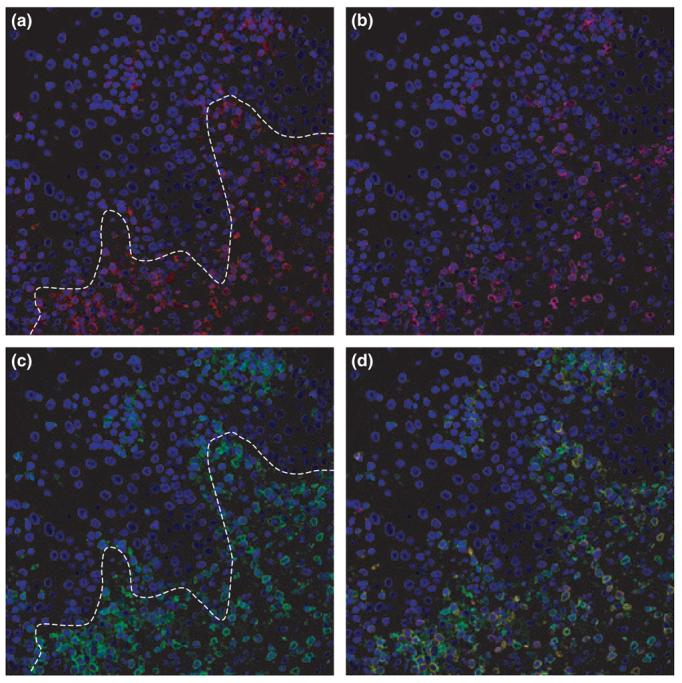
Immunohistology of oral cGVHD. Immunofluorescent staining and confocal imaging for CD3 (red, a), CD8 (cyan, b), and CD45RO, a marker for effector-memory T cells (green, c). Nuclei are stained with DAPI, blue. Note predominance of CD8 cells in the infiltrate (overlap, d). Line shows the approximate position of the basement membrane.
Salivary gland changes
Initial observations
In 1977, a paper was published describing clinical features of a 25-year-old male who received a BMT for acute myelogenous leukemia (Lawley et al, 1977).
Approximately 8 months following transplant, he presented with features of cutaneous scleroderma, xerophthalmia, and xerostomia. Results of the Schirmer's tear test, which quantifies tear production, and the minor salivary gland biopsy suggested that the patient had Sjögren's syndrome. That same year, another case series described four patients with complaints of xerophthalmia and xerostomia who had received allogeneic BMT and subsequently developed cGVHD. Three patients had keratoconjunctivitis sicca (KCS) similar to that found in patients with Sjögren's syndrome, and all four had significant minor salivary gland lymphocytic infiltration. Other features of various autoimmune diseases were present, including sclerodermatous skin and discoid lupus erythematosus-like lesions (Gratwhol et al, 1977).
Prevalence and risk factors for GHVD salivary gland disease and Sjogren's-like syndrome
Since the original descriptions, multiple reports of a Sjögren-type syndrome in patients with GVHD have been published (Izutsu et al, 1983a,b; Janin-Mercier et al, 1987; Lindahl et al, 1988; Rouquette-Gally et al, 1988; Nakhleh et al, 1989). However, as different case definitions have been used to define GVHD salivary gland disease, it is difficult to determine the prevalence and time course of salivary gland pathology after transplant (Table 2). In several studies, salivary gland infiltration was more common if patients had oral mucosal GVHD. Other factors, such as conditioning regimens, transplant cell source, GVHD prophylaxis protocols and patient age, may influence the development of oral and ocular lymphocytic infiltration post-transplant. In particular, data suggest that external beam total body irradiation during conditioning is a risk factor for GVHD salivary gland disease (Heimdahl et al, 1985; Dahllof et al, 1988; Brattstrom et al, 1991; Dens et al, 1996). Other studies suggest that autoimmune markers such as serum anti-nuclear antibodies are associated with GVHD salivary gland disease (Gratwhol et al, 1977; Rouquette-Gally et al, 1988), and salivary flow rate assessments across time suggest that there is an initial depression of flow after conditioning that may recover with time (Dens et al, 1996).
Table 2.
Histopathologic studies of minor glands and clinical findings of GVHD salivary gland disease
| Author, year | Patients | Cells |
Time post-transplant |
Salivary pathology criteria |
Biopsy controls |
Subjective findings |
Salivary flow findings |
Scintigraphy | Summary findings |
|---|---|---|---|---|---|---|---|---|---|
| Lawley et al, 1977 | 1 male with GVHD | BM | Greenspan and Daniels | None | Patient had oral dryness | ↓ Stimulated parotid flow | No | Patient also had features of scleroderma | |
| Gratwhol et al, 1977 | 4 with GVHD | BM | 14–19 months | Talal | 2 autologous BMT patients | 1 of 6 had dry mouth; 3 of 6 had dry eyes | Two with GVHD had ↓ parotid flows | 1 of 5 was abnormal | Lymphocytic infiltrates and acinar atrophy in 4/4 withGVHDand 0/2 controls |
| Shulman et al, 1980 | 20 patients with GVHD | BM | 13–365 days | Qualitative assessment | 32 controls without GVHD | Not reported systematically | None | None | One of the first papers describing the spec trum of systemic clinico-pathologic features of GVHD |
| Sale et al, 1981 | 37 patients with GVHD | BM | 75–2172 days | Grade I and Grade II | 47 grafted patients without GVHD | Not reported | None | None | Grade I findings were sensitive for GVHD (>80%), but only 50–56% specific. Grade II findings less sensitive but more specific for GVHD. No association of histo logic findings and irradiation |
| Izutsu et al, 1983a,b | 11 with chronic GVHD | BM | 76–704 days | Qualitative assessment | 6 allogeneic patients without GVHD and 4 syngeneic patients | Not reported systematically | Slight ↓ compared to healthy controls | No | 5/10 GVHD glands abnormal vs 1/8 controls. GVHD saliva had ↓ salivary IgA, and ↑ salivary Na+, IgG and albumin |
| Janin-Mercier et al, 1987 | 60 allogeneic and 8 syngeneic | BM | 100 days; some followed up to 3 years | Qualitative assessment | 8 syngeneic patients | Not reported systematically | No | Yes; graded 0–IV on maximal rate of emptying; | Minor glands = Class II in 21/60 allogeneic patients and 0/8 syngeneic. Scintigraphy abnormal in 46 GVHD patients and 8/8 syngeneic patients |
| Nakhleh et al, 1989 | 34 | BM | 24–1653 days | Qualitative assessment | None | No | No | No | GVHD in the salivary glands predicts GVHD in other organs |
| Horn et al, 1995 | 59 | BM | Mean = 136 days | Horn criteria | None | No | No | No | No association with oral histologic changes and BMT outcome |
| Hiroki et al, 1996 | 11 with chronic GVHD | BM | Not given | Scored 0, 1+, and 2+ | Compared with 24 Sjogren's patients | 8/11 reported oral dryness | ↓ Stimulated whole flow 4/11 GVHD patients | Abnormal in 3/11 GVHD patients | HLA-DR, ICAM-1, and E-selection expressed on ductal and endothe lial cells in areas of lymphocytic infiltration. CD4/CD8 ratio = 0.9 in GVHD glands |
| Nakamura et al, 1996 | 18 patients with GVHD | BM | Not given | Scored 0, 1+, and 2+ | 19 BMT patients without GVHD | + oral dryness in 11/18 with GVHD and 10/19 without | ↓ Whole stim. flow in 8/18 GVHD patients and 7/19 non-GVHD patients | Abnormal in 3/11 GVHD and 0/4 non-GVHD patients | 16/18 GVHD patients had 1+ or 2+ salivary biopsy vs 5/19 non-GVHD (differs at P < 0.001). No difference in acinar atrophy or destruction |
| Alborghetti et al, 2005 | 14 patients with oral GVHD | BM = 7; PBSC = 7 | 100–1006 days | Horn criteria | 9 BMT patients without GVHD | 2/14 GVHD + oral dryness | 14/14 by clinical exam | No | Acinar volume ↓ in GVHD group; fibrosis ↑ in GVHD. Biopsies evaluated without clinical diagnosis |
Keratoconjunctivitis sicca can be diagnosed without invasive methods; therefore, eye examinations are performed more often than salivary gland biopsies in patients post-transplant. The prevalence of KCS after BMT was 19% in a multicenter retrospective cohort study of 248 persons, and was more common in those with cGVHD. Other risk factors for KCS were female sex, age > 20 years, conditioning with single dose irradiation and methotrexate for prevention of GVHD (Tichelli et al, 1996).
Two recent studies suggest that salivary glands may be less affected by modern conditioning, which may be non-myeloablative and not use irradiation. In one study, salivary gland function was judged as normal in 47 of 49 pediatric patients who were treated with HSCT for a variety of conditions. Notably, these patients were young, many had not received total body irradiation as part of conditioning, and the source of the transplanted cells was stem cells rather than bone marrow cells (Tichelli et al, 1996; Treister et al, 2005). In another prospective cohort study of 20 adult patients receiving HSCT, 99mTc-pertechenate scintigraphy was employed to evaluate early changes in major salivary gland function following transplant (Coracin et al, 2006). Scans before HSCT as well as those at Days +30, +60 and, +100 post-transplant did not demonstrate functional changes over time. However, concurrent 67Ga scanning, an imaging method that detects inflammation, revealed inflammatory infiltration following HSCT, primarily in submandibular glands, up to Day +100 after transplant. Together, these findings suggest that salivary glands are still impacted by modern transplant techniques, but the long-term consequences that modern HSCT has on salivary gland function have not been defined.
Salivary compositional changes after HSCT
As salivary glands constitute part of the mucosal immune system, a few studies have examined changes in salivary constituents after transplant. Izutsu et al (1983b) reported that patients (n = 12) with cGVHD of salivary glands had decreased salivary immunoglobulin A (IgA) and inorganic phosphate, with increased [Na+], [Cl−], albumin and immunoglobulin G (IgG) as compared to transplant patients without GVHD (n = 10) or healthy controls (n = 8). These observations were confirmed by others (Nagler and Nagler, 2004). In another study of 61 subjects, elevated salivary [Na+] from the minor salivary glands was highly predictive of cGVHD in patients, especially those who had not had total body irradiation (Izutsu et al, 1983a). Other reported salivary changes in patients with cGVHD include elevated salivary epidermal growth factor (Nagler et al, 1996).
To achieve complete immune reconstitution after transplant, grafted cells must migrate to mucosal tissues, receive appropriate signals from antigen-presenting cells, and produce secretory immunoglobulins. In successfully grafted patients, secretory IgA can be detected in saliva within 6–12 months of transplant, and secre-tory IgA recovery precedes serum IgA by 2–3 months (Steinbrenner et al, 2005). Depressed levels of serum IgA (Fujimaki et al, 2001), salivary IgA (Izutsu et al, 1985) and IgA-bearing plasma cells (Loughran et al, 1990) within minor salivary glands are positively associated with the presence or development of cGVHD, and may explain the increased susceptibility of these patients to sinopulmonary infections.
A recent study demonstrated that transplant has a significant impact on the salivary proteome. Serially collected saliva samples from 41 patients undergoing allogeneic-HSCT were evaluated at three time points for 6 months after transplant. Multiple changes in salivary proteome were detected by SELDI-TOF mass spectrometry across time, with significant elevations of lactoferrin and secretory leukocyte protease inhibitor that persisted at least 6 months post-transplant (Imanguli et al, 2007).
Histopathology of salivary glands in GVHD
Three features characterize the histopathologic changes in GVHD salivary gland disease: lymphocytic infiltration of the salivary gland ducts, individual ductal epithelial cell necrosis (apoptosis), and destruction of acinar (at least 10%) tissues with periductal fibrosis (Soares et al, 2005; Shulman et al, 2006). Children may also exhibit oncocytic ductal metaplasia. A challenge for those evaluating biopsy specimens is distinguishing past disease from active disease. Dense fibrosis and acinar destruction most probably reflect past disease, while fibroplasia, acinar and periductal inflammation and ductal damage most probably reflect current GVHD activity. The lymphocyte infiltrate primarily contains T lymphocytes, with a slight predominance of CD8 vs CD4 cells and macrophages (Hiroki et al, 1996; Soares et al, 2005). Expression of HLA-DR is found on ductal epithelial cells associated with lymphocytic infiltration (Lindahl et al, 1988; Hiroki et al, 1996).
The hallmark of Sjögren's syndrome is a lymphoplasmocytic periductal infiltrate within the major or minor salivary glands, and three validated scoring methods are used to assess minor gland biopsies obtained for disease diagnosis. The presence of a lymphoplasmocytic cluster of at least 50 cells is termed a ‘focus’. Pathologists often use the focus score (Greenspan et al, 1974), a number between 0 and 12 that is generated by counting the foci in a 4-mm2 representative section of salivary tissue, or the Chisholm–Mason scale (Chisholm and Mason, 1968) to semi-quantify the mononuclear infiltrate. A focus score of 1 (which equals a Chisholm–Mason score of 3) is consistent with Sjögren's syndrome in the modified American–European criteria for Sjögren's syndrome (Vitali et al, 2002). The other scale often used to evaluate biopsies is the Tarpley scale (Tarpley et al, 1974), which assesses both salivary gland fibrosis and lymphocytic infiltration.
Most GVHD literature does not specify what, if any, criteria were used to evaluate and diagnose GVHD involving the salivary glands. Literature often states lymphocytic foci or infiltrates were noted in the glands, but focus scores usually were not reported (Table 2). While two histologic grading systems (Sale et al, 1981; Horn et al, 1995) have been proposed for cGVHD of minor salivary gland evolution that incorporate amount of lymphocytic infiltration with destruction of glandular acini and fibrosis, these scales are not used extensively. One small study compared the utility of the Horn scale to distinguish patients with and without GVHD salivary gland disease (Alborghetti et al, 2005), but the different proposed scales have not been validated using both normal minor gland tissues and tissues from transplanted patients.
Treatment
Multiple factors must be considered when treating a patient with oral GVHD. Even though oral GVHD may have serious health consequences, GVHD of the liver or lungs is life-threatening. Therefore, therapeutic decisions must include consideration of the patients' medical regimens for non-oral GVHD conditions and, as such, cooperation between treating physicians and dentists is mandated. Furthermore, individual response variation for oral GVHD is expected. The clinician may need to try various therapeutics and possibly therapeutic combinations to manage successfully oral GVHD symptoms. The primary treatment goals are to diminish patient pain, maintain the patient's ability to eat, increase the patient's quality of life and prevent destruction of oral tissues and dentition.
Pharmacotherapy for oral GVHD may be systemic, topical, or injectable (Imanguli et al, 2006; Couriel et al, 2006). In general, these agents have not been tested in well designed, randomized controlled trials, and trials of therapies for systemic GVHD may not include oral assessments. Therefore, the evidence for treatment efficacy should be considered preliminary.
Systemic pharmacotherapy for oral mucosal lesions
Systemic immunosuppressive therapy is used for extensive cGVHD involving multiple organs or body sites. The primary limitation of systemic immunosuppression is the increased risk for opportunistic infections, a leading cause of mortality in HSCT patients. Furthermore, there is the possibility of reduced graft-versus-tumor effect (Couriel et al, 2006; Imanguli et al, 2006). The two systemic immunosuppressive drugs used most commonly are cyclosporine and systemic corticosteroids, either alone or in combination. Cyclosporine, a calcineurin inhibitor, suppresses T-cell proliferation and prevents transcription of genes for IL-2, IL-2 receptor, and IFN-γ. Therefore, patients taking cyclosporine are at increased risk for oral mucosal infections such as oral candidiasis and herpetic infections. Systemic corticosteroids also predispose patients to fungal and viral infection. Therefore, clinicians managing severe mucosal lesions in GVHD patients must consider infectious agents as possible etiologic causes or contributors when patients are maintained using long-term systemic immunosuppression.
Other systemic agents used for systemic GVHD include tacrolimus (FK-506) and sirolimus (rapamycin), which inhibit T-cell proliferation similarly to cyclosporine, and pentostatin and mycophenolate mofetil (MMF), which suppress both T and B lymphocytes (Couriel et al, 2006; Imanguli et al, 2006). GI and hematologic toxicity can limit MMF use, and the infectious susceptibilities of patients taking this drug are significant (Krejci et al, 2005).
Hydroxychloroquine, an antimalarial drug with anti-inflammatory properties, clofazimine and thalidomide, which have anti-inflammatory properties including decreasing TNF-α activity, have also been tested as treatments for GVHD (Gilman et al, 2000).
Several trials evaluated thalidomide in cGVHD with modest overall responses. Systemic thalidomide is associated with multiple side effects which limit its tolerable dose and reduce its therapeutic benefit (Parker et al, 1995; Biagi et al, 2001; Imanguli et al, 2006).
Emerging systemic therapies being tested in patients with recalcitrant disease include monoclonal antibodies to block mediators of inflammation, such as the anti-TNF-α antibodies infliximab and etanercept, and the anti-IL-2 receptor antibody daclizumab (Imanguli et al, 2006). Rituximab, an anti-CD20 monoclonal antibody, has demonstrated promise for treatment of GVHD (Ratanatharathorn et al, 2003; Zaja et al, 2007). The drug was effective in over 50% of patients with refractory cGVHD and may have a beneficial impact upon survival. Cutler et al (2006) reported a clinical response rate of 70% in the treatment of steroid refractory GVHD (Kim, 2007).
A non-pharmacologic treatment for systemic GVHD is extracorporeal photophoresis (ECP), a process that separates the patient's mononuclear cells through apheresis and exposes them to ultraviolet light A (UVA). The cells are subsequently re-infused in the patient. Though not completely elucidated, the process is believed to induce apoptosis of alloreactive T lymphocytes, normalize the CD4/CD8 ratio and induce regulatory T cells. Preliminary data suggest that the process is efficacious for oral cGVHD, but the procedure is limited by its long duration (4 h) and the availability of ECP facilities (Imanguli et al, 2006).
Topical and local therapy
Topical and local therapy for oral GVHD offer several advantages, including fewer systemic side effects and drug interactions, the ability to intensify therapy to one specific area while preventing systemic host immuno-suppression, and maintenance of graft-versus-tumor effects. Despite these possible advantages, there are few controlled trials which have examined the efficacy of topical treatments for oral GVHD or have compared topical and systemic approaches for management of oral GVHD (Imanguli et al, 2006).
Corticosteroids
Topical corticosteroids, commonly used for many oral mucosal inflammatory conditions, are the most popular local therapy for oral cGVHD. Agents tested in studies include topical budesonide rinse (Elad et al, 2003) and topical dexamethasone rinse (0.1 mg cc−1) (Wolff et al, 2004). Other topical agents that might be used include flucinonide, clobetasol, beclomethasone, and triamcin-alone (Couriel et al, 2006; Imanguli et al, 2006; Kim, 2007).
Cyclosporine and tacrolimus
Topical cyclosporine has been tested in small open label studies for oral GVHD (Epstein and Reece, 1994) and topical tacrolimus is presently used in dermatology for atopic dermatitis and cutaneous cGVHD (Choi and Nghiem, 2001). Recently, there have been reports indicating possible efficacy of topical tacrolimus ointment in oral cGVHD. Preliminary findings suggest that it has therapeutic benefit with limited side-effects (Eckardt et al, 2004; Albert et al, 2007).
Local phototherapy
Exposure to UVA after oral administration of 8-methoxypsoralen (PUVA) causes cross-linkage of DNA, leading to cellular apoptosis. Rapidly proliferating cells such as activated T lymphocytes are particularly sensitive to effects of PUVA. (Yoo et al, 1996; Imanguli et al, 2006). Small studies tested PUVA for treatment of oral cGVHD, with preliminary reports of success. However, PUVA therapy may increase the risk for squamous cell and basal cell carcinoma in transplanted patients (Imanguli et al, 2006).
Treatment GVHD salivary gland disease
Guidelines for the treatment of patient with decreased salivary flow are designed to increase patient comfort and decrease caries risk (Atkinson et al, 2005). Agents to increase patient comfort include cholinergic agonists pilocarpine or cevimeline HCl that increase resting salivary flow rates, ‘artificial’ salivas or coating agents that moisten the mouth, and sugarless candies, sugarless mints or sugarless gums that stimulate salivary flow through mechanical and gustatory stimuli. Patients with reduced salivary flow have an increased risk for caries and oral fungal infections. Topical fluorides should be prescribed as needed for patients with active carious lesions. Other options to reduce caries include concentrated fluoride varnish (such as 5% sodium fluoride containing fluoride at 25 000 ppm) and monitoring the patient every 3 months to restore new lesions as needed.
Treatment studies of cholinergic agonists for HSCT patients with significant salivary complaints have been very small, consisting of one double-blind, placebo-controlled study and a few open label trials (Nagler and Nagler, 1999, 2001; Carpenter et al, 2006; Agha-Hosseini et al, 2007). The only controlled study found pilocarpine four times per day minimally increased salivary flow at one time point in the study and decreased xerostomia complaints, though it is unclear how perceptions of dryness were collected (Agha-Hosseini et al, 2007).
Study limitations
Most therapeutic trials for oral GVHD were not randomized, had no placebo group and were too small to control for confounding systemic therapies. Clinical outcomes were not graded using standardized scales, making interpretation of the data difficult. Large trials of systemic therapies often evaluate the mouth using the oral mucositis WHO grading scale, which was not developed to assess oral GVHD. Though two standardized scales for grading oral GVHD exist, (Piboonniyom et al, 2005; Pavletic et al, 2006), they need to be validated in prospective studies.
Limitations of GVHD salivary gland disease studies
Most studies of salivary gland pathology after transplantation are very small and should be interpreted with caution. At this time, there is no accepted case definition of GVHD salivary gland disease to use in clinical studies. Some investigations only use patient perceptions of oral dryness, which may not indicate salivary gland pathology. Studies comparing flow rates of transplanted patients with and without salivary gland disease should be large enough to account for the wide variance in salivary flow rates of healthy populations, and control for medication usage (Ship et al, 1991; Yeh et al, 1998).
Histologic interpretation of minor salivary glands has it own set of challenges. At least five different grading systems (Greenspan, Chisholm–Mason, Sale, Horn, and Talal, Table 2) were used to diagnosis GVHD salivary gland disease in various studies, and those developed for GVHD (Sale, Horn, and Talal) have not been extensively validated in studies that include healthy un-transplanted controls. This is essential, as modest lymphocytic infiltration with an occasional focus of lymphocytes (Radfar et al, 2002) is found in normal tissues. Therefore, one could expect that salivary glands would be re-populated after transplant with some lymphocytes. In addition, salivary gland fibrosis, a reported feature of GVHD salivary gland disease, occurs with age in normal glands (Syrjanen, 1984). More comprehensive, larger studies of the histopathologic changes associated with GVHD salivary gland disease are needed. Evaluators scoring minor gland biopsy specimens should not know the group assignment of specimens they are evaluating.
Conclusion
Chronic GVHD remains the most significant long-term challenge in the allogeneic HSCT. Despite high significance and prevalence, patho-physiology of cGVHD remains elusive and progress in the development of effective therapeutic and preventive strategies has been slow. Intensive basic, translational and patient-oriented research in this important area is critically needed to make allo-HSCT a more predictable and successful treatment modality. In addition, given the close clinical and histopathologic similarities between cGVHD and many autoimmune disorders, we believe that cGVHD serves as a unique human model of autoimmunity. Therefore, progress in cGVHD research may benefit not only those post-allo-HSCT but also a much larger community of patients.
Acknowledgements
The authors would like to thank Dr Fran Hakim for her continual guidance. This work was in part funded by the Center for Cancer Research, Intramural Program of the National Cancer Institute and the Intramural and Extramural Program of the National Institute of Dental and Craniofacial Research.
These opinions are of the authors and not of the National Cancer Institute, National Institute of Dental and Craniofacial Research, National Institutes of Health or the US government. The definitive version of this article is available at www.blackwell-synergy.com
References
- Agha-Hosseini F, Mirzaii-Dizgah I, Ghavamzadeh L, Ghavamzadeh A, Tohidast-Acrad Z. Effect of pilocarpine hydrochloride on unstimulated whole saliva flow rate and composition in patients with chronic graft-versus-host disease (cGVHD) Bone Marrow Transplant. 2007;39:431–434. doi: 10.1038/sj.bmt.1705621. [DOI] [PubMed] [Google Scholar]
- Albert MH, Becker B, Schuster FR, et al. Oral graft vs. host disease in children – treatment with topical tacrolimus ointment. Pediatr Transplant. 2007;11:306–311. doi: 10.1111/j.1399-3046.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- Alborghetti MR, Correa ME, Adam RL, et al. Late effects of chronic graft-vs.-host disease in minor salivary glands. J Oral Pathol Med. 2005;34:486–493. doi: 10.1111/j.1600-0714.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- Alpdogan O, Muriglan SJ, Eng JM, et al. IL-7 enhances peripheral T cell reconstitution after allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2003;112:1095–1107. doi: 10.1172/JCI17865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–1475. doi: 10.1056/NEJMp078166. [DOI] [PubMed] [Google Scholar]
- Atkinson JC, Grisius M, Massey W. Salivary hypofunction and xerostomia: diagnosis and treatment. Dent Clin North Am. 2005;49:309–326. doi: 10.1016/j.cden.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Baird K, Pavletic SZ. Chronic graft versus host disease. Curr Opin Hematol. 2006;13:426–435. doi: 10.1097/01.moh.0000245689.47333.ff. [DOI] [PubMed] [Google Scholar]
- Beelen DW, Elmaagacli A, Muller KD, Hirche H, Schaefer UW. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999;93:3267–3275. [PubMed] [Google Scholar]
- Biagi JJ, Mileshkin L, Grigg AP, Westerman DW, Prince HM. Efficacy of thalidomide therapy for extramedullary relapse of myeloma following allogeneic transplantation. Bone Marrow Transplant. 2001;28:1145–1150. doi: 10.1038/sj.bmt.1703292. [DOI] [PubMed] [Google Scholar]
- Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966;62:21–78. [PubMed] [Google Scholar]
- Blazar BR, Weisdorf DJ, Defor T, et al. Phase 1/2 randomized, placebo-control trial of palifermin to prevent graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT) Blood. 2006;108:3216–3222. doi: 10.1182/blood-2006-04-017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brattstrom C, Tollemar J, Ringden O, Bergstrom K, Tyden G. Isoamylase levels in bone marrow transplant patients are affected by total body irradiation and not by graft-versus-host disease. Transpl Int. 1991;4:96–98. doi: 10.1007/BF00336405. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Schubert MM, Flowers ME. Cevimeline reduced mouth dryness and increased salivary flow in patients with xerostomia complicating chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2006;12:792–794. doi: 10.1016/j.bbmt.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007;110:3804–3813. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjogren's disease. J Clin Pathol. 1968;21:656–660. doi: 10.1136/jcp.21.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CJ, Nghiem P. Tacrolimus ointment in the treatment of chronic cutaneous graft-vs-host disease: a case series of 18 patients. Arch Dermatol. 2001;137:1202–1206. doi: 10.1001/archderm.137.9.1202. [DOI] [PubMed] [Google Scholar]
- Clark FJ, Gregg R, Piper K, et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4+CD25high regulatory T cells. Blood. 2004;103:2410–2416. doi: 10.1182/blood-2003-06-2073. [DOI] [PubMed] [Google Scholar]
- Cooke KR, Gerbitz A, Crawford JM, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;107:1581–1589. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coracin FL, Pizzigatti Correa ME, Camargo EE, et al. Major salivary gland damage in allogeneic hematopoietic progenitor cell transplantation assessed by scintigraphic methods. Bone Marrow Transplant. 2006;37:955–959. doi: 10.1038/sj.bmt.1705351. [DOI] [PubMed] [Google Scholar]
- Couriel DR, Saliba RM, Giralt S, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10:178–185. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2006;12:375–396. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105:3802–3811. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahllof G, Heimdahl A, Bolme P, Lonnqvist B, Ringden O. Oral condition in children treated with bone marrow transplantation. Bone Marrow Transplant. 1988;3:43–51. [PubMed] [Google Scholar]
- Dens F, Boogaerts M, Boute P, et al. Caries-related salivary microorganisms and salivary flow rate in bone marrow recipients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:38–43. doi: 10.1016/s1079-2104(96)80145-4. [DOI] [PubMed] [Google Scholar]
- Eckardt A, Starke O, Stadler M, Reuter C, Hertenstein B. Severe oral chronic graft-versus-host disease following allogeneic bone marrow transplantation: highly effective treatment with topical tacrolimus. Oral Oncol. 2004;40:811–814. doi: 10.1016/j.oraloncology.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Elad S, Or R, Garfunkel AA, Shapira MY. Budesonide: a novel treatment for oral chronic graft versus host disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:308–311. doi: 10.1067/moe.2003.23. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Reece DE. Topical cyclosporin A for treatment of oral chronic graft-versus-host disease. Bone Marrow Transplant. 1994;13:81–86. [PubMed] [Google Scholar]
- Epstein JB, Ransier A, Sherlock CH, Spinelli JJ, Reece D. Acyclovir prophylaxis of oral herpes virus during bone marrow transplantation. Eur J Cancer B Oral Oncol. 1996;32B:158–162. doi: 10.1016/0964-1955(95)00091-7. [DOI] [PubMed] [Google Scholar]
- Farmer ER. Human cutaneous graft-versus-host disease. J Invest Dermatol. 1985;85:124s–128s. doi: 10.1111/1523-1747.ep12275636. [DOI] [PubMed] [Google Scholar]
- Ferrara JL, Reddy P. Pathophysiology of graft-versus-host disease. Semin Hematol. 2006;43:3–10. doi: 10.1053/j.seminhematol.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Flowers ME, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100:415–419. doi: 10.1182/blood-2002-01-0011. [DOI] [PubMed] [Google Scholar]
- Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108:2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki K, Maruta A, Yoshida M, et al. Immune reconstitution assessed during five years after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2001;27:1275–1281. doi: 10.1038/sj.bmt.1703056. [DOI] [PubMed] [Google Scholar]
- Fukushi N, Arase H, Wang B, et al. Thymus: a direct target tissue in graft-versus-host reaction after allogeneic bone marrow transplantation that results in abrogation of induction of self-tolerance. Proc Natl Acad Sci USA. 1990;87:6301–6305. doi: 10.1073/pnas.87.16.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia FVMJ, Pascual-Lopez M, Elices M, Dauden E, Garcia-Diez A, Fraga J. Superficial mucoceles and lichenoid graft versus host disease: report of three cases. Acta Derm Venereol. 2002;82:453–455. doi: 10.1080/000155502762064610. [DOI] [PubMed] [Google Scholar]
- Gilman AL, Chan KW, Mogul A, et al. Hydroxychloroquine for the treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:327–334. doi: 10.1016/s1083-8791(00)70058-9. [DOI] [PubMed] [Google Scholar]
- Goulmy E. Human minor histocompatibility antigens. Curr Opin Immunol. 1996;8:75–81. doi: 10.1016/s0952-7915(96)80108-7. [DOI] [PubMed] [Google Scholar]
- Gratwhol AA, Moutsopoulos HM, Chused TM, et al. Sjogren-type syndrome after allogeneic bone-marrow transplantation. Ann Intern Med. 1977;87:703–706. doi: 10.7326/0003-4819-87-6-703. [DOI] [PubMed] [Google Scholar]
- Grazia Roncarolo M, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjogren's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:217–229. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- Hakim FT, Gress RE. Reconstitution of thymic function after stem cell transplantation in humans. Curr Opin Hematol. 2002;9:490–496. doi: 10.1097/00062752-200211000-00004. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Heidt PJ, Vossen JM. Experimental and clinical gnotobiotics: influence of the microflora on graft-versus-host disease after allogeneic bone marrow transplantation. J Med. 1992;23:161–173. [PubMed] [Google Scholar]
- Heimdahl A, Johnson G, Danielsson KH, Lonqvist B, Sundelin P, Ringden O. Oral condition of patients with leukemia and severe aplastic anemia. Follow-up 1 year after bone marrow transplantation. Oral Surg Oral Med Oral Pathol. 1985;60:498–504. doi: 10.1016/0030-4220(85)90238-5. [DOI] [PubMed] [Google Scholar]
- Hiroki A, Nakamura S, Shinohara M, et al. A comparison of glandular involvement between chronic graft-versus-host disease and Sjogren's syndrome. Int J Oral Maxillofac Surg. 1996;25:298–307. doi: 10.1016/s0901-5027(06)80062-7. [DOI] [PubMed] [Google Scholar]
- Horn TD, Rest EB, Mirenski Y, Corio RL, Zahurak ML, Vogelsang GB. The significance of oral mucosal and salivary gland pathology after allogeneic bone marrow transplantation. Arch Dermatol. 1995;131:964–965. doi: 10.1001/archderm.131.8.964. [DOI] [PubMed] [Google Scholar]
- Imanguli MM, Pavletic SZ, Guadagnini JP, Brahim JS, Atkinson JC. Chronic graft versus host disease of oral mucosa: review of available therapies. Med Oral Pathol Oral Radiol Endod. 2006;101:175–183. doi: 10.1016/j.tripleo.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Imanguli MM, Atkinson JC, Harvey KE, et al. Changes in salivary proteome following allogeneic hematopoietic stem cell transplantation. Exp Hematol. 2007;35:184–192. doi: 10.1016/j.exphem.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izutsu KT, Schubert MM, Truelove EL, et al. The predictive value of elevated labial saliva sodium concentration: its relation to labial gland pathology in bone marrow transplant recipients. Hum Pathol. 1983a;14:29–35. doi: 10.1016/s0046-8177(83)80043-4. [DOI] [PubMed] [Google Scholar]
- Izutsu KT, Sullivan KM, Schubert MM, et al. Disordered salivary immunoglobulin secretion and sodium transport in human chronic graft-versus-host disease. Transplantation. 1983b;35:441–446. doi: 10.1097/00007890-198305000-00010. [DOI] [PubMed] [Google Scholar]
- Izutsu KT, Menard TW, Schubert MM, et al. Graft versus host disease-related secretory immunoglobulin A deficiency in bone marrow transplant recipients. Findings in labial saliva. Lab Invest. 1985;52:292–297. [PubMed] [Google Scholar]
- Janin-Mercier A, Devergie A, Arrago JP, et al. Systemic evaluation of Sjogren-like syndrome after bone marrow transplantation in man. Transplantation. 1987;43:677–679. doi: 10.1097/00007890-198705000-00015. [DOI] [PubMed] [Google Scholar]
- Kim SS. Treatment options in steroid-refractory acute graft-versus-host disease following hematopoietic stem cell transplantation. Ann Pharmacother. 2007;41:1436–1444. doi: 10.1345/aph.1K179. [DOI] [PubMed] [Google Scholar]
- Kolb HJ, Socie G, Duell T, et al. Malignant neoplasms in long-term survivors of bone marrow transplantation. Late Effects Working Party of the European Cooperative Group for Blood and Marrow Transplantation and the European Late Effect Project Group. Ann Intern Med. 1999;131:738–744. doi: 10.7326/0003-4819-131-10-199911160-00004. [DOI] [PubMed] [Google Scholar]
- Krejci M, Doubek M, Buchler T, Brychtova Y, Vorlicek J, Mayer J. Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol. 2005;84:681–685. doi: 10.1007/s00277-005-1070-0. [DOI] [PubMed] [Google Scholar]
- Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- Lalla RV, Sonis ST, Peterson DP. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52:61–77. doi: 10.1016/j.cden.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- Lawley TJ, Peck GL, Moutsopoulos HM, Gratwohl AA, Deisseroth AB. Scleroderma, Sjogren-like syndrome, and chronic graft-versus-host disease. Ann Intern Med. 1977;87:707–709. doi: 10.7326/0003-4819-87-6-707. [DOI] [PubMed] [Google Scholar]
- Lew J, Smith J. Mucosal graft-vs-host disease. Oral Dis. 2007;13:519–529. doi: 10.1111/j.1601-0825.2007.01412.x. [DOI] [PubMed] [Google Scholar]
- Lindahl G, Lonnquist B, Hedfors E. Lymphocytic infiltration and HLA-DR expression of salivary glands in bone marrow transplant recipients: a prospective study. Clin Exp Immunol. 1988;72:267–273. [PMC free article] [PubMed] [Google Scholar]
- Ljungman P, de La Camara R, Milpied N, et al. Randomized study of valacyclovir as prophylaxis against cytomegalovirus reactivation in recipients of allogeneic bone marrow transplants. Blood. 2002;99:3050–3056. doi: 10.1182/blood.v99.8.3050. [DOI] [PubMed] [Google Scholar]
- Loughran TP, Jr, Sullivan K, Morton T, et al. Value of day 100 screening studies for predicting the development of chronic graft-versus-host disease after allogeneic bone marrow transplantation. Blood. 1990;76:228–234. [PubMed] [Google Scholar]
- Mackay F, Silveira PA, Brink R. B cells and the BAFF / APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–336. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Mak TWSM. Basic and clinical principles. Elsevier; London: 2006. The immune response. [Google Scholar]
- Marmont AM, Horowitz MM, Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–2130. [PubMed] [Google Scholar]
- Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty M, Kuentz M, Michallet M, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation: long-term results of a randomized study. Blood. 2002;100:3128–3134. doi: 10.1182/blood.V100.9.3128. [DOI] [PubMed] [Google Scholar]
- Nagler RM, Nagler A. Pilocarpine hydrochloride relieves xerostomia in chronic graft-versus-host disease: a sialometrical study. Bone Marrow Transplant. 1999;23:1007–1011. doi: 10.1038/sj.bmt.1701752. [DOI] [PubMed] [Google Scholar]
- Nagler RM, Nagler A. The effect of pilocarpine on salivary constituents in patients with chronic graft-versus-host disease. Arch Oral Biol. 2001;46:689–695. doi: 10.1016/s0003-9969(01)00035-8. [DOI] [PubMed] [Google Scholar]
- Nagler RM, Nagler A. The molecular basis of salivary gland involvement in graft–vs.–host disease. J Dent Res. 2004;83:98–103. doi: 10.1177/154405910408300203. [DOI] [PubMed] [Google Scholar]
- Nagler R, Marmary Y, Krausz Y, Chisin R, Markitziu A, Nagler A. Major salivary gland dysfunction in human acute and chronic graft-versus-host disease (GVHD) Bone Marrow Transplant. 1996;17:219–224. [PubMed] [Google Scholar]
- Nakamura S, Hiroki A, Shinohara M, et al. Oral involvement in chronic graft-versus-host disease after allogeneic bone marrow transplantation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:556–563. doi: 10.1016/s1079-2104(96)80203-4. [DOI] [PubMed] [Google Scholar]
- Nakhleh RE, Miller W, Snover DC. Significance of mucosal vs salivary gland changes in lip biopsies in the diagnosis of chronic graft-vs-host disease. Arch Pathol Lab Med. 1989;113:932–934. [PubMed] [Google Scholar]
- Nash RA, McSweeney PA, Nelson JL, et al. Allogeneic marrow transplantation in patients with severe systemic sclerosis: resolution of dermal fibrosis. Arthritis Rheum. 2006;54:1982–1986. doi: 10.1002/art.21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudorf S, Sanders J, Kobrinsky N, et al. Allogeneic bone marrow transplantation for children with acute myelocytic leukemia in first remission demonstrates a role for graft versus leukemia in the maintenance of disease-free survival. Blood. 2004;103:3655–3661. doi: 10.1182/blood-2003-08-2705. [DOI] [PubMed] [Google Scholar]
- O'Keefe CL, Gondek L, Davis R, et al. Molecular analysis of alloreactive CTL post-hemopoietic stem cell transplantation. J Immunol. 2007;179:2013–2022. doi: 10.4049/jimmunol.179.3.2013. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Okano A, Akamatsu S, et al. Rituximab is effective for steroid-refractory sclerodermatous chronic graft-versus-host disease. Leukemia. 2006;20:172–173. doi: 10.1038/sj.leu.2403996. [DOI] [PubMed] [Google Scholar]
- Parker PM, Chao N, Nademanee A, et al. Thalidomide as salvage therapy for chronic graft-versus-host disease. Blood. 1995;86:3604–3609. [PubMed] [Google Scholar]
- Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389–396. doi: 10.1016/j.exphem.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Pavletic SZ, Illei GG. The role of immune ablation and stem cell transplantation in severe SLE. Best Pract Res Clin Rheumatol. 2005;19:839–858. doi: 10.1016/j.berh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Pavletic SZ, Smith LM, Bishop MR, et al. Prognostic factors of chronic graft-versus-host disease after allogeneic blood stem-cell transplantation. Am J Hematol. 2005;78:265–274. doi: 10.1002/ajh.20275. [DOI] [PubMed] [Google Scholar]
- Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12:252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Piboonniyom SO, Treister N, Pitiphat W, Woo SB. Scoring system for monitoring oral lichenoid lesions: a preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:696–703. doi: 10.1016/j.tripleo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Przepiorka D, Anderlini P, Saliba R, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 2001;98:1695–1700. doi: 10.1182/blood.v98.6.1695. [DOI] [PubMed] [Google Scholar]
- Radfar L, Kleiner DE, Fox PC, Pillemer SR. Prevalence and clinical significance of lymphocytic foci in minor salivary glands of healthy volunteers. Arthritis Rheum. 2002;47:520–524. doi: 10.1002/art.10668. [DOI] [PubMed] [Google Scholar]
- Ratanatharathorn V, Carson E, Reynolds C, et al. Anti-CD20 chimeric monoclonal antibody treatment of refractory immune-mediated thrombocytopenia in a patient with chronic graft-versus-host disease. Ann Intern Med. 2000;133:275–279. doi: 10.7326/0003-4819-133-4-200008150-00011. [DOI] [PubMed] [Google Scholar]
- Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9:505–511. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- Reddy P, Ferrara JLM. Immunobiology of acute graft-versus-host disease. Blood Rev. 2003;17:187–194. doi: 10.1016/s0268-960x(03)00009-2. [DOI] [PubMed] [Google Scholar]
- Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- Roncarolo M-G, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- Rouquette-Gally AM, Boyeldieu D, Prost AC, Gluckman E. Autoimmunity after allogeneic bone marrow transplantation. A study of 53 long-term-surviving patients. Transplantation. 1988;46:238–240. doi: 10.1097/00007890-198808000-00010. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Sale GE, Shulman HM, Schubert MM, et al. Oral and ophthalmic pathology of graft versus host disease in man: predictive value of the lip biopsy. Hum Pathol. 1981;12:1022–1030. doi: 10.1016/s0046-8177(81)80260-2. [DOI] [PubMed] [Google Scholar]
- Saliba RM, de Lima M, Giralt S, et al. Hyperacute GVHD: risk factors, outcomes, and clinical implications. Blood. 2007;109:2751–2758. doi: 10.1182/blood-2006-07-034348. [DOI] [PubMed] [Google Scholar]
- Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13:6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari I, Altuntas F, Kocyigit I, et al. The effect of budesonide mouthwash on oral chronic graft versus host disease. Am J Hematol. 2007;82:349–356. doi: 10.1002/ajh.20814. [DOI] [PubMed] [Google Scholar]
- Sasazuki T, Juji T, Morishima Y, et al. Effect of matching of Class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. N Engl J Med. 1998;339:1177–1185. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- Schubert MM, Correa ME. Oral graft-versus-host disease. Dent Clin North Am. 2008;52:79–109. doi: 10.1016/j.cden.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Schubert MM, Sullivan KM. Recognition, incidence, and management of oral graft-versus-host disease. NCI Monogr. 1990;9:135–143. [PubMed] [Google Scholar]
- Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- Shimada K, Yokozawa T, Atsuta Y, et al. Solid tumors after hematopoietic stem cell transplantation in Japan: incidence, risk factors and prognosis. Bone Marrow Transplant. 2005;36:115–121. doi: 10.1038/sj.bmt.1705020. [DOI] [PubMed] [Google Scholar]
- Ship JA, Fox PC, Baum BJ. How much saliva is enough? ‘Normal’ function defined. J Am Dent Assoc. 1991;122:63–69. doi: 10.14219/jada.archive.1991.0098. [DOI] [PubMed] [Google Scholar]
- Shlomchik WD. Antigen presentation in graft-vs-host disease. Exp Hematol. 2003;31:1187–1197. doi: 10.1016/j.exphem.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12:31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Soares AB, Faria PR, Magna LA, et al. Chronic GVHD in minor salivary glands and oral mucosa: histopathological and immunohistochemical evaluation of 25 patients. J Oral Pathol Med. 2005;34:368–373. doi: 10.1111/j.1600-0714.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- Steinbrenner M, Hafer R, Gruhn B, et al. T-cell independent production of salivary secretory IgA after hematopoietic stem cell transplantation in children. Oral Microbiol Immunol. 2005;20:282–288. doi: 10.1111/j.1399-302X.2005.00226.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Tawara I, Toubai T, Reddy P. Pathophysiology of acute graft-versus-host disease: recent advances. Transl Res. 2007;150:197–214. doi: 10.1016/j.trsl.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svegliati S, Olivieri A, Campelli N, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110:237–241. doi: 10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- Syrjanen S. Age-related changes in structure of labial minor salivary glands. Age Ageing. 1984;13:159–165. doi: 10.1093/ageing/13.3.159. [DOI] [PubMed] [Google Scholar]
- Tarpley TM, Jr, Anderson LG, White CL. Minor salivary gland involvement in Sjogren's syndrome. Oral Surg Oral Med Oral Pathol. 1974;37:64–74. doi: 10.1016/0030-4220(74)90160-1. [DOI] [PubMed] [Google Scholar]
- Thomas X, Boiron JM, Huguet F, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22:4075–4086. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- Tichelli A, Duell T, Weiss M, et al. Late-onset keratoconjunctivitis sicca syndrome after bone marrow transplantation: incidence and risk factors. European Group or Blood and Marrow Transplantation (EBMT) Working Party on Late Effects. Bone Marrow Transplant. 1996;17:1105–1111. [PubMed] [Google Scholar]
- Treister NS, Woo SB, O'Holleran EW, Lehmann LE, Parsons SK, Guinan EC. Oral chronic graft-versus-host disease in pediatric patients after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:721–731. doi: 10.1016/j.bbmt.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Treister NS, Cook EF, Jr, Antin J, Lee SJ, Soiffer R, Woo S-B. Clinical evaluation of oral chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14:110–115. doi: 10.1016/j.bbmt.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Ueno NT, Childs RW. What's past is prologue: lessons learned and the need for further development of allogeneic hematopoietic stem cell transplantation for renal cell carcinoma. Biol Blood Marrow Transplant. 2007;13:31–33. doi: 10.1016/j.bbmt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechalekar A, Cranfield T, Sinclair D, Ganzckowski M. Occurrence of autoantibodies in chronic graft vs. host disease after allogeneic stem cell transplantation. Clin Lab Haematol. 2005;27:247–249. doi: 10.1111/j.1365-2257.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- Wolff D, Anders V, Corio R, et al. Oral PUVA and topical steroids for treatment of oral manifestations of chronic graft-vs.-host disease. Photodermatol Photoimmunol Photomed. 2004;20:184–190. doi: 10.1111/j.1600-0781.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Woo SB, Lee SJ, Schubert MM. Graft-vs.-host disease. Crit Rev Oral Biol Med. 1997;8:201–216. doi: 10.1177/10454411970080020701. [DOI] [PubMed] [Google Scholar]
- Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K, Choi U, Woltz PC, et al. Severe chronic graft-versus-host disease is characterized by a preponderance of CD4+ effector memory cells relative to central memory cells. Blood. 2004;103:3986–3988. doi: 10.1182/blood-2003-09-3286. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Horwitz ME, Kwatemaa A, et al. Unique abnormalities of CD4+ and CD8+ central memory cells associated with chronic graft-versus-host disease improve after extracorporeal photopheresis. Biol Blood Marrow Transplant. 2006;12:22–30. doi: 10.1016/j.bbmt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Yeh CK, Johnson DA, Dodds MW. Impact of aging on human salivary gland function: a community-based study. Aging (Milano) 1998;10:421–428. doi: 10.1007/BF03339889. [DOI] [PubMed] [Google Scholar]
- Yoo EK, Rook AH, Elenitsas R, Gasparro FP, Vowels BR. Apoptosis induction of ultraviolet light A and photochemotherapy in cutaneous T-cell Lymphoma: relevance to mechanism of therapeutic action. J Invest Dermatol. 1996;107:235–242. doi: 10.1111/1523-1747.ep12329711. [DOI] [PubMed] [Google Scholar]
- Zaja F, Bacigalupo A, Patriarca F, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40:273–277. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]


