Abstract
Hormone exposure, including testosterone and its metabolite estradiol, induces a myriad of effects during a critical period of brain development that are necessary for brain sexual differentiation. Nuclear volume, neuronal morphology and astrocyte complexity are examples of the wide range of effects by which testosterone and estradiol can induce permanent changes in the function of neurons for the purpose of reproduction in adulthood. This review will examine the multitude of mechanisms by which steroid hormones induce these permanent changes in brain structure and function. Elucidating how steroids alter brain development sheds light on how individual variation in neuronal phenotype is established during a critical period.
Keywords: sex differences, estrogen, astrocyte morphology, nuclear volume, dendritic spines, cell death
The brain is a target organ for gonadal steroids. In adulthood, hormone exposure is responsible for inducing many of the functions necessary for sexual receptivity and reproduction. In adult males, testosterone is necessary for spermatogenesis in the gonads as well as sexual behavior, which involves both motivation to seek a mating partner (appetitive behavior) and motor components (consummatory behavior). In adult females, fluctuating levels of estradiol and progesterone induce follicular development and ovulation in the gonads, but are also essential for appropriately timing behavioral sexual receptivity within the limited time window for fertilization. Establishing the myriad functions for steroid hormones in the adult brain remains an active research goal, but equally important and substantially less investigated are the effects of gonadal steroids on the developing brain. The immature brain is highly sensitive to the same hormones produced in adulthood and this hormone exposure during development is necessary to express many of the functions in adulthood described above. The goal of this review is to elucidate the current understanding of how steroids act on the developing brain to permanently organize neuroarchitectural sex differences that then mediate adult sex differences in physiology and behavior.
BACKGROUND
Both the gonads and brain begin as a bipotential organ and are then differentiated as male or female by a variety of cues in the developing environment. In genetic males (XY), the sex-determining region of the Y chromosome (SRY) contains the genes necessary to induce the formation of the testis (Koopman et al., 1990). In the absence of the SRY gene, in females (XX), the bipotential gonad becomes an ovary (Sinclair et al., 1990). During the last days of gestation in the rodent, and as early as the second trimester in primates, the testes of the developing male begin to produce significant quantities of testosterone (Rhoda et al., 1984; Weisz and Ward, 1980). Exposure of males to testosterone at this time induces the formation of the secondary sex characteristics, including the epididymis, vas deferens, and male genitalia (Jost, 1947). In addition, this testosterone exposure occurs at a time when the brain is particularly sensitive to hormone exposure, also known as the critical period for sexual differentiation of the brain. A critical period is defined as a restricted window of development, characterized by a heightened sensitivity to an environmental stimulus, wherein certain events must occur or forever be precluded. The concept of a critical developmental period applies to multiple neurophysiological endpoints, including the visual system (Desai et al., 2002; Wong, 1999; Zhang and Poo, 2001), development of motor neurons (Hanson and Landmesser, 2004; Haverkamp and Oppenheim, 1986), control of whisker movement in the rat (Schlaggar and O'Leary, 1991; Schlaggar and O'Leary, 1993), vocal learning in finches (Iyengar and Bottjer, 2002) and even some aspects of language development in humans (Ruben, 1997). Sexual differentiation of the bi-potential brain occurs during a restricted time point in all species examined to-date (Arnold and Gorski, 1984; MacLusky and Naftolin, 1981; Nevison et al., 1997; Swaab, 2004).
The laboratory rat is arguably the best characterized species for sexual differentiation and sex differences in the rodent brain are robust and reliable. In the male rat, the testes synthesize and release testosterone as early as embryonic day 18, and this exposure is necessary to induce a phenotypic male brain. From embryonic day 18 until around postnatal day 10, exogenous treatment of females with testosterone can override development as a female, and induce a phenotypic male brain. After postnatal day 10, treatment of females with testosterone has no effect on the development of the bipotential brain as it has been permanently induced to be phenotypically female ((Arnold and Gorski, 1984; MacLusky and Naftolin, 1981). This lack of responsiveness to exogenous steroids in the female is used to operationally define the end of the sensitive period. In primates, sexual differentiation of the brain begins and ends prenatally but may also require a later pubertal component for its completion (see (Wallen, 2005)for review), a phenomenon that also appears true for the rodent (Sisk and Foster, 2004; Sisk and Zehr, 2005). Nonetheless, testosterone exposure during the critical period is an essential requirement for masculinization of the brain and guarantees that the “brain sex” matches the gonadal sex. The organization of the brain that occurs during this critical period, followed by the activational effects of hormones on sex behavior in adulthood, constitute what has been named the Organizational/Activational Hypothesis of the brain and behavior.
Early studies performed in the guinea pig by Phoenix, Goy, Gerall and Young (Phoenix et al., 1959), were the basis for the Organizational/Activational Hypothesis. Not long after, other groups determined that treatment of females rodents with estradiol could induce masculinization of the brain just as well, if not better, than testosterone (Booth, 1977; Feder and Whalen, 1965; McEwen et al., 1977), leading to The Aromatization Hypothesis. A critical argument for this hypothesis was the discovery that the developing fetus has high levels of circulating alpha-fetoprotein, a protein that potently binds estradiol thereby sequestering the maternal estradiol in the fetal circulation (Andrews et al., 1982). Coincident with this finding was the discovery that brain nuclei that are sexually dimorphic express high levels of the enzyme P450 aromatase (Naftolin and MacLusky, 1984; Reddy et al., 1974), the enzyme that converts testosterone to estradiol. Blocking of neuronal aromatase or absence of this key enzyme during development prevents differentiation of the male rodent brain (Bakker et al., 1993; Bakker et al., 2004). In the developing male rat, testosterone secreted from the testes is not bound by alpha-fetoprotein and freely enters the brain where it is locally converted to estradiol in specific nuclei. Consequently, neonatal males have more than double the levels of estradiol than females in brain regions subject to sexual differentiation (Amateau et al., 2004; Rhoda et al., 1984). Moreover, high levels of the estrogen receptor (ER) are concentrated in these same brain regions and ER is essential for transducing the steroid signal, initiating the cascade of cellular changes leading to masculinization (McCarthy et al., 1993; Shughrue et al., 1997).
The normal development of the male rodent brain requires completion of two distinct processes: masculinization and defeminization. Masculinization is the organization of a neural substrate permissive to the expression of adult male sex behavior. Defeminization is the loss of capacity as an adult to respond to the activational effects of estradiol and progesterone to induce female sex behavior. Both processes oppose the process of Feminization, which occurs in the absence of critical levels of neuronal estradiol and is the process leading to adult female-typical behavior (Figure 1). While feminization is a default process, occurring in the absence of a particular stimulus, estradiol, it can nonetheless be considered an active process as there are surely specific cellular events that must occur in an organized fashion to generate the appropriate neuronal network for mediating female sex behavior in adulthood. What those cellular events might be remains entirely unknown. Studies attempting to elucidate the mechanistic basis of sex differences in the brain can benefit from the robust and reliable endpoint of adult sexual behavior. The separate processes of masculinization, defeminization and feminization, which result in separate behavioral outputs, allow us to attribute the many effects of estradiol in the brain to a specific process represented by a behavior.
Figure 1. The multiple processes of sexual differentiation.
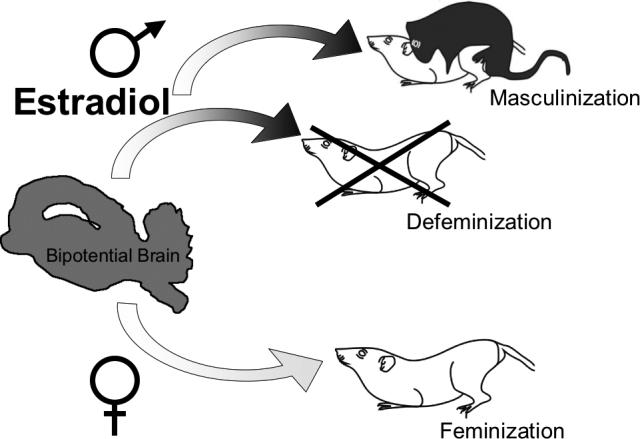
Estradiol induces sexual differentiation of the male brain by two separate processes, masculinization and defeminization. Masculinization is the active process of inducing a neural circuit that allows for the expression of male sexual behavior in adulthood. Defeminization is also an active process to eliminate or obscure the capacity to express female sex behavior in adulthood. In the normal female, feminization occurs in the absence of estradiol exposure and is the process leading to the capacity to express female sex behavior in adulthood.
SEX DIFFERENCES IN THE BRAIN
Early studies focused on two different types of sex differences in the brain: large volumetric differences of entire structures or regions, sometimes visible to the naked eye, and small microscopic differences in the morphometry of individual cells. On the macroscopic scale, Roger Gorski discovered the sexually dimorphic nucleus of the preoptic area (SDN-POA), named for the fact that this nucleus of cells is nearly 5 times larger in males than in females (Gorski et al., 1978), and embedded in a brain region necessary for the control of male sex behavior, the preoptic area (POA) (Larsson and Heimer, 1964). Other groups found sex differences in the neuronal morphology and synaptic patterning of the POA neurons (Pfaff, 1966; Raisman and Field, 1973). Sex differences such as these are microscopic but the magnitude of the differences is just as large. Both types of sex differences, macroscopic and microscopic, represent the biological underpinnings for behavior in adulthood. Importantly, both types of sex differences can be established by estradiol exposure during development.
STEROID REGULATION OF CELL DEATH AND ITS ROLE IN ESTABLISHING VOLUMETRIC SEX DIFFERENCES
Sex differences in the size of certain nuclei are generally induced by hormone exposure, or the lack thereof, which stimulates or prevents cell death in these areas. This is evidenced by the SDN-POA, the spinal nucleus of the bulbocavernosus (SNB), the principle bed nucleus of the stria terminalis (BNSTp), and the anteroventral periventricular nucleus (AVPV). Each of these subnuclei is critically involved in the regulation of reproductive behavior or physiology. The SDN-POA has been implicated in partner preference in sheep (Roselli et al., 2004). The SNB is a motor nucleus of the spinal cord that innervates the penile muscles and contains many more neurons in males than in females as a result of neonatal testosterone exposure (Freeman et al., 1996; Nordeen et al., 1985). The BNSTp projects to the AVPV and together these nuclei form part of a functional circuit controlling sexually dimorphic gonadotropin secretion from the anterior pituitary (De Vries and Simerly, 2002; Gu and Simerly, 1997; Herbison, 1998). In the SDN, SNB, and BNSTp, neonatal testosterone and/or estradiol decreases naturally occurring cell death, resulting in a larger nucleus in males than females (Breedlove and Arnold, 1983; Davis et al., 1996; del Abril et al., 1987; Nordeen et al., 1985). Conversely, in the AVPV, testosterone/estradiol increases cell death during the critical period, creating a significantly smaller nucleus in males than in females (Murakami and Arai, 1989; Simerly et al., 1985). These examples provide evidence that one steroid can differentially affect the same cellular process, apoptosis, depending on the brain region of action. Two major classes of signaling proteins mediate the process of apoptosis, one being the anti-apoptotic proteins such as Bcl-2, the other being the pro-apoptotic proteins, such as Bax. In transgenic mice that over-express Bcl-2, the survival-promoting cell signaling protein, the sex difference in the size of the SNB is significantly reduced; suggesting that in the normal male mouse, testosterone might up-regulate anti-apoptotic proteins such as Bcl-2 to inhibit cell death in this nucleus (Zup et al., 2003).
Conversely, the AVPV is significantly larger in females than males, with an overall increase in cell number, specifically dopaminergic neurons (Forger et al., 2004; Simerly et al., 1985; Sumida et al., 1993). Reciprocal to this is the number of cells in the BNSTp, where there are significantly fewer cells in females than in males (Guillamon et al., 1988; Hines et al., 1992). Mice lacking functional Bax, a pro-apoptotic protein, show increased cell number in the AVPV and the BNST, eliminating any hormonally induced sex difference in both of these regions (Gotsiridze et al., 2007).
Another example of macroscopic sex differences in the brain include projections from one brain region to another, such as the projection from the bed nucleus of the stria terminalis to the AVPV, which contains nearly ten times the number of fibers in males as in females (Gu and Simerly, 1997). This sex difference is established by testosterone during the critical period. However, rather than modulating the cellular mechanisms of apoptosis, the neurons of the AVPV are impacted by testosterone to induce synthesis of a target-derived trophic factor to promote outgrowth of neurites in order to form the connections originating from the BNST (Ibanez et al., 2001). The identity of this trophic factor has not yet been established.
SEX DIFFERENCES IN CELLULAR MORPHOLOGY ESTABLISHED BY EARLY ESTRADIOL EXPOSURE
In addition to the volumetric and cell number differences induced by early hormone exposure in the brain, there are also robust sex differences in synaptic connectivity and cellular morphology. The preoptic area (POA), the arcuate nucleus (ARC) and the ventromedial nucleus of the hypothalamus (VMN) are subnuclei critical for the control of reproductive functions in adulthood, and each one exhibits profound sex differences in cell morphology and synaptic patterning. These differences have been identified via multiple techniques including electron microscopy, Golgi-Cox impregnation, immunocytochemistry and western blot analysis of dendritic spine proteins. Electron microscopy of the POA, ARC, and VMN reveals sex differences in the type and number of synapses found on developing neurons (Matsumoto and Arai, 1986b; Mong et al., 2001; Raisman and Field, 1973), which allow for changes in neuronal sensitivity to synaptic inputs to ultimately affect neuronal function. Synaptic patterning refers to the frequency and density of synaptic contacts on individual neurons in a particular brain region. There are three basic types of synapses; 1) axosomatic synapses, referring to an axon terminating on the soma of a second neuron, 2) axodendritic synapses when an axon terminates on the dendrite of a second neuron, and 3) axodendritic spine synapses when an axon terminates on a dendritic spine of a dendrite. Dendritic spines are the major sites of excitatory input to neurons. Spine number and shape are sensitive to electrical and chemical activity in the neuron and, therefore, the basis of plasticity in many brain regions (Bourne and Harris, 2007; Hayashi and Majewska, 2005). In conjunction with the changes seen at the synaptic level using electron microscopy, these brain regions have corresponding changes in the number of dendritic spines on these neurons, which can be visualized by Golgi-Cox impregnation of individual neurons. Western blot analysis of dendritic spine proteins, such as spinophilin, a protein located preferentially in the head and necks of dendritic spines, is also a reliable method for analyzing dendritic spine number (Amateau and McCarthy, 2002a; Brake et al., 2001; Li et al., 2004; Todd et al., 2007). Associated with the changes in synaptic patterning and dendritic spines, is the length and branching of neuronal dendrites as well as the morphology of the neighboring astrocytes, a type of glia closely associated with synapses. There is a close relationship between neuronal morphology and astrocytic morphology (Fields and Stevens-Graham, 2002; Hansson and Ronnback, 2003). Immunocytochemical detection of the protein glial fibrillary acidic protein (GFAP), allows for detailed morphological examination of astrocytes and sex differences in astrocyte complexity and morphology have been identified in both the POA and the ARC nucleus (Amateau and McCarthy, 2002b; Mong et al., 1999). The communication between astrocytes and neurons seems to vary between these two brain regions, relying predominantly on GABA in the ARC (Mong et al., 2002), and glutamate in the POA (McCarthy, 2008).
Despite the fact that estradiol exposure in the developing brain works to achieve a single goal – induce a male-typic brain – estradiol utilizes distinct mechanisms to establish sex differences in each brain region. Not only does this speak to the versatility of estradiol action in the brain, but raises questions of why? Why would estradiol use separate mechanisms to reach the same goal? Different mechanisms may relate to differences in plasticity and permanency of estradiol action in each region during development. Separate mechanisms may be a way by which estradiol selectively induces separate processes, such as masculinization and defeminization, while effectively excluding the development of other brain functions. Alternatively, the many mechanisms of estradiol action might relate to the ability of estradiol to exploit preexisting cell types and signaling mechanisms that are region specific. Regardless of the reasons, the outcome is that a far greater variability can be achieved between individuals than would otherwise occur with a single mechanism for estradiol action, as there are multiple nodal points that can be independently modulated. This point will be elaborated on further after a more detailed discussion of the particular mechanisms for estradiol action that have so far been established.
ESTRADIOL-INDUCED SEX DIFFERENCES OF THE PREOPTIC AREA REQUIRE PROSTAGLANDIN E2
The SDN-POA is the most well-known sex difference in the rodent brain. However, surrounding this small sexually dimorphic nucleus is a forest of neurons whose morphology and connectivity exhibit sex differences induced by estradiol exposure during development. The POA is a brain region necessary for male sex behavior in adulthood, and therefore a prime target for estradiol-mediated sex differences. In 1973, Raisman and Field discovered a difference in the number and distribution of synapses in the POA (Raisman and Field, 1973). Since then, we have determined that males have significantly more dendritic spines than females in the developing POA (Amateau and McCarthy, 2002a). Also in the POA, males have more complex astrocytes, characterized by an increase in the number of processes and branches extending from these cells (Amateau and McCarthy, 2002b). Both of these sex differences are established by estradiol exposure during the critical period of development.
A major unanswered question was the mechanism by which estradiol mediates changes in neuronal and glial morphology in the developing POA. The answer turned out to be quite surprising, with the discovery that a lipid molecule, prostaglandin E2 (PGE2), could mimic the effects of estradiol during the critical period to induce an increase in dendritic spines (Amateau and McCarthy, 2002a). Prostaglandins are synthesized from fatty acids and maintained at basal levels. Cyclooxygenase 2, or COX-2, is an inducible form of the upstream enzyme necessary for prostaglandin production, and can be stimulated by many factors; most well known of which is inflammation and the subsequent production of cytokines. In mature animals, PGE2 in the POA is the primary initiator of fever (Lazarus et al., 2007). In the developing POA, COX-2 is higher in males than in females and treatment of females with estradiol increases mRNA for COX-2 in this region. Moreover, treatment of neonatal females with estradiol increases levels of PGE2 in the developing POA by 7-fold. Treatment of neonatal females with estradiol plus indomethacin, an inhibitor of the COX-2 enzyme, completely blocks estradiol-induced increases in dendritic spines in the developing POA (Amateau and McCarthy, 2004). Thus estradiol exposure in the developing POA stimulates COX-2 to increase production of prostaglandins, specifically PGE2, which can independently increase dendritic spines in this area to induce sex differences. The effects of PGE2 on dendritic spines in this region are partially blocked by antagonizing the AMPA-subtype glutamate receptor (Amateau and McCarthy, 2002a). This suggests that PGE2 enhances neuronal excitation at the ionotropic glutamate receptor to induce dendritic spines in the POA by promoting the release of glutamate from astrocytes (Figure 2), a common mechanism seen in other brain regions (Bezzi et al., 1998; Newman, 2003; Zhang et al., 2004). However, PGE2 may also increase dendritic spines via direct actions of PGE2 on one or a combination of the prostanoid receptors, EP1−4 (Burks et al., 2007).
Figure 2. Estradiol induces PGE2 synthesis to initiate changes in both neuronal and astrocyte morphology of the developing POA.
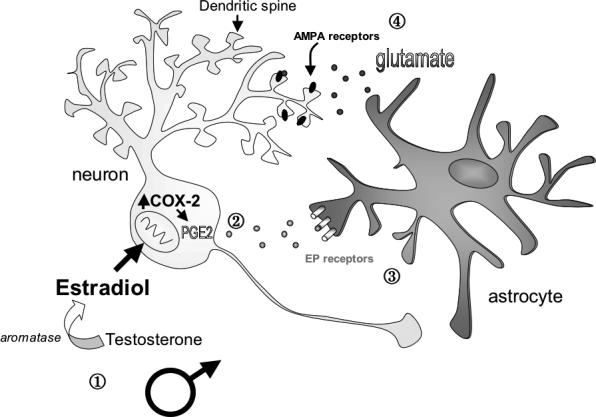
In a working model of the developing male preoptic area (POA), estradiol synthesized from testosterone up-regulates the synthesis of COX-2, an inducible enzyme necessary for prostaglandin synthesis. Increased COX-2 leads to an increase in prostaglandin E2 (PGE2) synthesis. Estradiol requires PGE2 synthesis and downstream activation of the EP receptor to induce changes in astrocyte morphology, including increased branches and complexity. PGE2 also increases the dendritic spine density on POA neurons. The effects of PGE2 on dendritic spines in this region can be partially blocked by pretreatment with NBQX, an AMPA glutamate receptor antagonist (Amateau and McCarthy, 2002a). This suggests that PGE2 enhances neuronal excitation at the ionotropic glutamate receptor to induce dendritic spines in the POA, possibly by promoting the release of glutamate from astrocytes
In addition to the effects of estradiol on the morphology of neurons in the developing POA, estradiol also induces sexual differentiation of the surrounding astrocytes. Of all glia, astrocytes are one of the most abundant cells in the brain. Both their shape and their close proximity to neuronal synapses make them an intricate part of the development of new synapses as well as being important to mature neurotransmission (Araque et al., 1999a; Araque et al., 1999b). In the developing POA, astrocytes in the male have a greater number of primary processes, and the average length of these processes is longer than astrocytes in the female (Amateau and McCarthy, 2002b). Treatment of newborn females with estradiol increases the astrocyte primary process length and number to the levels seen in males, indicating that the sex difference in astrocyte morphology is established via estradiol exposure (Amateau and McCarthy, 2002b). The effect of estradiol on astrocyte morphology is partly inhibited by the COX-2 inhibitor, indomethacin, and partly mimicked by exogenous PGE2 administration to females during development (McCarthy, 2008). Thus, at least part of the mechanism by which estradiol induces astrocytic morphological changes involves production of PGE2.
To test whether the effects of PGE2 in the developing preoptic area constitute permanent changes in the sexual differentiation of the brain, the effect of neonatal PGE2 administration or COX-2 inhibition was assessed on adult sex behavior. Specifically, females treated with PGE2 on the day of birth expressed the full complement of male sex behavior (exclusive of ejaculation) when given testosterone in adulthood, indicating that their brains were permanently masculinized by PGE2 (Amateau and McCarthy, 2004). However, females treated with PGE2 on the day of birth also express normal female sex behavior when given estradiol and progesterone as adults, indicating that the brain was not defeminized (Todd et al., 2005). Males treated with indomethacin at birth, which blocks PGE2 production via inhibition of COX-2, exhibited no sex behavior as adults, indicating they were completely defeminized but not masculinized. Thus, estradiol-induced changes in POA neuronal and astrocyte morphology via PGE2 are a necessary component of brain masculinization during the critical period but have no effect on estradiol-induced defeminization of the brain and behavior. Female rodents treated with PGE2 neonatally that express adult sex behavior that has been masculinized and not defeminized are capable of still displaying normal maternal behavior (Todd et al., 2005), a behavior additionally controlled by the POA (Numan, 1974). Therefore, despite large changes in the neuronal and astrocytic morphology of the POA during development, PGE2 had no effect on other hormonally dependent behaviors, such as female sex behavior and maternal behavior. These findings suggest that the effects of estradiol via PGE2 may be a mechanism by which estradiol selectively induces brain masculinization, leaving the development of other brain functions effectively untouched.
ESTRADIOL-INDUCED SEX DIFFERENCES IN THE DEVELOPING VENTROMEDIAL NUCLEUS OF THE MEDIOBASAL HYPOTHALAMUS
Just caudal to the POA, the mediobasal hypothalamus (MBH) is another key target for estradiol and a site of many sex differences during development and adulthood. A central region in the MBH, the ventromedial nucleus (VMN) is necessary for female sex behavior. In adulthood, injection of estradiol directly into, or electrical stimulation of the VMN facilitates female sex behavior, while lesions of the VMN prevent sexual receptivity (Mathews and Edwards, 1977; Pfaff and Sakuma, 1979a; Pfaff and Sakuma, 1979b). The VMN is characterized by its well-defined oval shape and sparse thin dendrites (Millhouse, 1973). In males, the VMN is only slightly larger than in females yet male VMN neurons have more than three times as many dendritic spine and shaft synapses as females (Matsumoto and Arai, 1983; Matsumoto and Arai, 1986b). This sex difference in synaptic patterning is detected as early as postnatal day 2 and is still present at postnatal day 100 (Matsumoto and Arai, 1986a; Pozzo Miller and Aoki, 1991). The male pattern can be induced in females by treatment with either testosterone or estradiol within the first few days of life (Matsumoto and Arai, 1986a; Pozzo Miller and Aoki, 1991). Blocking estradiol production in males using the aromatase inhibitor, Letrozole, prevents sexual differentiation of the VMN – thereby inducing the female phenotype of dendritic spines during development and the behavioral profile of an adult female (Lewis et al., 1995). In the adult female brain there is synaptic plasticity induced by hormonal changes associated with the estrous cycle or exogenous estradiol treatment and these changes correlate with changes in female sex receptivity (Calizo and Flanagan-Cato, 2000; Frankfurt et al., 1990). Whether the capacity of steroid-induced plasticity is lost in the adult male VMN has not been carefully explored but is inferred from the ineffectiveness of treatment with female hormones to induce feminine behavior in adult males.
Despite the similar estradiol-induced increase in dendritic spines in both the POA and the VMN during the critical period, PGE2 does not mediate the effects of estradiol in the developing VMN as it does in the developing POA. Treatment of neonatal females with PGE2 at birth, a paradigm that effectively mimics estradiol treatment and increases dendritic spines in the POA, had no effect on dendritic spine levels in the VMN (Todd et al., 2005). These results indicate that two regions close in proximity to each other and both responsive to estradiol are sexually differentiated through different mechanisms.
In an attempt to elucidate what is the mechanism of estradiol-mediated sexual differentiation of the VMN, early work focused on the amino acid transmitter, GABA (gamma-aminobutyric acid). Estrogen sensitive GABAergic neurons are found in the anterior and mediobasal hypothalamus (Flugge et al., 1986) and newborn males have twice as much glutamic acid decarboxylase (GAD – the rate-limiting enzyme in GABA synthesis) and GABA as females (Davis et al., 1996; Davis et al., 1999). This sex difference is hormonally established and decreasing GAD in neonatal males with an antisense oligonucleotide targeted against GAD mRNA disrupts the differentiation of sex behavior. The GABAA receptor is an ionotropic receptor permeable to chloride. During development, an increase in the intracellular chloride concentration allows chloride ions to flow out of the cell to depolarize the membrane upon GABAA receptor activation with muscimol (Obrietan and van den Pol, 1995). As the brain matures, GABA gradually changes from being depolarizing and excitatory to hyperpolarizing and inhibitory (Rohrbough and Spitzer, 1996). Estradiol exposure delays this change in hypothalamic neurons, extending the duration of time during which muscimol-induced GABAA receptor activation is excitatory (Perrot-Sinal et al., 2001). In doing so, estradiol mediates sex differences in intracellular signaling, including the phosphorylation of the cyclic AMP response element binding protein (pCREB) in the neonatal VMN (Auger et al., 2001a; Auger et al., 2001b) (Figure 3). Despite this dramatic effect of estradiol on the GABAergic system in the developing hypothalamus, there has been no clear link established between the organization of morphological sex differences in the VMN and depolarizing GABA (Todd et al., 2007). Reversal of the estradiol-induced chloride gradient would be an exciting first step in understanding the functional significance of this effect of estradiol in the developing hypothalamus.
Figure 3. Estradiol and neurotransmitter function in the developing MBH.
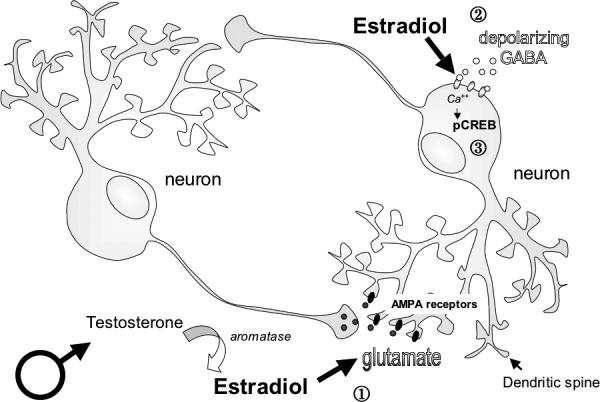
In the developing male mediobasal hypothalamus (MBH), estradiol synthesized from testosterone initiates a host of changes in neurotransmitter function.
By enhancing glutamatergic transmission, estradiol can increase the number of dendritic spines and branches on developing hypothalamic neurons. Blocking glutamate receptors, AMPA and NMDA, blocks estradiol-induced increase in dendritic spines in this region (Todd et al., 2007).
Estradiol also enhances excitatory GABAergic neurotransmission in MBH neurons by extending the duration of time during which GABA is excitatory and increasing the depolarization caused by an efflux of chloride ions (Perrot-Sinal et al., 2001). In doing so, estradiol significantly enhances calcium entry into the cell and mediates sex differences in intracellular signaling, including the phosphorylation of the cyclic AMP response element binding protein (pCREB) (Auger et al., 2001a; Auger et al., 2001b).
More recent work in the developing hypothalamus has focused on the glutamatergic neurotransmitter system. Glutamate is the primary neurotransmitter in the VMN (Ziegler et al., 2002) and is a known modulator of dendritic spine formation and maintenance in many brain regions (Malenka and Nicoll, 1993; McKinney et al., 1999; Pozzo-Miller et al., 1999). Treatment of females with the ionotropic AMPA glutamate receptor antagonist, NBQX, effectively blocks estradiol-induced increases in dendritic spines in the hypothalamus but has no effect on basal levels of dendritic spines, suggesting that all estradiol-induced increases in dendritic spines in the VMN requires enhancement of the glutamatergic system in this area (Todd et al., 2007)(Figure 3). In the neighboring POA, glutamate receptor activation was necessary for approximately half of estradiol-induced increases in dendritic spines. These results again highlight the differing mechanisms by which estradiol can increase dendritic spines and induce sex differences in the developing brain.
ESTRADIOL-INDUCED SEX DIFFERENCES IN THE ARCUATE NUCLEUS REQUIRE GABAERGIC TRANSMISSION
Though the GABAergic neurotransmitter system plays no role in estradiol-induced morphological sex differences in the developing VMN, this system has been found to play a major role in the sex differences induced by estradiol in the developing arcuate nucleus, a small nucleus at the base of the hypothalamus that regulates feeding (Stricker-Krongrad et al., 1998), anterior pituitary function (Micevych et al., 2003) and female sexual receptivity (Dewing et al., 2007). During development, both the neurons and well-developed astrocytes in the arcuate exhibit robust sex differences in morphology. In males, neurons of the developing arcuate have a lower density of dendritic spines synapses than females (Mong et al., 1999; Mong et al., 2002). Conversely, developing astrocytes in the male arcuate are highly complex in comparison to females, having longer primary processes and more frequent branching. Treatment of females with testosterone or estradiol neonatally not only decreases dendritic spine density but also increases astrocytic complexity (Mong et al., 1999). Once adults, females that were treated with estradiol neonatally have an increase in astrocyte surface area that is not significantly different from the surface area seen in adult males (Mong et al., 1999), indicating a permanent steroid hormone mediated organization of astrocyte morphology.
The coordinated changes observed in arcuate neuronal and astrocyte morphology suggest that cell-to-cell communication is an important component of the steroid-mediated differentiation of this brain region. The amino acid transmitter, GABA, is made exclusively in neurons but can act on astrocytes, which express GABAA receptors. Knocking down GAD in the neonatal arcuate prevents estradiol-induced astrocyte differentiation but has no effect on female astrocytes. Likewise, treating females with the GABAA agonist, muscimol induces male astrocyte morphology but has no effect when given to males (Mong et al., 2002). Given that estradiol up regulates GABA in the developing arcuate, it is evident that neurons are the primary site of estradiol action, resulting in increased synthesis and release of GABA that then acts on neighboring astrocytes to induce their differentiation (McCarthy, 2008) (Figure 4). Whether or how the change in astrocyte morphology then impacts on neuronal morphology in the arcuate remains unknown. In the adult female brain, astrocyte and neuronal morphology change in a coordinated fashion across the estrous cycle in this brain region (Garcia-Segura et al., 1994a; Garcia-Segura et al., 1994b; Witkin et al., 1991). When estradiol levels are high during the estrous cycle, arcuate astrocyte surface area is increased, accompanied by a decrease in synaptic connectivity. The opposite occurs when estradiol levels are low, with a decrease in astrocyte surface area and a corresponding increase in synaptic connectivity, implicating a negative correlation between astrocyte complexity and synaptic connectivity. The change in arcuate synaptic profile across the estrous cycle correlates with changes in LH-secretion from the anterior pituitary but a precise causal link has not been established.
Figure 4. Estradiol induction of cellular changes in the ARC requires GABA.
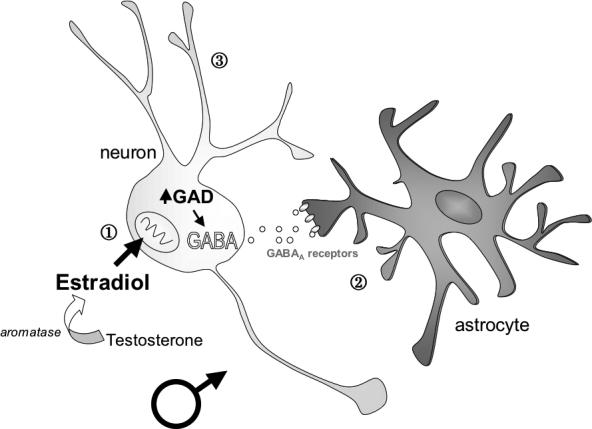
In a working model of the developing male arcuate nucleus (ARC), estradiol synthesized from testosterone up-regulates the formation of Glutamic Acid Decarboxylase (GAD), the rate-limiting enzyme for GABA synthesis (Davis et al., 1996). This increased GABA synthesis and transmission is necessary for estradiol-induced changes in astrocyte morphology, including an increase in the length and complexity of processes (Mong et al., 2002). In conjunction with an increase in astrocyte complexity, estradiol also decreases the density of dendritic spines on neighboring neurons in the developing ARC (Mong et al., 1999).
ESTRADIOL AND BRAIN DEFEMINIZATION: A POSSIBLE NEGATIVE REGULATION
Much of the work looking at the effects of estradiol in the developing and adult brain finds estradiol to be a positive modulator of signaling pathways and gene transcription. This includes many of the developmental estradiol effects described in this review, specifically apoptotic proteins in sexually dimorphic nuclei, PGE2 in the POA, glutamatergic transmission in the MBH, and GAD in the ARC. This makes sense, as the function of estradiol during the critical period of sexual differentiation is to initiate a change in entire circuits underlying sex behavior. One function of estradiol is to defeminize the brain, suppressing or erasing the female developmental pathway. Therefore, defeminization is an active process in the male brain, initiated by estradiol and would be predicted to involve induction of new proteins and signaling pathways. Conversely, feminization is the formation of a female brain from a bi-potential brain that occurs in the absence of neonatal critical levels of estradiol. One might hypothesize that if levels of a particular signaling molecule were high in the female brain and were suppressed by estradiol in the male brain, this would suggest that signaling molecule is a component of the process of brain feminization. Little work has been done to identify these possible mechanisms, perhaps due to the difficulty of identifying genes that would be turned off or proteins that would be down regulated in the presence of estradiol or elevated in the normal female brain. How would one know where to start?
Using a high-throughput proteomics approach, two proteins were identified as elevated in the developing female hypothalamus compared to the male: focal adhesion kinase (FAK) and paxillin. Both proteins are members of the focal adhesion complex family of proteins and are active in processes such as cell adhesion, migration, and growth. FAK and paxillin are significantly higher in the female hypothalamus compared to the male on the day of birth and treatment of females with estradiol suppresses FAK within only 6 hours to levels seen in males (Speert et al., 2007). Paxillin expression in the female is reduced after two days of estradiol treatment (Speert et al., 2007). These proteins are known to inhibit branching and outgrowth of neurites in developing neurons (Li et al., 2004; Liu et al., 2004; Rico et al., 2004; Robles and Gomez, 2006). We know from previous experiments that males have more dendritic spines and branches on hypothalamic neurons than females (Todd et al., 2007), and it is possible FAK and paxillin are negative regulators of dendritic growth in females (Figure 5). The suppression of protein expression by estradiol in the developing brain is relatively rare and suggests that not only does estradiol inhibit transcription or translation of this protein, with the rapid down-regulation by 6 hours; it may actively enhance degradation of FAK. A related topic of interest is the relationship between estradiol-induced masculinization and defeminization in the developing MBH. Are these two separate estradiol-mediated processes coexisting in the same brain region, or are they part of the same process of “yin” and “yang”, where one happens only in the absence of the other? Understanding more about the relationship between these processes will help us to better understand the processes themselves.
Figure 5. Estradiol and the negative regulation of FAK and paxillin.
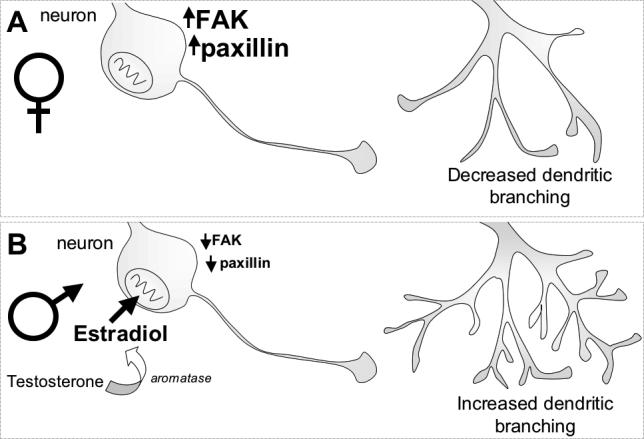
A. In the normal developing female mediobasal hypothalamus (MBH), expression of focal adhesion kinase (FAK) and paxillin is high. These proteins have been shown to inhibit the outgrowth of neuronal processes, such as dendrites.
B. In the normal developing male MBH, estradiol synthesized from testosterone suppresses the production of FAK and paxillin. Estradiol inhibits production of FAK within 6 hours and paxillin within 48 hours (Speert et al., 2007). By down-regulating these proteins, estradiol may block the inhibition of neurite outgrowth caused by FAK and paxillin thereby increasing dendritic branching in the male.
CONCLUSIONS
Estradiol induces permanent changes in different brain regions during a critical period of development via distinct mechanisms. An emerging principle is the importance of neuronal – astrocytic communication, however the nature and direction of this communication is regionally specific. As described above, there is a negative correlation between the astrocyte complexity and dendritic spine density in the arcuate. In contrast, in the POA, increases in dendritic spines are positively correlated with astrocyte complexity. In the VMN of the hypothalamus, estradiol increases dendritic spines but to-date no effect has been detected on the under-developed astrocytes in this region. Differences in estradiol mechanisms from one brain region to another provide valuable insight into general mechanisms of estradiol action in the brain as well insight into how sex differences are established and potential sources of individual variability in physiology and behavior can occur. For instance, genetic variation in the gene(s) coding for the cyclooxogenase enzymes (COX-1 and COX-2) could lead to differences in estradiol-mediated masculinization of the POA that would be entirely independent of genetic or experientially induced differences in glutamate receptors in the VMN that are modulated by estradiol. Likewise, variation in GAD or GABAA receptors could alter responsiveness of both neurons and astrocytes to estradiol in the developing arcuate nucleus. Thus one hormone, estradiol, could have both subtle and profoundly different effects across different individuals with each brain region varying in its responsiveness independent of the others.
Estradiol binds to one of two estrogen receptor (ER) isoforms, ER-alpha and ER-beta. Understanding the differences between these two isoforms and their function is emerging. The classical model of estradiol action involves binding to the nuclear ER, alpha or beta, and regulation of gene transcription via its interactions at estrogen response elements (EREs) located in the promoter region of genes modulated by estradiol. ER alpha is the predominant isoform found in the developing hypothalamus and in regions which are known to be sexually dimorphic (Shughrue et al., 1997). Male mice lacking a functional ER-alpha show low levels of male sex behavior, possibly due to the organizational or activational effects of the receptor that are yet to be determined (Ogawa et al., 1999; Wersinger et al., 1997). Recent work also suggests that the process of estradiol-mediated defeminization is induced by activation of ER-beta, however, the exact mechanism is not known (Kudwa et al., 2005). However, the examples discussed in this review demonstrate that the effects of estradiol are not cell autonomous and underscore the idea that there are global effects of estradiol in establishing entire functional circuits, which do not appear to impact solely on cells that express the ER.
Why would estradiol have multiple mechanisms in order to achieve a single goal? One possible explanation is that mechanisms of estradiol action are restricted by preexisting cell types or signaling pathways endogenous to each brain region: PGE2 in the POA, GABA in the arcuate and glutamate in the VMN. A second possible explanation is that multiple mechanisms of estradiol exposure during development are necessary for establishing multiple functional endpoints in adulthood. Remember that the induction of a male phenotypic brain requires both a gain of function, male sex behavior, as well as a loss of function, female sex behavior. One might hypothesize that both male and female sex behavior are controlled by the same brain regions, yet the expression of either behavior is mediated by differences established during development in the responsiveness or plasticity of the cells in these brain regions to adult hormone exposure. For example, the POA, a brain region critical for male sex behavior, might be “turned on” by early estradiol effects via PGE2 – allowing for the capacity of POA neurons to respond to the hormonal and environmental status of the animal necessary for the expression of male sex behavior in adulthood. Conversely, the VMN, a brain region critical for female sex behavior, must be “turned off” by early estradiol effects via glutamate – inhibiting the capacity of VMN neurons to respond to the hormonal and environmental status of the animal in order to repress female sex behavior in adulthood. This gain or loss of function upon developmental estradiol exposure corresponds to the specific cellular morphological changes seen during the critical period, but it is unclear how the individual changes in dendritic spines and astrocytes seen in each brain region retain that “memory” of early estradiol exposure. Therefore, further research is necessary to understand how adult neuronal plasticity in response to steroids is constrained by early steroid effects. Equally under investigated is the potential for epigenetic modification of the chromatin and DNA of specific genes to impart a memory or permanency of response to specific neurons in particular brain regions. The increasing prevalence of endocrine disrupting or mimicking compounds in our food and local environment, combined with the marked sex differences in the prevalence of numerous disorders of mental health (reviewed in (McCarthy, 2008)), highlight the compelling need for a greater understanding of the nature of steroid hormone action on the developing brain.
References
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145(6):2906–17. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nature Neuroscience. 2004;7(6):643–50. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostaglandin-E2. J Neurosci. 2002a;22(19):8586–96. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. Journal of Neuroendocrinology. 2002b;14(11):904–10. doi: 10.1046/j.1365-2826.2002.00858.x. [DOI] [PubMed] [Google Scholar]
- Andrews GK, Dziadek M, Tamaoki T. Expression and methylation of the mouse alpha-fetoprotein gene in embryonic, adult, and neoplastic tissues. The Journal of Biological Chemistry. 1982;257(9):5148–53. [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends in Neurosciences. 1999a;22(5):208–15. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Astrocyte-induced modulation of synaptic transmission. Canadian Journal of Physiology and Pharmacology. 1999b;77(9):699–706. [PubMed] [Google Scholar]
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annual Review of Neuroscience. 1984;7:413–42. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- Auger AP, Hexter DP, McCarthy MM. Sex difference in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Research. 2001a;890(1):110–7. doi: 10.1016/s0006-8993(00)03151-6. [DOI] [PubMed] [Google Scholar]
- Auger AP, Perrot-Sinal TS, McCarthy MM. Excitatory versus inhibitory GABA as a divergence point in steroid-mediated sexual differentiation of the brain. Proceedings of the National Academy of Sciences of the United States of America. 2001b;98(14):8059–64. doi: 10.1073/pnas.131016298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Brand T, van Ophemert J, Slob AK. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behavioral Neuroscience. 1993;107(3):480–7. doi: 10.1037//0735-7044.107.3.480. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Hormones and Behavior. 2004;46(1):1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391(6664):281–5. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Booth JE. Sexual behaviour of neonatally castrated rats injected during infancy with oestrogen and dihydrotestosterone. The Journal of Endocrinology. 1977;72(2):135–41. doi: 10.1677/joe.0.0720135. [DOI] [PubMed] [Google Scholar]
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Current Opinion in Neurobiology. 2007;17(3):381–6. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Brake WG, Alves SE, Dunlop JC, Lee SJ, Bulloch K, Allen PB, Greengard P, McEwen BS. Novel target sites for estrogen action in the dorsal hippocampus: an examination of synaptic proteins. Endocrinology. 2001;142(3):1284–1289. doi: 10.1210/endo.142.3.8036. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1983;3(2):424–32. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks SR, Wright CL, McCarthy MM. Exploration of prostanoid receptor subtype regulating estradiol and prostaglandin E2 induction of spinophilin in developing preoptic area neurons. Neuroscience. 2007;146(3):1117–1127. doi: 10.1016/j.neuroscience.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calizo LH, Flanagan-Cato LM. Estrogen selectively regulates spine density within the dendritic arbor of rat ventromedial hypothalamic neurons. J Neurosci. 2000;20(4):1589–96. doi: 10.1523/JNEUROSCI.20-04-01589.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AM, Grattan DR, Selmanoff M, McCarthy MM. Sex differences in glutamic acid decarboxylase mRNA in neonatal rat brain: implications for sexual differentiation. Hormones and Behavior. 1996;30(4):538–52. doi: 10.1006/hbeh.1996.0057. [DOI] [PubMed] [Google Scholar]
- Davis AM, Ward SC, Selmanoff M, Herbison AE, McCarthy MM. Developmental sex differences in amino acid neurotransmitter levels in hypothalamic and limbic areas of rat brain. Neuroscience. 1999;90(4):1471–82. doi: 10.1016/s0306-4522(98)00511-9. [DOI] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734(1−2):10–18. [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB. Anatomy, Development, and Function of Sexually Dimorphic Neural Circuits in the Mammalian Brain. Hormones, Brain, and Behavior. 2002;5:137–92. [Google Scholar]
- del Abril A, Segovia S, Guillamon A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Res. 1987;429(2):295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nature Neuroscience. 2002;5(8):783–9. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J. Neurosci. 2007;27(35):9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder HH, Whalen RE. Feminine Behavior in Neonatally Castrated and Estrogen-Treated Male Rats. Science (New York, N. Y. 1965;147:306–7. doi: 10.1126/science.147.3655.306. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science (New York, N. Y. 2002;298(5593):556–62. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugge G, Oertel WH, Wuttke W. Evidence for estrogen-receptive GABAergic neurons in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology. 1986;43(1):1–5. doi: 10.1159/000124500. [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(37):13666–71. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology. 1990;51(5):530–5. doi: 10.1159/000125387. [DOI] [PubMed] [Google Scholar]
- Freeman LM, Watson NV, Breedlove SM. Androgen spares androgen-insensitive motoneurons from apoptosis in the spinal nucleus of the bulbocavernosus in rats. Hormones and Behavior. 1996;30(4):424–33. doi: 10.1006/hbeh.1996.0047. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Chowen JA, Duenas M, Torres-Aleman I, Naftolin F. Gonadal steroids as promoters of neuro-glial plasticity. Psychoneuroendocrinology. 1994a;19(5−7):445–53. doi: 10.1016/0306-4530(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Luquin S, Parducz A, Naftolin F. Gonadal hormone regulation of glial fibrillary acidic protein immunoreactivity and glial ultrastructure in the rat neuroendocrine hypothalamus. Glia. 1994b;10(1):59–69. doi: 10.1002/glia.440100108. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Research. 1978;148(2):333–46. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Gotsiridze T, Kang N, Jacob D, Forger NG. Development of sex differences in the principal nucleus of the bed nucleus of the stria terminalis of mice: role of Bax-dependent cell death. Dev. Neurobiol. 2007;67(3):355–362. doi: 10.1002/dneu.20353. [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. The Journal of Comparative Neurology. 1997;384(1):142–64. [PubMed] [Google Scholar]
- Guillamon A, Segovia S, del Abril A. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Research. 1988;44(2):281–90. doi: 10.1016/0165-3806(88)90226-x. [DOI] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43(5):687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Hansson E, Ronnback L. Glial neuronal signaling in the central nervous system. Faseb J. 2003;17(3):341–8. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- Haverkamp LJ, Oppenheim RW. Behavioral development in the absence of neural activity: effects of chronic immobilization on amphibian embryos. J Neurosci. 1986;6(5):1332–7. doi: 10.1523/JNEUROSCI.06-05-01332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Majewska AK. Dendritic spine geometry: functional implication and regulation. Neuron. 2005;46(4):529–32. doi: 10.1016/j.neuron.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr. Rev. 1998;19(3):302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Research. 1992;579(2):321–6. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Ibanez MA, Gu G, Simerly RB. Target-dependent sexual differentiation of a limbic-hypothalamic neural pathway. J Neurosci. 2001;21(15):5652–9. doi: 10.1523/JNEUROSCI.21-15-05652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Bottjer SW. The role of auditory experience in the formation of neural circuits underlying vocal learning in zebra finches. J Neurosci. 2002;22(3):946–58. doi: 10.1523/JNEUROSCI.22-03-00946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost A. Recherches sur la differenciation sexuelle de l'embryon de lapin. Arch Microsc Morph Exp. 1947;(36):271–315. [Google Scholar]
- Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348(6300):450–2. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Bodo C, Gustafsson JA, Rissman EF. A previously uncharacterized role for estrogen receptor beta: defeminization of male brain and behavior. Proc. Natl. Acad. Sci. U. S. A. 2005;102(12):4608–4612. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K, Heimer L. Mating Behaviour of Male Rats after Lesions in the Preoptic Area. Nature. 1964;202:413–4. doi: 10.1038/202413a0. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10(9):1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Lewis C, McEwen BS, Frankfurt M. Estrogen-induction of dendritic spines in ventromedial hypothalamus and hippocampus: effects of neonatal aromatase blockade and adult GDX. Brain Research. 1995;87(1):91–5. doi: 10.1016/0165-3806(95)00052-f. [DOI] [PubMed] [Google Scholar]
- Li W, Lee J, Vikis HG, et al. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nature Neuroscience. 2004;7(11):1213–21. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Beggs H, Jurgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nature Neuroscience. 2004;7(11):1222–32. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science (New York, N. Y. 1981;211(4488):1294–302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends in Neurosciences. 1993;16(12):521–7. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Mathews D, Edwards DA. Involvement of the ventromedial and anterior hypothalamic nuclei in the hormonal induction of receptivity in the female rat. Physiology & Behavior. 1977;19(2):319–26. doi: 10.1016/0031-9384(77)90345-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Development of sexual dimorphism in synaptic organization in the ventromedial nucleus of the hypothalamus in rats. Neuroscience Letters. 1986a;68(2):165–8. doi: 10.1016/0304-3940(86)90135-7. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Male-female difference in synaptic organization of the ventromedial nucleus of the hypothalamus in the rat. Neuroendocrinology. 1986b;42(3):232–6. doi: 10.1159/000124445. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinologia Japonica. 1983;30(3):277–80. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol. Rev. 2008;88(1):91–134. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Schlenker EH, Pfaff DW. Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology. 1993;133(2):433–9. doi: 10.1210/endo.133.2.8344188. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lieberburg I, Maclusky N, Plapinger L. Do estrogen receptors play a role in the sexual differentiation of the rat brain? Journal of Steroid Biochemistry. 1977;8(5):593–8. doi: 10.1016/0022-4731(77)90267-9. [DOI] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nature Neuroscience. 1999;2(1):44–9. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology. 2003;78(1):29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. The organization of the ventromedial hypothalamic nucleus. Brain Research. 1973;55(1):71–87. [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19(4):1464–72. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Nunez JL, McCarthy MM. GABA mediates steroid-induced astrocyte differentiation in the neonatal rat hypothalamus. Journal of Neuroendocrinology. 2002;14(1):45–55. doi: 10.1046/j.1365-2826.2002.00737.x. [DOI] [PubMed] [Google Scholar]
- Mong JA, Roberts RC, Kelly JJ, McCarthy MM. Gonadal steroids reduce the density of axospinous synapses in the developing rat arcuate nucleus: an electron microscopy analysis. The Journal of Comparative Neurology. 2001;432(2):259–67. doi: 10.1002/cne.1101. [DOI] [PubMed] [Google Scholar]
- Murakami S, Arai Y. Neuronal death in the developing sexually dimorphic periventricular nucleus of the preoptic area in the female rat: effect of neonatal androgen treatment. Neurosci. Lett. 1989;102(2−3):185–190. doi: 10.1016/0304-3940(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Naftolin F, MacLusky N. Aromatization hypothesis revisited. 1984 [Google Scholar]
- Nevison CM, Brown GR, Dixson AF. Effects of altering testosterone in early infancy on social behaviour in captive yearling rhesus monkeys. Physiology & Behavior. 1997;62(6):1397–403. doi: 10.1016/s0031-9384(97)00209-6. [DOI] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends in Neurosciences. 2003;26(10):536–42. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science (New York, N. Y. 1985;229(4714):671–3. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Numan M. Medial preoptic area and maternal behavior in the female rat. Journal of Comparative and Physiological Psychology. 1974;87(4):746–59. doi: 10.1037/h0036974. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J Neurosci. 1995;15(7 Pt 1):5065–77. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96(22):12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Davis AM, Gregerson KA, Kao JP, McCarthy MM. Estradiol enhances excitatory gamma-aminobutyric [corrected] acid-mediated calcium signaling in neonatal hypothalamic neurons. Endocrinology. 2001;142(6):2238–43. doi: 10.1210/endo.142.6.8180. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Morphological changes in the brains of adult male rats after neonatal castration. The Journal of Endocrinology. 1966;36(4):415–6. doi: 10.1677/joe.0.0360415. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. The Journal of Physiology. 1979a;288:203–10. [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. The Journal of Physiology. 1979b;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pozzo Miller LD, Aoki A. Stereological analysis of the hypothalamic ventromedial nucleus. II. Hormone-induced changes in the synaptogenic pattern. Brain Res. Dev. Brain Res. 1991;61(2):189–196. doi: 10.1016/0165-3806(91)90131-2. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Inoue T, Murphy DD. Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices. Journal of Neurophysiology. 1999;81(3):1404–11. doi: 10.1152/jn.1999.81.3.1404. [DOI] [PubMed] [Google Scholar]
- Raisman G, Field PM. Sexual dimorphism in the neuropil of the preoptic area of the rat and its dependence on neonatal androgen. Brain Research. 1973;54:1–29. doi: 10.1016/0006-8993(73)90030-9. [DOI] [PubMed] [Google Scholar]
- Reddy VV, Naftolin F, Ryan KJ. Conversion of androstenedione to estrone by neural tissues from fetal and neonatal rats. Endocrinology. 1974;94(1):117–21. doi: 10.1210/endo-94-1-117. [DOI] [PubMed] [Google Scholar]
- Rhoda J, Corbier P, Roffi J. Gonadal steroid concentrations in serum and hypothalamus of the rat at birth: aromatization of testosterone to 17 beta-estradiol. Endocrinology. 1984;114(5):1754–60. doi: 10.1210/endo-114-5-1754. [DOI] [PubMed] [Google Scholar]
- Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nature Neuroscience. 2004;7(10):1059–69. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nature Neuroscience. 2006;9(10):1274–83. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Spitzer NC. Regulation of intracellular Cl− levels by Na(+)-dependent Cl− cotransport distinguishes depolarizing from hyperpolarizing GABAA receptor-mediated responses in spinal neurons. J Neurosci. 1996;16(1):82–91. doi: 10.1523/JNEUROSCI.16-01-00082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145(2):478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. A time frame of critical/sensitive periods of language development. Acta Oto-Laryngologica. 1997;117(2):202–5. doi: 10.3109/00016489709117769. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, O'Leary DD. Patterning of the barrel field in somatosensory cortex with implications for the specification of neocortical areas. Perspectives on Developmental Neurobiology. 1993;1(2):81–91. [PubMed] [Google Scholar]
- Schlaggar BL, O'Leary DD. Potential of visual cortex to develop an array of functional units unique to somatosensory cortex. Science (New York, N. Y. 1991;252(5012):1556–60. doi: 10.1126/science.2047863. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. The Journal of Comparative Neurology. 1997;388(4):507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylaseimmunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985;40(6):501–10. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346(6281):240–4. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat. Neurosci. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005;26(3−4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Speert DB, Konkle AT, Zup SL, Schwarz JM, Shiroor C, Taylor ME, McCarthy MM. Focal adhesion kinase and paxillin: novel regulators of brain sexual differentiation? Endocrinology. 2007;148(7):3391–3401. doi: 10.1210/en.2006-0845. [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Burlet C, Beck B. Behavioral deficits in monosodium glutamate rats: specific changes in the structure of feeding behavior. Life Sci. 1998;62(23):2127–2132. doi: 10.1016/s0024-3205(98)00187-8. [DOI] [PubMed] [Google Scholar]
- Sumida H, Nishizuka M, Kano Y, Arai Y. Sex differences in the anteroventral periventricular nucleus of the preoptic area and in the related effects of androgen in prenatal rats. Neuroscience Letters. 1993;151(1):41–4. doi: 10.1016/0304-3940(93)90040-r. [DOI] [PubMed] [Google Scholar]
- Swaab DF. Sexual differentiation of the human brain: relevance for gender identity, transsexualism and sexual orientation. Gynecol Endocrinol. 2004;19(6):301–12. doi: 10.1080/09513590400018231. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, McCarthy MM. Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation. Horm. Behav. 2005;48(5):512–521. doi: 10.1016/j.yhbeh.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Schwarz JM, Mong JA, McCarthy MM. Glutamate AMPA/kainate receptors, not GABA(A) receptors, mediate estradiol-induced sex differences in the hypothalamus. Dev. Neurobiol. 2007;67(3):304–315. doi: 10.1002/dneu.20337. [DOI] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front. Neuroendocrinol. 2005;26(1):7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106(1):306–16. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm. Behav. 1997;32(3):176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Witkin JW, Ferin M, Popilskis SJ, Silverman AJ. Effects of gonadal steroids on the ultrastructure of GnRH neurons in the rhesus monkey: synaptic input and glial apposition. Endocrinology. 1991;129(2):1083–92. doi: 10.1210/endo-129-2-1083. [DOI] [PubMed] [Google Scholar]
- Wong RO. Retinal waves and visual system development. Annual Review of Neuroscience. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nature Neuroscience. 2001;4(Suppl):1207–14. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Pangrsic T, Kreft M, Krzan M, Li N, Sul JY, Halassa M, Van Bockstaele E, Zorec R, Haydon PG. Fusion-related release of glutamate from astrocytes. The Journal of Biological Chemistry. 2004;279(13):12724–33. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. The Journal of Comparative Neurology. 2002;448(3):217–29. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]
- Zup SL, Carrier H, Waters EM, Tabor A, Bengston L, Rosen GJ, Simerly RB, Forger NG. Overexpression of bcl-2 reduces sex differences in neuron number in the brain and spinal cord. J Neurosci. 2003;23(6):2357–62. doi: 10.1523/JNEUROSCI.23-06-02357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


