Abstract
We have recently identified large glucagon-expressing neurons that densely ramify neurites in the peripheral edge of the retina and regulate the proliferation of progenitors in the circumferential marginal zone (CMZ) of the postnatal chicken eye (Fischer et al., 2005). However, nothing is known about the transmitters and proteins that are expressed by the glucagon-expressing neurons in the avian retina. We used antibodies to cell-distinguishing markers to better characterize the different types of glucagon-expressing neurons. We found that the large glucagon-expressing neurons were immunoreactive for substance P, neurofilament, Pax6, AP2α, HuD, calretinin, trkB and trkC. Colocalization of glucagon and substance P in the large glucagon-expressing neurons indicates that these cells are the “bullwhip cells” that have been briefly described by Ehrlich, Keyser and Karten (1987). Similar to the bullwhip cells, the conventional glucagon-expressing amacrine cells were immunoreactive for calretinin, HuD, Pax6, and AP2α. Unlike bullwhip cells, the conventional glucagon-expressing amacrine cells were immunoreactive for GABA. While glucagon-immunoreactive amacrine cells were negative for substance P in central regions of the retina, a subset of this type of amacrine cell was immunoreactive for substance P in far peripheral regions of the retina. An additional type of glucagon/substance P-expressing neuron, resembling the bullwhip cells, was found in far peripheral and dorsal regions of the retina. Based on morphology, distribution within the retina, and histological markers, we conclude that there may be 4 different types of glucagon-expressing neurons in the avian retina.
Keywords: retina, substance P, glucagon, chicken
Introduction
The retina of vertebrates is home to many diverse types of cells that can be segregated into 6 general categories. These categories of cells include rod and cone photoreceptors, horizontal cells, bipolar cells, amacrine cells, ganglion cells and Müller glia. The neuronal cell types in the retina can be further sub-divided based on morphology, physiology, transmitters and protein-expression profiles. Based on such criteria, for example, there are at least 30 different types of amacrine cells (MacNeil et al., 1999; Masland, 2001; Masland, 2004). One type of retinal neuron that has recently been the focus of studies regarding visually guided ocular growth is the glucagon-expressing amacrine cell of the avian retina. Glucagon-expressing amacrine cells have been shown to respond to hyperopic- or myopic-defocus (Bitzer and Schaeffel, 2002; Fischer et al., 1999a). Furthermore, levels of retinal glucagon are influenced by visual stimuli that regulate ocular growth (Feldkaemper and Schaeffel, 2002), and exogenous glucagon and antagonist to glucagon receptors influence vision-guided ocular growth (Vessey et al., 2005a; Vessey et al., 2005b). These findings have implicated the glucagon-producing retinal amacrine cells in the regulation of vision-guided ocular growth.
Glucagon is a 29-amino acid peptide that is a member of the VIP-secretin family of peptide hormones and is highly conserved across species. Glucagon and related peptides are derived from pro-glucagon mRNA and pro-peptide by tissue-specific processing of the precursor peptide (in mammals) or alternative splicing of the mRNA (in chicken and fish) (Irwin and Wong, 1995). Proglucagon can give rise to 5 secreted bioactive peptides; glucagon, mini-glucagon, oxyntomodulin, glucagon-like peptides 1 and 2 (GLP1 and GLP2) that are cleaved from separate regions of the propeptide. Glucagon is known to be expressed by a homogeneous class of interneurons comprising 1–2% of the amacrine cells in the avian retina (Ekman and Tornqvist, 1985; Kuwayama et al., 1982; Tornqvist and Ehinger, 1983; Tornqvist et al., 1981). However, there is brief mention in the literature of additional types of glucagon-immunoreactive neurons in the pigeon retina (Karten and Brecha, 1983), and a “dense fiber plexus” immunoreactive for glucagon in the periphery of the chick retina (Kiyama et al., 1985). Consistent with these reports, we have recently demonstrated that there are at least 2 additional types of large glucagon-expressing neurons (LGENs) in the chicken retina whose neurites ramify densely within the peripheral edge of the retina and these cells may regulate the proliferation of neural progenitors within the circumferential marginal zone (CMZ) (Fischer et al., 2005). One type of LGEN has a unipolar morphology, is found only in the ventral retina, and forms an axon that projects into the CMZ where their terminals are densely ramified. On average, there are only about 240 LGENs per retina and these cells are found only in ventral and mid-peripheral regions of the postnatal chick retina. The second type of LGEN was termed mini-LGEN because these cells have a morphology similar to that of the LGENs but have smaller somata and are found only in dorsal regions of the retina. We found that GLP1 may be made only by the LGENs, but not by conventional glucagon-expressing amacrine cells (CGACs) (Fischer et al., 2005), indicating that LGENs and CGACs differ not only in morphology but also in the ability to generate glucagon and GLP1 from the glucagon propeptide. Despite implications of important functions within the eye, little is known about the transmitters or proteins that are expressed by the different types of glucagon-expressing neurons within the retina. Thus, the purpose of this study was to better characterize the glucagon-expressing neurons, in particular the LGENs, in the avian retina.
A study by Katayama-Kumoi and colleagues (1985) reported that immunoreactivity for glucagon and substance P is co-localized within retinal amacrine cells in far peripheral regions of the retina. In addition, this study mentions co-localization of glucagon and substance P immunoreactivities in “giant-sized cells (above 20 μm in diameter)” that are found near the pecten and in a “dense fiber plexus” in the peripheral retina (Katayama-Kumoi et al., 1985). Even though only a terse description and no representative photomicrographs are provided, it is likely that the “giant-sized cells” and the “dense fiber plexus” represent the LGENs and their neurites within the CMZ, respectively. A subsequent study from Ehrlich et al (1987) characterized the distribution of substance P-immunoreactivity in retinal ganglion cells and the optic tectum of the chick. This study provides a brief description and one high-quality micrograph of substance P-immunoreactive cells in the inner nuclear layer (INL) that strongly resemble the LGENs. These cells were termed “bullwhip cells” because of their unique morphology (Ehrlich et al., 1987). Thus, one purpose of this study was to assay whether substance P-immunoreactive bullwhip cells and the LGENs are the same cell type.
We report here that the LGENs express a number of proteins that are common to both amacrine and ganglion cells and have a histological profile that is distinctly different from that of CGACs. Furthermore, we found that CGACs are distributed asymmetrically across the retina, with higher densities residing in a central region of retina, immediately dorsal to the region of LGENs.
Methods and Materials
Animals
The use of animals in these experiments was in accordance with the guidelines established by the National Institutes of Health and the Ohio State University. Newly hatched leghorn chickens (Gallus gallus domesticus) were obtained from the Department of Animal Sciences at the Ohio State University and kept on a cycle of 12 hours light, 12 hours dark (lights on at 7:00 am). Chicks were housed in a stainless steel brooder at about 30°C and received water and Purinatm chick starter ad libitum.
Fixation, sectioning and immunocytochemistry
Tissues were fixed, sectioned and immunolabeled as described elsewhere (Fischer et al., 1998; Fischer and Stell, 1999). In short, enucleated eyes were hemisected equatorially and the gel vitreous removed from the posterior eye cup. Eye cups were fixed (4% paraformaldehyde plus 3% sucrose in 0.1 M phosphate buffer, pH 7.4, 30 min at 20°C), washed three times in PBS (phosphate-buffered saline; 0.05 M sodium phosphate, 195 mM NaCl, pH 7.4), cryoprotected in PBS plus 30% sucrose, immersed in embedding medium (OCT-compound; Tissue-Tek), and freeze-mounted onto sectioning blocks. Vertical sections, nominally 14 μm thick, were cut in the nasotemporal plane of the eye, and thaw-mounted onto SuperFrost Plustm slides (Fisher Scientific). Sections were air-dried and stored at −20°C until use.
Sections were thawed, ringed with rubber cement, washed three times in PBS, covered with primary antibody solution (200 μl of antiserum diluted in PBS plus 5% normal goat serum, 0.2% Triton X-100, and 0.01% NaN3), and incubated for about 24 hr at 20°C in a humidified chamber. The slides were washed three times in PBS, covered with secondary antibody solution, and incubated for at least 1 hr at 20°C in a humidified chamber. Finally, samples were washed three times in PBS, rubber cement removed from the slides, and coverglass mounted on 4:1 (v:v) glycerol to water.
Whole-mount preparations of the retina were prepared as described elsewhere (Fischer et al., 2002; Fischer and Reh, 2002; Fischer et al., 1996). In short, fixed retinas were dissected away from the pigmented epithelium, choroid and sclera, cryprotected in 20% sucrose in PBS and taken through 3 cycles of freezing and thawing. Samples were washed 3 times in PBS, placed in 250 μl of primary antibody, and incubated at room temperature on an oscillating shaker for 24 hr. The primary antibody was aspirated, samples washed 3 times in PBS, 250 μl of secondary antibody solution added, and tissues incubated at room temperature on an oscillating shaker for 24 hr. Finally, samples were washed 3 times in PBS and mounted under coverglass in 4:1 (v:v) glycerol to water.
Working dilutions and sources of antibodies used in this study included; mouse anti-glucagon was raised to full-length human glucagon was and used at 1:400 (Dr. M. Gregor, University of Tübingen via the Center for Ulcer Research and Education, UCLA), rabbit anti-GLP1 was raised to amino acids 1–19 of human GLP1 and used at 1:400 (4660-1604; Biogenesis Ltd.), mouse anti-Pax6 was raised to amino acids 1–223 of chicken Pax6 and used at 1:50 (PAX6; Developmental Studies Hybridoma Bank), mouse anti-AP2α was raised to the DNA binding domain of human AP2α and used at 1:200 (3B5; Developmental Studies Hybridoma Bank), mouse anti-Brn3a was raised to amino acids 186–224 of rodent Brn-3a and used at 1:400 (mab1585; Chemicon), mouse anti-Islet1 was raised to the C-terminal of chicken Islet1 and used at 1:50 (40.2D6; Developmental Studies Hybridoma Bank), mouse anti-Islet2 was raised to recombinant full-length chicken Islet2 and used at 1:50 (51.4H9; Developmental Studies Hybridoma Bank), rat anti-substance P was raised to the 5–8 C-terminal fragment of human substance P and used at 1:400 (ab6338; Abcam); rabbit anti-trkA/B/C were raised to the extracellular domains of chicken trkA, trkB and trkC, and used at 1:5000 (Dr. F. Lefcort, Montana State University), mouse anti-neurofilament was raised to gel-excised bovine NF-M, is known to recognize a phosphate-independent epitope in the C-terminal of NF-M, and was used at 1:2000 (RMO270, Dr. V. Lee, University of Pennsylvania), mouse anti-HuC/D was raised to a peptide (QAQRFRLDNLLN, common to human HuC and HuD) conjugated to keyhole limpet hemocyanin and used at 1:200 (16A11; Invitrogen -Molecular Probes), mouse anti-calbindin was raised to purified full-length bovine kidney calbindin and used at 1:1000 (D28; Sigma-Aldrich), rabbit anti-calretinin was raised to full-length recombinant human calretinin and used at 1:1000 (7699/4; Swant Immunochemicals), mouse anti-parvalbumin at 1:1000 was raised to purified full-length frog muscle parvalbumin and used at 1:1000 (P3088, Sigma-Aldrich), rabbit anti-GABA was raised to GABA-gluteraldehyde-BSA and used at 1:100 (AB131; Chemicon), and rat anti-glycine was raised to glycine-paraformaldehyde-thyroglobulin and used at 1:1000 (Dr. D. Pow, University of Queensland). We evaluated the specificity of primary antibodies by comparison with published examples of results and assays for specificity. None of the observed labeling was due to non-specific binding of secondary antibody or auto-fluorescence in the fixed tissues because sections labeled with secondary antibodies alone were devoid of fluorescence. Secondary antibodies included goat-anti-rabbit-Alexa488/568, goat-anti-rat-Alexa488 and goat-anti-mouse-Alexa488/568 (Invitrogen - Molecular Probes) diluted to 1:1000 in PBS plus 0.2% Triton X-100.
Photography, measurements, cell counts, and statistical analyses
Photomicrographs were taken by using a Leica DM5000B microscope equipped with epifluorescence and a 12 megapixel Leica DC500 digital camera. Confocal microscopy was done by using a Ziess LSM 510 meta at the Campus Microscopy and Imaging Facility at The Ohio State University. Images were optimized for color, brightness and contrast, and overlay images composed by using Adobe Photoshop™6.0. Counts of double-labeled cells were made on at least 5 sections from 3 different animals. Counts of the LGENs were made on at least 20 cells; the sparse distribution of the LGENs limited the identification of large numbers of double-labeled cells in retinal sections. In whole-mount preparations and transverse retinal sections the LGENs were distinguished from the conventional glucagon-immunoreactive amacrine cells based on the size of their somata; the LGENs were at least 13 μm in diameter whereas the conventional amacrine cells were less than 6 μm in diameter. The topographical distribution and density of cell bodies for the GLP1/glucagon-immunoreactive cells were counted from whole-mount preparation, and means and standard deviations calculated on data sets from at least 5 individuals. To avoid the possibility of region-specific differences within the retina, cell counts were consistently made from the same region of retina for each data set.
Results
Substance P
To assay whether glucagon and substance P are co-localized in bullwhip cells and in neurites clustered within the CMZ we double-labeled retinal sections with antibodies to glucagon and substance P. We used a rat monoclonal antibody to substance P that has been reported to recognize amacrine, bullwhip and ganglion cells in the chicken retina (Ehrlich et al., 1987; Katayama-Kumoi et al., 1985). In addition, we used a monoclonal antibody to glucagon that has been reported to recognize glucagon and proglucagon (Gregor and Riecken, 1985), and is known to selectively label amacrine cells and LGENs in the chicken retina (Fischer et al., 1999a; Fischer et al., 2005).
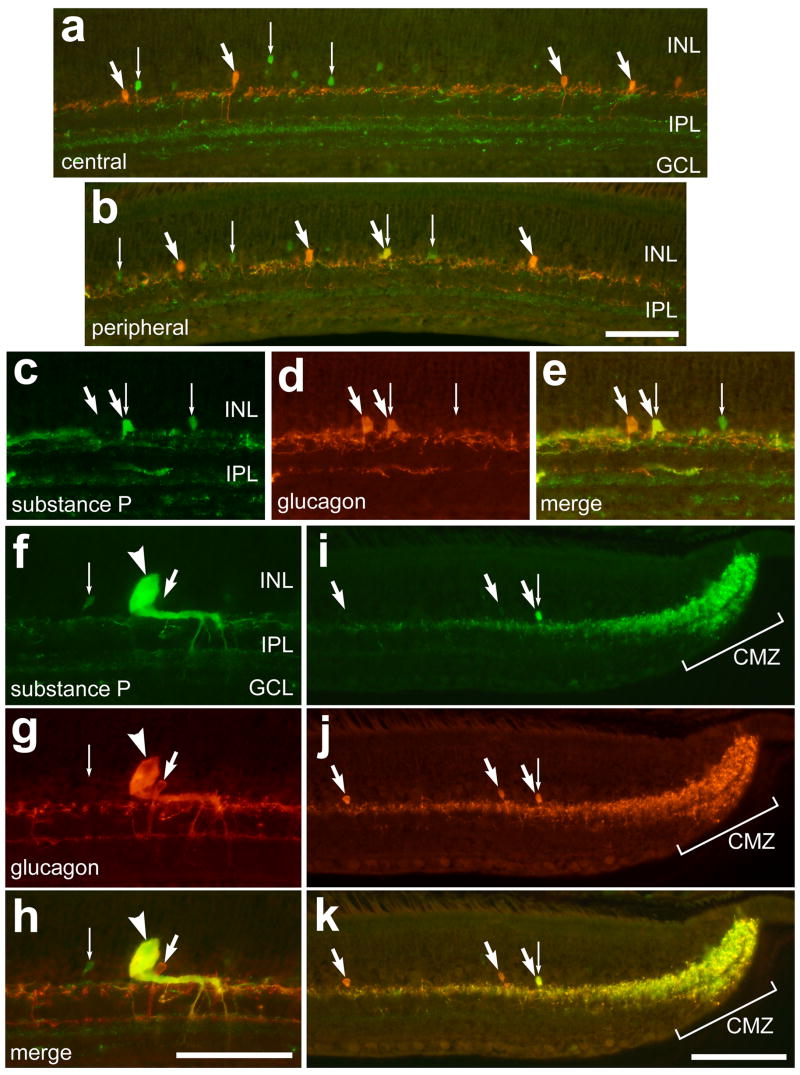
Similar to the report from Katayama-Kumoi and colleagues (1985), we found no overlap of immunoreactivities for glucagon and substance P in amacrine cells in central regions of the retina (Fig. 1a), whereas peripheral regions contained amacrine cells that were immunoreactive for glucagon and substance P (Figs. 1b–e). We found that about 40% (42.3 ± 14.0%) of the glucagon-positive amacrine cells within 500 μm of the CMZ were immunoreactive for substance P (Fig. 2a–c). In addition, we found that all bullwhip cells (n = 44; Figs. 1f–h) and the neurites that are densely clustered within the CMZ (Figs. 1i–k) are immunoreactive for glucagon and substance P. The overlap of glucagon- and substance P-immunoreactivities in neurites was very high within the CMZ, and this overlap decreased with increasing distance into the neural retina (Fig. 1k). Glucagon and substance P immunoreactivities overlapped completely within the dorsal, ventral, nasal and temporal regions of the CMZ (data not shown). Since Ehrlich, Keyser and Karten (1987) aptly named the large substance P-immunoreactive neurons in the ventral retina nearly 20 years ago, we feel that it is appropriate to rename the LGENs as bullwhip cells.
Figure 1.
Immunoreactivities for glucagon and substance P are co-localized to bullwhip cells and neurites within the CMZ. Vertical section of the retina and CMZ were labeled with antibodies to substance P (green; a–c, f and i) and glucagon (red; a, b, d, g and j). Sections were obtained from central (a), peripheral (b–e), ventral (f–h) and far peripheral (i–k) regions of the retina. Small arrows indicate cells immunolabeled for substance P alone, the large arrows indicate cells labeled for glucagon alone, the combination of a large and small arrow indicates amacrine cells labeled for substance P and glucagon, and the arrow-heads indicate a bullwhip cell labeled for substance P and glucagon. The calibration bar (50 μm) in panel b applies to panels a and b, the bar in h applies to c–h, and the bar in k applies to i–k. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, CMZ – circumferential marginal zone.
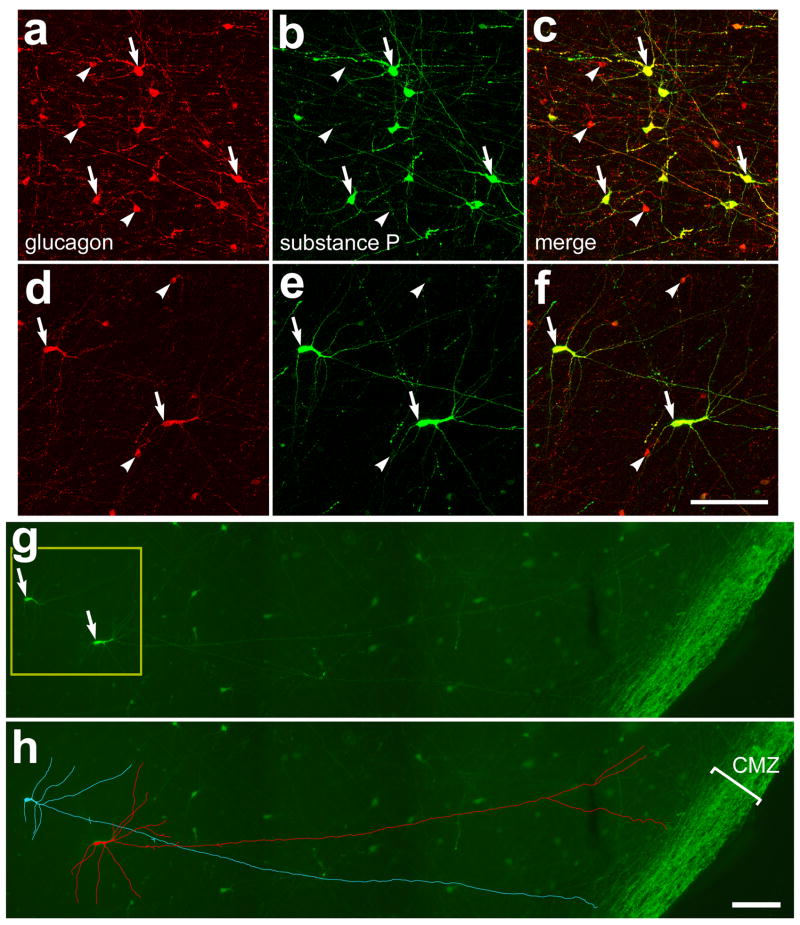
Figure 2.
Glucagon and substance P are co-localized to mini-bullwhip cells and CGACs in far peripheral regions of the dorsal retina. Flat-mounts of the far dorsal retina were labeled with antibodies to glucagon (red) and substance P (green). Arrows indicate cells labeled for glucagon and substance P, and arrow-heads indicate cells labeled for glucagon alone. The images in panels a–f were obtained by using confocal microscopy and projection of ten 1μm-thick optical sections. The images in panels a–c represent a typical field of view of cells that are found within 400 μm of the dorsal CMZ. The images in panels d–f represent a typical field of cells that are between 400 and 800 μm from the CMZ. Panels d–f are two-fold enlargements of the field indicated by the yellow box in panel g. Panels g and h are a montage of 6 images that were obtained by using standard epifluorescence and digital microscopy. Panel h is the same field of view as g with the immunolabeling for substance P within 2 different cells digitally traced (using Adobe Photoshoptm 6) in blue or red to better illustrate the morphology of the mini-bullwhip cells. The calibration bar (50 μm) in panel f applies to panels a–f, and the bar in h applies to g and h. Abbreviation: CMZ – circumferential marginal zone.
In addition to CGACs and bullwhip cells, there may be a third type of glucagon-expressing neuron within the avian retina (Fischer et al., 2005; Karten and Brecha, 1983). Similar to the bullwhip cells, this third type of glucagon-immunoreactive neuron have somata that are larger than CGACs (but smaller than those of bullwhip cells) and these cells produce neurites that project into the CMZ (Fischer et al., 2005; Karten and Brecha, 1983). Unlike the bullwhip cells which are found between 1 and 4 mm from the ventral CMZ (Fischer et al., 2005), the bullwhip-like glucagon-immunoreactive cells are found only in dorsal regions of the retina within 1 mm of the CMZ. In vertical sections of the retina, the bullwhip-like cells were difficult to identify and distinguish from CGACs because of their sparse distribution and soma size which was only a few microns larger than the somata of CGACs. Accordingly, we were restricted to making observations of this cell type in whole-mount preparations of the retina. We found that all (n = 51) of the mid-sized glucagon-expressing cells in dorsal/peripheral regions of the retina were immunoreactive for substance P (Figs. 2d–f). Similar to the bullwhip cells, the mid-sized cells that are found in the far dorsal retina produced at least one lengthy neurite (>300 μm) that projected toward the periphery of the retina and ramified within the CMZ (Figs. 2g and h). This is consistent with our previous finding that the mid-sized glucagon-immunoreactive cells, that are found immediately dorsal to the zone of bullwhip cells, produce 3–4 lengthy neurites that project into the CMZ (Fischer et al., 2005). Because the mid-sized glucagon-immunoreactive cells shared similarities with the bullwhip cells, but had smaller somata and were found only in dorsal and far peripheral regions of the retina, we termed them mini-bullwhip cells. As noted in our previous study (Fischer et al., 2005) the morphology of the mini-bullwhip cells is far more variable than that of the bullwhip cells, which have a highly regular and unique morphology. By comparison, the mini-bullwhip cells have a unipolar morphology in retinal regions immediately dorsal to the zone of bullwhip cells (Fischer et al., 2005), and this morphology becomes predominantly multipolar in far dorsal regions of the retina (Figs. 2d–f).
Islet1, Islet2, Brn3a and neurofilament
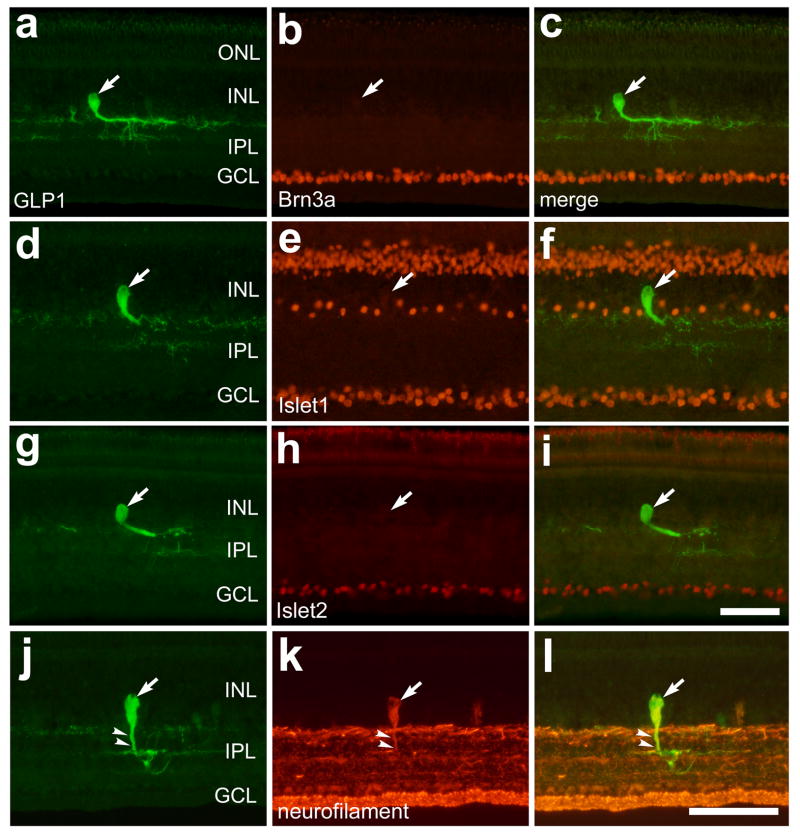
To assess whether bullwhip cells or CGACs express proteins that are expressed by ganglion cells, we double-labeled retinal sections with antibodies to GLP1 to identify the bullwhip cells and CGACs and either Brn3a, Islet1, Islet2, or neurofilament to identify ganglion cells. Brn3a is a POU-domain homeotic transcription factor that is required for the differentiation and survival of retinal ganglion cells (Gan et al., 1996; Liu et al., 1996; Xiang et al., 1993). In the avian retina, Brn3a is known to be expressed by >90% of the ganglion cells in the ganglion cell layer (GCL) but not those displaced to the INL (Fischer et al., 2002). Islet1 is a LIM-domain homeotic transcription factor that is expressed by nearly 80% of the orthotopic ganglion cells and by all displaced ganglion cells in the INL of the chick retina (Fischer et al., 2002). Like Islet1, Islet2 is a LIM-domain homeotic transcription factor, but is only expressed by a subset of orthotopic ganglion cells that project contralaterally (Pak et al., 2004). We used monoclonal antibodies to Islet1, Islet2 and neurofilament that have reported specificity for their respective antigens and well-established patterns of labeling in the chicken retina (Fischer et al., 2002; Pak et al., 2004). In addition, we used a polyclonal antiserum to GLP1 that includes IgG species that recognize GLP1 and glucagon, and has been shown to co-label glucagon-immunoreactive structures in the chick retina (Fischer et al., 2005).
We found that GLP1/glucagon-immunoreactive bullwhip cells were not immunoreactive for Brn3a (n = 24; Figs. 3a–c), Islet1 (n = 37; Figs. 3d–f), or Islet2 (n = 27; Figs. 3g–i). These findings suggest that bullwhip cells are not an unusual type of ganglion cell that is displaced to the INL. However, we found that bullwhip cells were immunoreactive for neurofilament, which is also expressed by all orthotopic and displaced ganglion cells (Fischer et al., 2002), and efferent target cells (ETCs) in the avian retina (Fischer and Stell, 1999). The somata and primary neurite of the bullwhip cells were immunoreactive for neurofilament (Figs. 3j–l). The bullwhip cell in figures 3j–l is atypical because the primary neurite does not turn and remain in the sclerad IPL. Instead, the neurite courses into deeper layers of the IPL allowing for better visualization of neurofilament within the primary neurite which might otherwise be obscured by neurofilament-immunoreactivity in processes that are present in the sclerad IPL.
Figure 3.
Bullwhip cells express neurofilament, but do not express Islet1, Islet2 or Brn3a. Vertical sections of the ventral retina were labeled with antibodies to GLP1 (a, d, g and j) and Brn3a (b), Islet1 (e), Islet2 (h) or neurofilament (k). The micrographs in the column on the right are overlay images derived from the two panels to the left. The large arrows indicate GLP1-immunoreactive bullwhip cells and small arrows in panels j–l indicate the primary neurite of a bullwhip cell that is labeled for GLP1 and neurofilament. The calibration bar (50 μm) in panel i applies to panels a–c and g–i, and the bar in l applies to d–f and j–l. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
Pax6 and AP2α
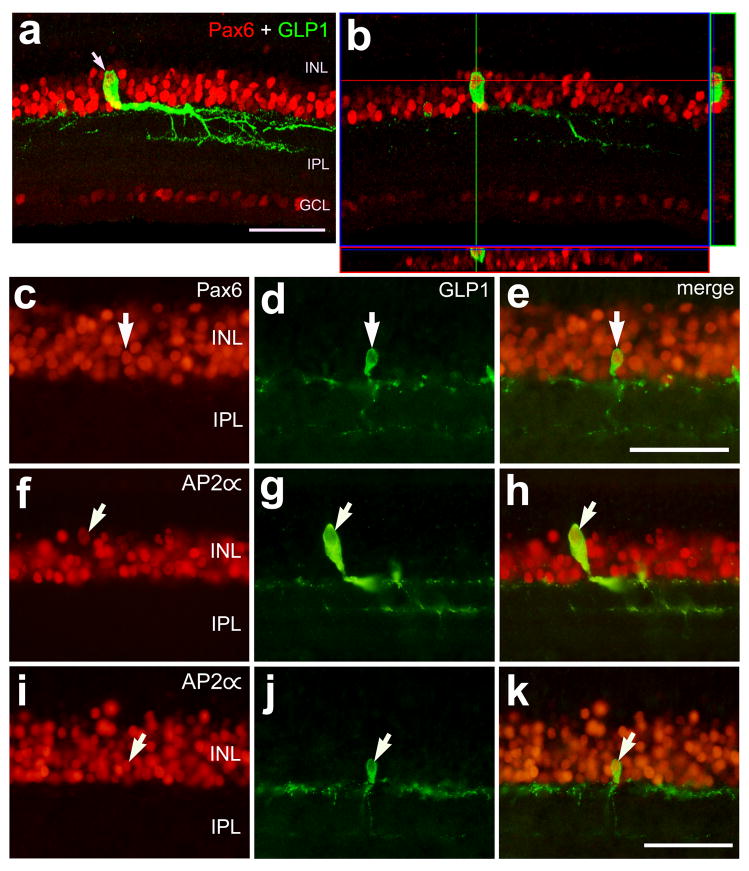
To assess whether glucagon-positive cells express transcription factors that are expressed by inner retinal neurons, we labeled retinal sections with antibodies to GLP1/glucagon and Pax6 or AP2α. We used a monoclonal antibody to Pax6 that is known to label horizontal, amacrine, ganglion cells and progenitors in the avian retina (Fischer et al., 2002; Fischer and Reh, 2000; Fischer and Reh, 2001), consistent with reports of Pax6 expression in most vertebrate species (Marquardt, 2003). We used an antibody to AP2α that has demonstrated specificity in the ocular tissues of mice (West-Mays et al., 1999) and produced immunolabeling consistent with a previous report in the chick retina that used a different source of AP2α antibodies (Bisgrove and Godbout, 1999).
We found that all bullwhip cells (n = 29) and CGACs (n = 78) were immunoreactive for Pax6 (Figs. 4a–e). We found that immunoreactivity for AP2α is found in the nuclei of many cells in the amacrine layer of the INL (Figs. 4f and i). Similar to distribution of Pax6-immunolabeling in the glucagon-expressing neurons, we found that all bullwhip cells (n = 26) and CGACs (n = 62) were immunoreactive for AP2α (Figs. 4f–k).
Figure 4.
Bullwhip cells and conventional glucagon-expressing amacrine cells (CGACs) express the transcription factors Pax6 and AP2α. Vertical sections of the ventral retina were labeled with antibodies to GLP1 (green; a,b,d,e,g,h,j and k) and Pax6 (red; a–c and e) or AP2α (red; f,h,i and k). The images in panels a and b were obtained using confocal microscopy and the images in panels c–k were obtained using epifluorescence microscopy. The image in panel a was generated from the projection of a Z-series of eight 1μm-thick optical sections. The image in panel b represents a single optical section and the orthogonal projection of a Z-series centered on the nucleus of a bullwhip cell. Arrows indicate bullwhip cells (a and f–h) and CGACs (c–e and i–k) labeled for GLP1 and Pax6 or AP2α. The calibration bar (50 μm) in panel a applies to panels a and b, the bar in panel e applies to c–e, and the bar in k applies to f–k. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL –ganglion cell layer.
HuD, parvalbumin, calretinin and calbindin
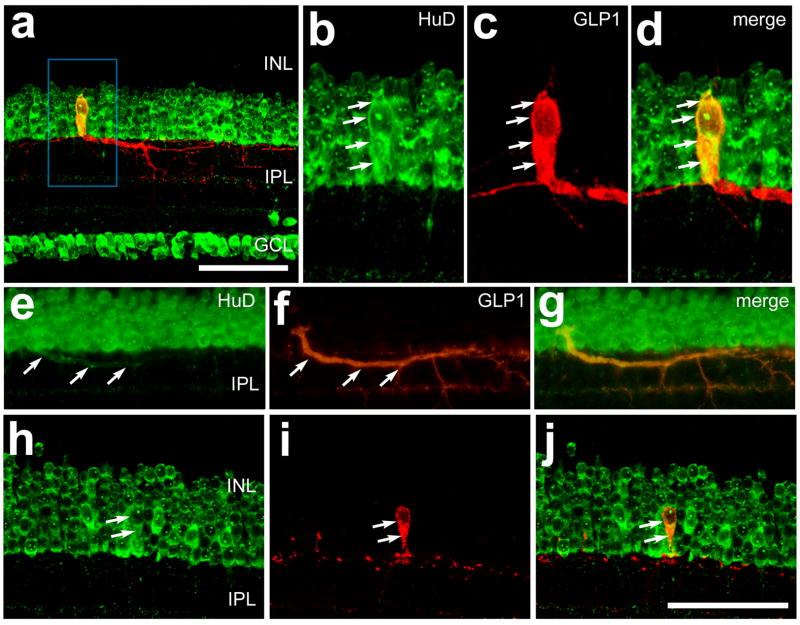
In addition to transcription factors, we assayed whether glucagon-immunoreactive neurons expressed cytoplasmic proteins that are known to be expressed by inner retinal neurons. HuD is an RNA-binding protein that may play a role in trafficking of mRNA into the axons of neurons to mediate stimulus-dependent translation (Perrone-Bizzozero and Bolognani, 2002; Smith et al., 2004). We used an antibody to HuD that is known to label most, if not all, amacrine and ganglion cells in the chick retina (Fischer and Reh, 2000). We found that all bullwhip cells (n = 35) were immunoreactive for HuD (Figs. 5a–d). In some instances we observed immunoreactivity for HuD within the primary neurite of the bullwhip cells (Fig. 5e–g). Similar to the bullwhip cells, all CGACs (n = 121) were immunoreactive for HuD (Figs. 5h–j), consistent with the hypothesis that all amacrine cells in the avian retina express HuD.
Figure 5.
Bullwhip cells and conventional glucagon-expressing amacrine cells (CGACs) express the RNA-binding protein HuD. Vertical sections of the retina were labeled with antibodies to HuD (green) and GLP1 (red). Arrows indicate a bullwhip cell labeled for GLP1 and HuD (b–d), HuD-immunoreactivity within the primary neurite of a bullwhip cell (e–g) or a CGAC that is immunoreactive for HuD and GLP1 (h–j). The images in panels a–d and h–j were obtained by using confocal microscopy and projection of four 1μm-thick optical sections. The images in panels e–g were obtained using epifluoresence microscopy. The calibration bar (50 μm) in panel a applies to panel a alone, and the bar in panel j applies to e–j. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
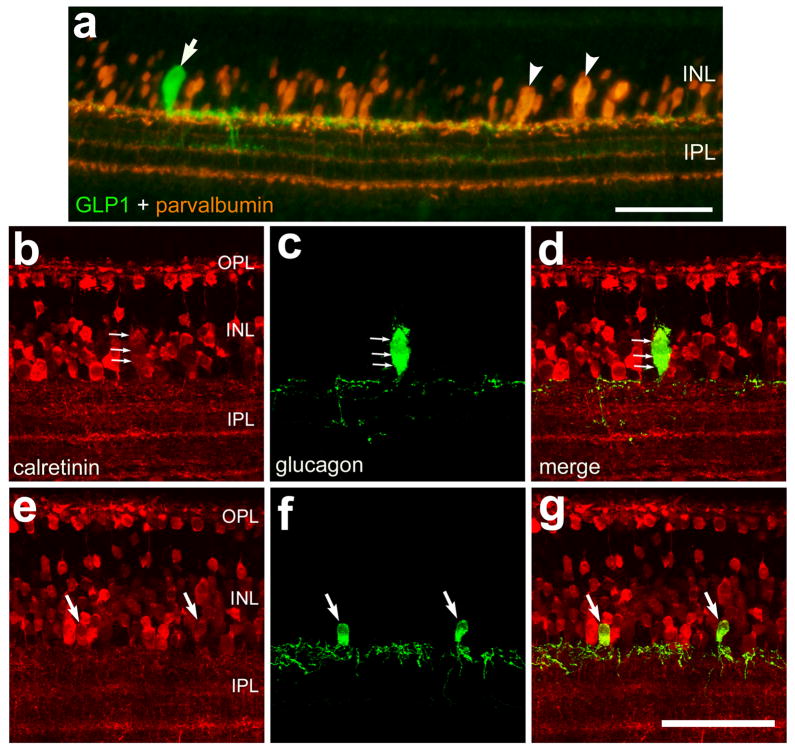
The calcium-binding proteins parvalbumin, calretinin and calbindin are known to be expressed by inner retinal neurons of the avian retina (Ellis et al., 1991; Pasteels et al., 1990; Pochet et al., 1989; Rogers et al., 1990; Rogers, 1989). We used antibodies to parvalbumin, calretinin and calbindin that have demonstrated specific labeling in the chicken retina (Fischer et al., 1999b; Fischer and Reh, 2000; Fischer et al., 1998; Fischer and Stell, 1999). Previous reports have shown that efferent target cells (ETCs), a distinct type of axon-forming neuron that is found only in ventral regions of the avian retina, expresses parvalbumin, nNOS and neurofilament (de Carvalho et al., 1996; Fischer and Stell, 1999; Morgan et al., 1994; Ramon Y Cajal, 1972; Uchiyama and Ito, 1993). Thus, we assayed whether bullwhip cells are immunoreactive for parvalbumin. We found that the bullwhip cells, unlike the ETCs, failed to label with antibodies to parvalbumin (Fig. 6a). Similarly, CGACs were not immunoreactive for parvalbumin (data not shown). To assess whether bullwhip cells and CGACs express calcium-binding proteins other than parvalbumin, we labeled retinal sections with antibodies to GLP1/glucagon and calretinin or calbindin. Calretinin and calbindin are known to be expressed by subsets of ganglion, amacrine, bipolar and horizontal cells in the avian retina (Ellis et al., 1991; Fischer et al., 1999b; Fischer et al., 1998; Fischer et al., 1999c; Rogers, 1989). Although bullwhip cells and CGACs failed to label with antibodies to calbindin (data not shown), bullwhip cells (n = 31) and CGACs (n = 91) were immunoreactive for calretinin (Figs. 6b–f). The level of immunofluorescence in bullwhip cells and CGACs was relatively low compared to levels seen in some other amacrine cells and in horizontal cells.
Figure 6.
Bullwhip cells and CGACs are immunoreactive for calretinin but not parvalbumin. Vertical sections of the retina were labeled with antibodies to GLP1 (green; a) or glucagon (green; c,d,f and g) and parvalbumin (red; a) or calretinin (red; b,d,e and g). Each image in panels b–g was generated from the projection of a Z-series of four 1μm-thick confocal optical sections. In panel a the arrow indicates a bullwhip cell that is immunoreactive for GLP1 and the arrow-heads indicate efferent target cells (ETCs). In panels b–d the small arrows indicate a bullwhip cell that is labeled for calretinin and glucagon. In panels e–g the arrows indicate CGACs that are labeled for calretinin and glucagon. The calibration bar (50 μm) in panel a applies to panel a alone and the bar in g applies to b–g. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
GABA and glycine
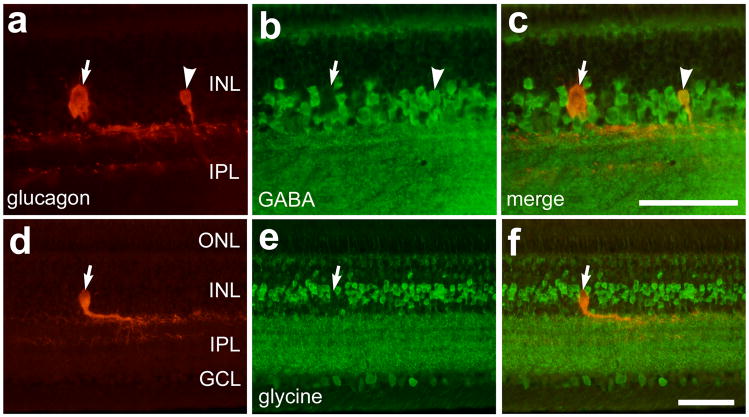
To determine whether bullwhip cells contain inhibitory neurotransmitters we applied antibodies to GABA and glycine to sections of the ventral retina. We used antibodies to GABA and glycine that have demonstrated specific labeling in the chicken retina (Fischer et al., 1999c). We found that all of the CGACs (n = 91) were strongly immunoreactive for GABA (Fig. 7a–c), while the bullwhip cells were not immunoreactive for GABA (Figs. 7a–c). By comparison, neither bullwhip cells nor CGACs were immunoreactive for glycine (Figs. 7d–f). These findings suggest a fundamental difference between bullwhip cells and amacrine cells.
Figure 7.
Bullwhip cells are not immunoreactive for GABA or glycine, and CGACs are immunoreactive for GABA but not glycine. Vertical sections of the retina were labeled with antibodies to GLP1 (red; a,c,d and f) and GABA (green; b and c) or glycine (green; e and f). Arrows indicate GLP1-positive bullwhip cells that are negative for GABA or glycine, and the arrow-heads indicate a CGAC that is labeled for GLP1 and GABA. The calibration bar (50 μm) in panel c applies to panels a–c, and the bar in f applies to d–f. Abbreviations: OPL – outer plexiform layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
Bullwhip cells are immunoreactive for receptors to neurotrophins
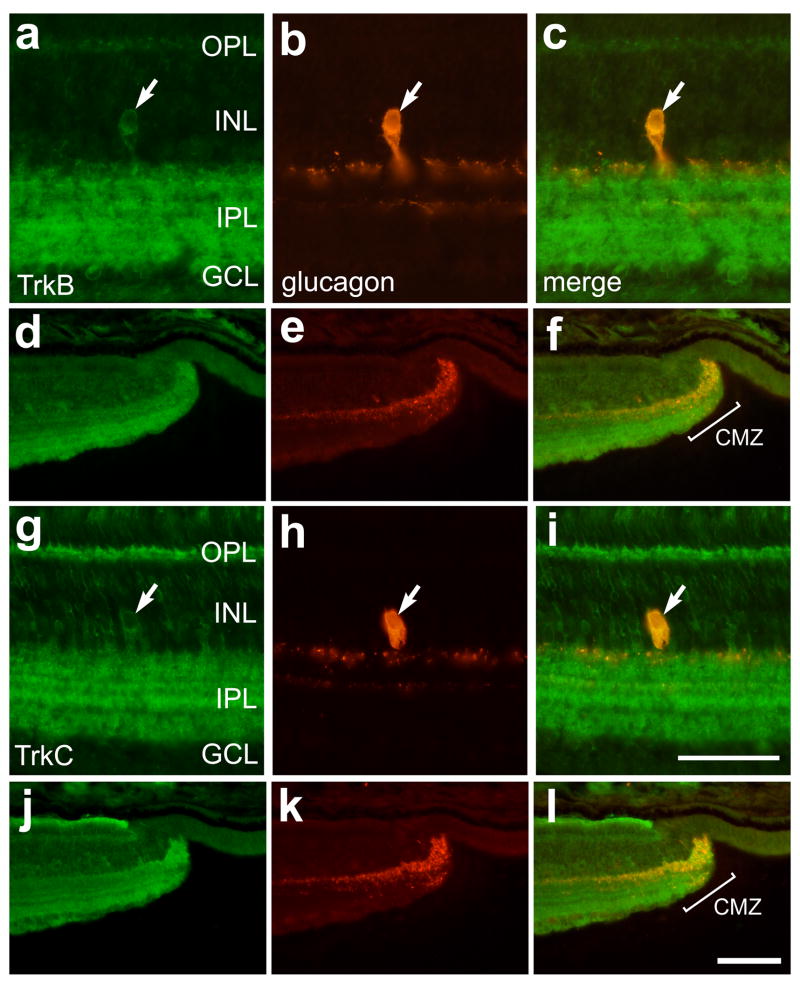
Axon-forming cells within the CNS often derive trophic support from target cells and, thus, express receptors for trophic factors. To assess whether the axon-forming bullwhip cells express receptors for neurotrophins we double-labeled retinal sections with antibodies to glucagon and to trkA, trkB and trkC. The antibodies to the trk’s have been widely used in the chicken CNS, including the retina, to identify cells that express neurotrophin receptors (Gallo et al., 1997; Karlsson et al., 1998; Lefcort et al., 1996). We found that bullwhip cells and CGACs were not immunoreactive for trkA (data not shown). By comparison, bullwhip cells were immunoreactive for trkB (Figs. 8a–c) and trkC (Figs. 8g–i) while CGACs were not (data not shown). Since bullwhip cells densely ramify terminal arbors in the retinal margin (Fischer et al., 2005), we tested whether trkB- and trkC-immunoreactivities are present in the CMZ. We found immunoreactivities for trkB and trkC at the peripheral edge of the retina, coincident with the dense clustering of glucagon-immunoreactive neurites within the CMZ (Figs. 8d–f and 8j–l).
Figure 8.
Bullwhip cells are immunoreactive for trkB and trkC. Vertical sections of the ventral retina (a–c and g–i) and CMZ (d–f and j–l) were labeled with antibodies to trkB (green; a,c,d and f) or trkC (g,i, j and l) and glucagon (red; b,e,h and k). The column of micrographs on the right are overlay images to the two panels to the left. Arrows indicate bullwhip cells that are immunolabeled for glucagon and trkB or trkC. The calibration bar (50 μm) in panel i applies to all panels a–c and g–i, and the bar in l applies to d–f and j–l. Abbreviations: OPL – outer plexiform layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
A summary of the immunolabeling in CGACs, bullwhip cells, ganglion and amacrine cells is provided in table 1.
Table 1.
Summary of immunolabeling for different markers in CGACs, bullwhip cells, ganglion cells and amacrine cells.
| CGAC | bullwhip cell | Ganglion cells | Amacrine cells* | |
|---|---|---|---|---|
| Brn3a | − | − | +++ | − |
| Islet1 | − | − | +++ | + |
| Islet2 | − | − | ++ | − |
| Pax6 | +++ | +++ | +++ | +++ |
| AP2α | +++ | +++ | − | ++ |
| Neurofilament | − | ++ | +++ | − |
| HuD | +++ | +++ | +++ | +++ |
| Calretinin | +++ | ++ | ++ | ++ |
| GABA | +++ | − | − | ++ |
| Substance P | +** | +++ | ++ | + |
| TrkB | − | ++ | ++ | + |
| TrKC | − | ++ | ++ | + |
− no labeling, + little labeling, ++ moderate labeling, +++ wide-spread labeling
non-glucagonergic amacrine cells,
only in peripheral retina
Distribution of glucagon-expressing cells in the retina
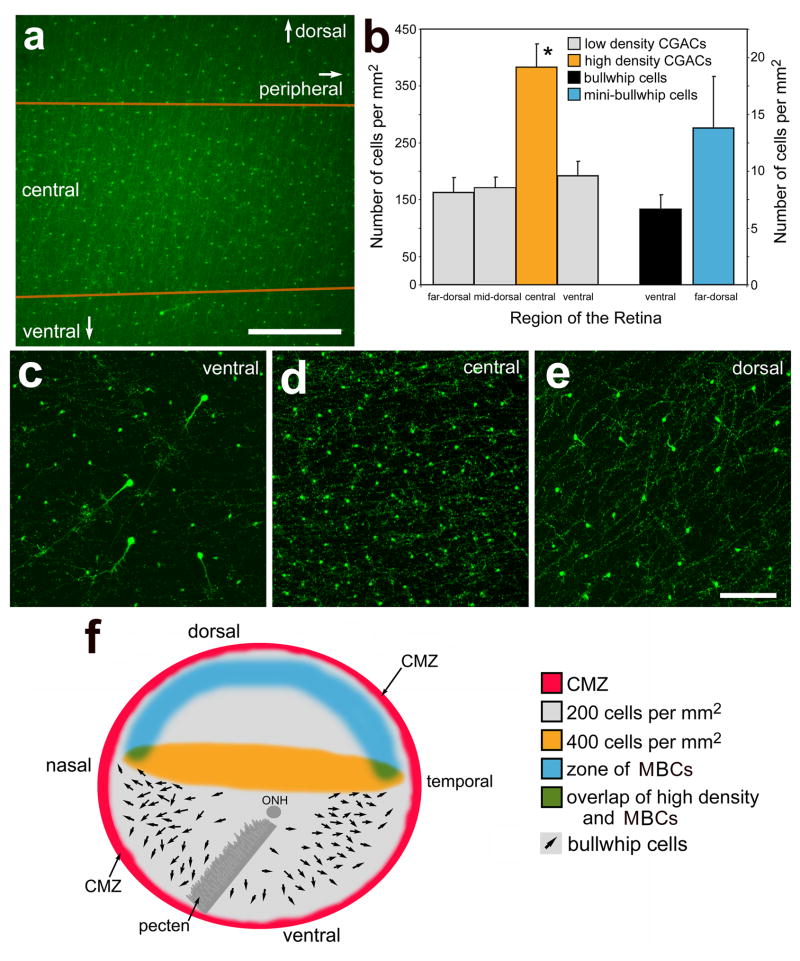
We found that the distribution of CGACs differed across the retina. The density of CGACs was highest in a region of central retina (about 700 μm wide) that spanned between the temporal and nasal edges of the retina (Figs. 9a, d and f). Consistent with a prior report (Kiyama et al., 1985), we found CGACs at nearly 400 cells per mm2 in the central retina (Figs. 9a, b and d). The area of retina that contained the high density of CGACs was elliptical and stretched between the nasal and temoral CMZ (Fig. 9f). By comparison, the CGACs are found at nearly 200 cells per mm2 in all other regions of the retina (Figs. 9b, c, e and f). The transition from areas of high- to low-density CGACs was abrupt, occurring within 200 μm (Fig. 9a). By comparison, the bullwhip cells are found in ventral and mid-peripheral regions of the retina at low-density, about 7 cells per mm2 (Figs. 9b, c and f). The mean distance between a bullwhip cell and the nearest CGACs was about 23 μm (23.1 ± 17.4 μm; n = 30), with distances ranging from 4 to 59 μm, suggesting that spacing between bullwhip cells and CGACs is not patterned in a regular manner. In some instances we found CGACs directly adjacent to bullwhip cells (see Figs. 1f–h). We found that the mini-bullwhip cells were found only in the far peripheral (within 1.0 mm of the CMZ) and dorsal regions of the retina (Figs. 9b and f). In comparison to bullwhip cells, the mini-bullwhip cells were about twice as abundant per mm2 of retina (Fig. 9b)
Figure 9.
Glucagon-expressing neurons are differentially distributed across the retina. Panel a is a low-magnification image of a central region of the retina. The area between the orange lines indicates the region of retina that contains a higher density of CGACs compared to dorsal and ventral regions of the retina. Panel b is a histogram illustrating the mean number of CGACs per 1 mm2 in ventral, central, dorsal and far dorsal regions of the retina, the number of bullwhip cells per 1 mm2 in the ventral retina, and the number of mini-bullwhip cells per 1 mm2 in the dorsal retina. The y-axis scale on the left applies to the CGACs and the scale on the right applies to the bullwhip cells and mini-bullwhip cells. Error bars represent the standard deviation of the sample mean (n = 8). Significance of difference was assessed using ANOVA (*p < 0.0005). Panels c–e are confocal micrographs of whole-mounted retinas labeled with antibodies to glucagon. Images were collected from ventral (c), central (d) and dorsal (e) regions of the retina. Each image represents the projection of a Z-series of between 12 and 15 1μm-thick confocal optical sections. The calibration bar (200 μm) in panel a applies to a alone, and the bar (50 μm) in e applies to c–e. Panels f is a schematic illustration of the distribution of the CMZ (red), low density CGACs (grey), high density CGACs (orange), mini-bullwhip cells (blue), and bullwhip cells (arrows) within the right eye of a postnatal chicken. Panel f is modified from Fischer et al. (2005) to provide a more complete topographical distribution of the different types of glucagon-expressing neurons in the chicken retina. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, CMZ – circumferential marginal zone.
Discussion
We report here that glucagon-expressing retinal neurons are a heterogeneous population of cells that are differentially distributed across the retina. We found that there may be as many as 4 different types of glucagon-immunoreactive neurons within the retina, consistent with previous reports (Fischer et al., 2005; Karten and Brecha, 1983; Kiyama et al., 1985; Kuwayama et al., 1982). The different types of glucagon-expressing neurons differ with respect to morphology, distribution within the retina, types of proteins expressed, and the transmitters that they accumulate. The different types of glucagon-positive cells include the conventional amacrine cells that are immunoreactive for glucagon alone, amacrine cells that are immunoreactive for glucagon and substance P, bullwhip cells and mini-bullwhip cells.
The bullwhip cells do not express some of the transcription factors that are expressed by ganglion cells. These transcription factors include Brn3a, Islet1 and Islet2 (Table 1). These findings suggest that the phenotypic characteristics of bullwhip cells that are similar to those of ganglion cells (i.e. large somata, axons, and neurofilament protein) do not require the transcriptional activity of Brn3a, Islet1 or Islet2. By comparison, the bullwhip cells express AP2α, a transcription factor that is expressed by amacrine cells, and Pax6, a transcription factor that is expressed by amacrine, ganglion and horizontal cells (Belecky-Adams et al., 1997; Fischer and Reh, 2000). Taken together, these findings imply that the unique characteristics of bullwhip cells are likely to be bestowed by transcription factors in addition to Pax6 and AP2α.
The bullwhip and mini-bullwhip cells are distinctly different from CGACs. These differences are apparent not only from the different morphologies, but also from distinct histological profiles (see Table 1). It is unlikely that bullwhip cells are an extreme variation of the CGACs. If this were the case, then one might expect these cells to coordinate their patterning within the retina. The bullwhip cells are distributed in a quasi-regular pattern, with a minimum distance between cells of about 70 μm (Fischer et al., 2005). The CGACs do not adhere to this minimum spacing from bullwhip cells. CGACs are occasionally found within 5 μm of bullwhip cells, but never within 30 μm of each other (see Figs. 1g and 9c). This observation suggests that signals that pattern the spacing of bullwhip cells and CGACs are not shared between these cell types, consistent with the hypothesis that these cell types are not homotypic.
It is possible that the mini-bullwhip cells are a variation of the bullwhip cells and differ merely by subtle changes in morphology, somata size and distribution within the retina. Similarly, it is possible that the CGACs that are scattered across all regions of the retina can be classified a singular type of amacrine cell. Although many of the CGACs that are found in far peripheral regions of the retina express substance P and all CGACs in central regions of the retina do not, these cells appear to have similar morphologies. Further studies are required to assay whether bullwhip and mini-bullwhip cells, as well as substance P-positive CGACs and substance P-negative CGACs, can be distinguished from one another by histological markers or physiological differences.
We have reported elsewhere that glucagon, GLP1 and glucagon antagonist regulate the proliferation and number of progenitors in the CMZ (Fischer et al., 2005). We found that the neurites that are densely clustered within the CMZ are immunoreactive for glucagon, GLP1 and substance P, consistent with prior reports (Brecha, 1983; Fischer et al., 2005; Karten and Brecha, 1983; Katayama-Kumoi et al., 1985; Kiyama et al., 1985). However, it remains unknown whether substance P influences the neural progenitors in the CMZ. It is possible that the neurites immunoreactive for glucagon and substance P that are clustered within the CMZ arise from CGACs in far peripheral regions of the retina in addition to bullwhip and mini-bullwhip cells. Consistent with a prior report (Katayama-Kumoi et al., 1985), we found that the incidence of amacrine cells that are immunoreactive for both glucagon and substance P increases with increasing proximity to the CMZ.
The bullwhip cells share some properties with another unique type of neuron in the avian retina, the efferent target cell (ETC). Like the bullwhip cells, the ETCs of the chick retina are found only in ventral regions, have large somata located among the amacrine cells of the INL, form axons that remain within the retina, and express neurofilament (Fischer and Stell, 1999). However, unlike the ETCs, the bullwhip cells do not express parvalbumin (see Fig. 6). Unlike the bullwhip cells, the ETCs do not express glucagon or GLP1. It has been argued that ETCs cannot be grouped into any of the five major categories of retinal neurons (Uchiyama et al., 2004). Similarly, the bullwhip cells cannot be easily assigned to any of the five major types of retinal neurons. The bullwhip cells share cell-distinguishing phenotypes with both amacrine and ganglion cells, including morphological features and the expression of a number of proteins (see Table 1). Although bullwhip cells may share many similarities with amacrine cells, surveys of the different types of amacrine cells in the mammalian retina have not identified large, unipolar, axon-forming cells (MacNeil et al., 1999). In addition, the bullwhip cells are not immunoreactive for glycine or GABA, unlike other types of amacrine cells. This finding suggests that the bullwhip cells are not an unusual type of amacrine cell. Thus, bullwhip cells and ETCs do not fall into the conventional categories of retinal cell-types and might be grouped together as “unconventional” retinal cells.
We found that bullwhip cells were immunoreactive trkB and trkC, but not trkA. In addition, we identified immunoreactivity for trkB and trkC in the CMZ, where glucagon-immunoreactive neurites are heavily clustered. Many of the glucagon-positive neurites in the CMZ arise from bullwhip cells (Fischer et al., 2005), consistent with the hypothesis that bullwhip cells derive trophic support from cells within the CMZ. The preferred ligand of the trkB receptor is brain derived neurotrophic factor (BDNF) and the preferred ligand of the trkC receptor is neurotrophin-3 (NT-3; reviewed by (Lee et al., 2002). These observations suggest that bullwhip cells may require trophic support in the form of target-derived BDNF and/or NT-3. Alternatively, bullwhip cells could respond to trophic factors derived from sources near their somata, within ventral and mid-peripheral regions of the retina. For example, BDNF and NT-3 are generated within the neural retina (Hallbook et al., 1996) and could activate receptors on the somata of bullwhip cells. It is possible that, like ganglion cells, the bullwhip cells are over-produced during development and that only those cells that form appropriate connections and derive sufficient trophic support survive in the postnatal retina. The mean number of bullwhip cells remains constant, at about 240 cells per retina, and does not decrease during postnatal development (Fischer et al., 2005). We propose that bullwhip cells may compete for trophic support from retinal progenitors in the CMZ of the embryonic retina. However, it remains unknown whether cells within the CMZ produce neurotrophins.
We found that CGACs were concentrated in an elliptical region of central retina that spanned between the nasal and temporal CMZ (see Fig. 9). Similarly, Kiyama et al. (1985) reported that glucagon-immunoreactive amacrine cells are found at higher density in a circular region of the central retina. It is possible that the high density of CGACs in the central retina is positioned to influence the growth of the sclera. For example, CGACs have been implicated in the regulation of postnatal ocular growth (Bitzer and Schaeffel, 2002; Feldkaemper and Schaeffel, 2002; Fischer et al., 1999a), ocular growth is guided by retina-derived signals to the sclera (Wallman and Winawer, 2004), and scleral growth occurs from the addition of proteoglycans to the posterior pole of the eye (Rada et al., 1994), adjacent to central retina and the high density of CGACs. In addition, the original report that CGACs respond to the sign of defocus made observations only on retinal sections obtained from the central retina cut in the nasal-temporal plane (Fischer et al., 1999). It remains uncertain whether CGACs outside of the central retinal zone respond to visual cues that regulate ocular growth. It is clear, however, that equatorial expansion of the eye is inhibited by intravitreal injections of glucagon (Beloukhina et al., 2005), suggesting that the release of glucagon from peripheral regions of the retina may regulate the equatorial circumference of the eye. We conclude that the CGACs are found at high density across the central retina in the nasal-temporal plane and propose that these cells are well-positioned to regulate vision-guided growth of the sclera.
Conclusions
We conclude that there may be at least 4 different types of glucagon-expressing neurons in the avian retina. These types of cells include CGACs negative for substance P in the central retina, CGACs positive for substance P in the far peripheral retina, bullwhip cells in the ventral retina, and mini-bullwhip cells in the dorsal retina. Bullwhip cells are an unconventional type of retinal neuron that expresses a number of proteins that are shared by ganglion and amacrine cells. These proteins include neurofilament, HuD, Pax6, AP2α, calretinin, trkB and trkC. By comparison, the CGACs are immunoreactive for HuD, Pax6, AP2α, calretinin and GABA. The CGACs are asymmetrically distributed across the retina, with higher densities residing in a central zone that stretched between the nasal and temporal edges of the retina. By comparison, the bullwhip cells are found only in ventral and mid-peripheral regions of the retina and the mini-bullwhip cells are found only in dorsal and far-peripheral regions of the retina.
Acknowledgments
We thank Drs. W.K. Stell and G. Bishop for comments that contributed to the final form of this paper. We thank Drs. F. Lefcort and Virginia Lee for providing antibodies to the trk receptors and neurofilament, respectively. Expert technical assistance was provided by Ms. G. Omar and by Dr. R. Burry at the Campus Microscopy and Imaging Facility at The Ohio State University. The Islet1, Islet2, AP2α and Pax6 antibodies developed by Drs T. Jessell, T. Williams and A. Kawakami, respectively, were obtained from the Developmental Studies Hybridoma Bank developed under auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City. This work was supported by start-up funds provided by the Ohio State University and a grant (#0413795) from the National Science Foundation.
Grant Support: National Science Foundation grant #0413795
References
- Belecky-Adams T, Tomarev S, Li HS, Ploder L, McInnes RR, Sundin O, Adler R. Pax-6, Prox 1, and Chx10 homeobox gene expression correlates with phenotypic fate of retinal precursor cells. Invest Ophthalmol Vis Sci. 1997;38(7):1293–1303. [PubMed] [Google Scholar]
- Beloukhina N, Vessey KA, Stell WK. Glucagon prevents myopia via distal retina or RPE. Invest Ophthalmol Vis Sci. 2005;46:3337. [Google Scholar]
- Bisgrove DA, Godbout R. Differential expression of AP-2alpha and AP-2beta in the developing chick retina: repression of R-FABP promoter activity by AP-2. Dev Dyn. 1999;214(3):195–206. doi: 10.1002/(SICI)1097-0177(199903)214:3<195::AID-AJA3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Bitzer M, Schaeffel F. Defocus-induced changes in ZENK expression in the chicken retina. Invest Ophthalmol Vis Sci. 2002;43(1):246–252. [PubMed] [Google Scholar]
- Brecha N. A review of retinal neurotransmitters: Histochemical and biochemical studies. In: Emson PC, editor. Neurochemical Anatomy. New York: Raven; 1983. [Google Scholar]
- de Carvalho RP, de Faria MH, do Nascimento JL, Hokoc JN. Development of NADPH-diaphorase in the avian retina: regulation by calcium ions and relation to nitric oxide synthase. J Neurochem. 1996;67(3):1063–1071. doi: 10.1046/j.1471-4159.1996.67031063.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich D, Keyser KT, Karten HJ. Distribution of substance P-like immunoreactive retinal ganglion cells and their pattern of termination in the optic tectum of chick (Gallus gallus) J Comp Neurol. 1987;266(2):220–233. doi: 10.1002/cne.902660208. [DOI] [PubMed] [Google Scholar]
- Ekman R, Tornqvist K. Glucagon and VIP in the retina. Invest Ophthalmol Vis Sci. 1985;26(10):1405–1409. [PubMed] [Google Scholar]
- Ellis JH, Richards DE, Rogers JH. Calretinin and calbindin in the retina of the developing chick. Cell Tissue Res. 1991;264(2):197–208. doi: 10.1007/BF00313956. [DOI] [PubMed] [Google Scholar]
- Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis Neurosci. 2002;19(6):755–766. doi: 10.1017/s0952523802196064. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002;129(9):2283–2291. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, McGuire JJ, Schaeffel F, Stell WK. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci. 1999a;2(8):706–712. doi: 10.1038/11167. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Morgan IG, Stell WK. Colchicine causes excessive ocular growth and myopia in chicks. Vision Res. 1999b;39(4):685–697. doi: 10.1016/s0042-6989(98)00178-3. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Omar G, Walton NA, Verrill TA, Unson CG. Glucagon-expressing neurons within the retina regulate the proliferation of neural progenitors in the circumferential marginal zone of the avian eye. J Neurosci. 2005;25(44):10157–10166. doi: 10.1523/JNEUROSCI.3247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220(2):197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4(3):247–252. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Exogenous growth factors stimulate the regeneration of ganglion cells in the chicken retina. Dev Biol. 2002;251(2):367–379. doi: 10.1006/dbio.2002.0813. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reisch HM, Kyle AL, Stell WK. Characterization of the RFamide-like neuropeptides in the nervus terminalis of the goldfish (Carassius auratus) Regul Pept. 1996;62(2–3):73–87. doi: 10.1016/0167-0115(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Seltner RL, Poon J, Stell WK. Immunocytochemical characterization of quisqualic acid- and N-methyl-D-aspartate-induced excitotoxicity in the retina of chicks. J Comp Neurol. 1998;393(1):1–15. [PubMed] [Google Scholar]
- Fischer AJ, Stell WK. Nitric oxide synthase-containing cells in the retina, pigmented epithelium, choroid, and sclera of the chick eye. J Comp Neurol. 1999;405(1):1–14. doi: 10.1002/(sici)1096-9861(19990301)405:1<1::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Wallman J, Mertz JR, Stell WK. Localization of retinoid binding proteins, retinoid receptors, and retinaldehyde dehydrogenase in the chick eye. J Neurocytol. 1999c;28(7):597–609. doi: 10.1023/a:1007071406746. [DOI] [PubMed] [Google Scholar]
- Gallo G, Lefcort FB, Letourneau PC. The trkA receptor mediates growth cone turning toward a localized source of nerve growth factor. J Neurosci. 1997;17(14):5445–5454. doi: 10.1523/JNEUROSCI.17-14-05445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Xiang M, Zhou L, Wagner DS, Klein WH, Nathans J. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci U S A. 1996;93(9):3920–3925. doi: 10.1073/pnas.93.9.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor M, Riecken EO. Production and characterization of N-terminally and C-terminally directed monoclonal antibodies against pancreatic glucagon. Gastroenterology. 1985;89(3):571–580. doi: 10.1016/0016-5085(85)90453-6. [DOI] [PubMed] [Google Scholar]
- Hallbook F, Backstrom A, Kullander K, Ebendal T, Carri NG. Expression of neurotrophins and trk receptors in the avian retina. J Comp Neurol. 1996;364(4):664–676. doi: 10.1002/(SICI)1096-9861(19960122)364:4<664::AID-CNE5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Irwin DM, Wong J. Trout and chicken proglucagon: alternative splicing generates mRNA transcripts encoding glucagon-like peptide 2. Mol Endocrinol. 1995;9(3):267–277. doi: 10.1210/mend.9.3.7776976. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Clary DO, Lefcort FB, Reichardt LF, Karten HJ, Hallbook F. Nerve growth factor receptor TrkA is expressed by horizontal and amacrine cells during chicken retinal development. J Comp Neurol. 1998;400(3):408–416. [PMC free article] [PubMed] [Google Scholar]
- Karten HJ, Brecha N. Localization of neuroactive substances in the vertebrate retina: evidence for lamination in the inner plexiform layer. Vision Res. 1983;23(10):1197–1205. doi: 10.1016/0042-6989(83)90033-0. [DOI] [PubMed] [Google Scholar]
- Katayama-Kumoi Y, Kiyama H, Manabe R, Shiotani Y, Tohyama M. Co-existence of glucagon- and substance P-like immunoreactivity in the chicken retina. Neuroscience. 1985;16(2):417–424. doi: 10.1016/0306-4522(85)90013-2. [DOI] [PubMed] [Google Scholar]
- Kiyama H, Katayama-Kumoi Y, Kimmel J, Steinbusch H, Powell JF, Smith AD, Tohyama M. Three dimensional analysis of retinal neuropeptides and amine in the chick. Brain Res Bull. 1985;15(2):155–165. doi: 10.1016/0361-9230(85)90132-7. [DOI] [PubMed] [Google Scholar]
- Kuwayama Y, Ishimoto I, Fukuda M, Shimiza Y, Shiosaka S, Inagaki S, Senba E, Sakanaka M, Takagi H, Takatsuki K, Hara Y, Kawai Y, Tohyama M. Overall distribution of glucagon-like immunoreactivity in the chicken retina: an immunohistochemical study with flat-mounts. Invest Ophthalmol Vis Sci. 1982;22(5):681–686. [PubMed] [Google Scholar]
- Lee FS, Rajagopal R, Chao MV. Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev. 2002;13(1):11–17. doi: 10.1016/s1359-6101(01)00024-7. [DOI] [PubMed] [Google Scholar]
- Lefcort F, Clary DO, Rusoff AC, Reichardt LF. Inhibition of the NT-3 receptor TrkC, early in chick embryogenesis, results in severe reductions in multiple neuronal subpopulations in the dorsal root ganglia. J Neurosci. 1996;16(11):3704–3713. doi: 10.1523/JNEUROSCI.16-11-03704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YZ, Dawson SJ, Latchman DS. Alternative splicing of the Brn-3a and Brn-3b transcription factor RNAs is regulated in neuronal cells. J Mol Neurosci. 1996;7(1):77–85. doi: 10.1007/BF02736850. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413(2):305–326. [PubMed] [Google Scholar]
- Marquardt T. Transcriptional control of neuronal diversification in the retina. Prog Retin Eye Res. 2003;22(5):567–577. doi: 10.1016/s1350-9462(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol. 2001;11(4):431–436. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- Masland RH. Neuronal cell types. Curr Biol. 2004;14(13):R497–500. doi: 10.1016/j.cub.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Morgan IG, Miethke P, Li ZK. Is nitric oxide a transmitter of the centrifugal projection to the avian retina? Neurosci Lett. 1994;168(1–2):5–7. doi: 10.1016/0304-3940(94)90402-2. [DOI] [PubMed] [Google Scholar]
- Pak W, Hindges R, Lim YS, Pfaff SL, O’Leary DD. Magnitude of binocular vision controlled by islet-2 repression of a genetic program that specifies laterality of retinal axon pathfinding. Cell. 2004;119(4):567–578. doi: 10.1016/j.cell.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Pasteels B, Rogers J, Blachier F, Pochet R. Calbindin and calretinin localization in retina from different species. Vis Neurosci. 1990;5(1):1–16. doi: 10.1017/s0952523800000031. [DOI] [PubMed] [Google Scholar]
- Perrone-Bizzozero N, Bolognani F. Role of HuD and other RNA-binding proteins in neural development and plasticity. J Neurosci Res. 2002;68(2):121–126. doi: 10.1002/jnr.10175. [DOI] [PubMed] [Google Scholar]
- Pochet R, Blachier F, Malaisse W, Parmentier M, Pasteels B, Pohl V, Resibois A, Rogers J, Roman A. Calbindin-D28 in mammalian brain, retina, and endocrine pancreas: immunohistochemical comparison with calretinin. Adv Exp Med Biol. 1989;255:435–443. doi: 10.1007/978-1-4684-5679-0_46. [DOI] [PubMed] [Google Scholar]
- Rada JA, Matthews AL, Brenza H. Regional proteoglycan synthesis in the sclera of experimentally myopic chicks. Exp Eye Res. 1994;59(6):747–760. doi: 10.1006/exer.1994.1161. [DOI] [PubMed] [Google Scholar]
- Ramon Y, Cajal S. In: The structure of the retina. Thorpe SA, Glickstein M, translators. Springfield, Illinois: Charles C. Thomas; 1972. pp. 76–92. [Google Scholar]
- Rogers J, Khan M, Ellis J. Calretinin and other CaBPs in the nervous system. Adv Exp Med Biol. 1990;269:195–203. doi: 10.1007/978-1-4684-5754-4_32. [DOI] [PubMed] [Google Scholar]
- Rogers JH. Two calcium-binding proteins mark many chick sensory neurons. Neuroscience. 1989;31(3):697–709. doi: 10.1016/0306-4522(89)90434-x. [DOI] [PubMed] [Google Scholar]
- Smith CL, Afroz R, Bassell GJ, Furneaux HM, Perrone-Bizzozero NI, Burry RW. GAP-43 mRNA in growth cones is associated with HuD and ribosomes. J Neurobiol. 2004;61(2):222–235. doi: 10.1002/neu.20038. [DOI] [PubMed] [Google Scholar]
- Tornqvist K, Ehinger B. Glucagon immunoreactive neurons in the retina of different species. Graefes Arch Clin Exp Ophthalmol. 1983;220(1):1–5. doi: 10.1007/BF02307008. [DOI] [PubMed] [Google Scholar]
- Tornqvist K, Loren I, Hakanson R, Sundler F. Peptide-containing neurons in the chicken retina. Exp Eye Res. 1981;33(1):55–64. doi: 10.1016/s0014-4835(81)80081-4. [DOI] [PubMed] [Google Scholar]
- Uchiyama H, Aoki K, Yonezawa S, Arimura F, Ohno H. Retinal target cells of the centrifugal projection from the isthmo-optic nucleus. J Comp Neurol. 2004;476(2):146–153. doi: 10.1002/cne.20225. [DOI] [PubMed] [Google Scholar]
- Uchiyama H, Ito H. Target cells for the isthmo-optic fibers in the retina of the Japanese quail. Neurosci Lett. 1993;154(1–2):35–38. doi: 10.1016/0304-3940(93)90165-h. [DOI] [PubMed] [Google Scholar]
- Vessey KA, Lencses KA, Rushforth DA, Hruby VJ, Stell WK. Glucagon receptor agonists and antagonists affect the growth of the chick eye: a role for glucagonergic regulation of emmetropization? Invest Ophthalmol Vis Sci. 2005a;46(11):3922–3931. doi: 10.1167/iovs.04-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey KA, Rushforth DA, Stell WK. Glucagon- and secretin-related peptides differentially alter ocular growth and the development of form-deprivation myopia in chicks. Invest Ophthalmol Vis Sci. 2005b;46(11):3932–3942. doi: 10.1167/iovs.04-1027. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Zhang J, Nottoli T, Hagopian-Donaldson S, Libby D, Strissel KJ, Williams T. AP-2alpha transcription factor is required for early morphogenesis of the lens vesicle. Dev Biol. 1999;206(1):46–62. doi: 10.1006/dbio.1998.9132. [DOI] [PubMed] [Google Scholar]
- Xiang M, Zhou L, Peng YW, Eddy RL, Shows TB, Nathans J. Brn-3b: a POU domain gene expressed in a subset of retinal ganglion cells. Neuron. 1993;11(4):689–701. doi: 10.1016/0896-6273(93)90079-7. [DOI] [PubMed] [Google Scholar]