Abstract
This study evaluated a model of skin permeation to determine the depth of delivery of chlorhexidine into full-thickness excised human skin following topical application of 2% (wt/vol) aqueous chlorhexidine digluconate. Skin permeation studies were performed on full-thickness human skin using Franz diffusion cells with exposure to chlorhexidine for 2 min, 30 min, and 24 h. The concentration of chlorhexidine extracted from skin sections was determined to a depth of 1,500 μm following serial sectioning of the skin using a microtome and analysis by high-performance liquid chromatography. Poor penetration of chlorhexidine into skin following 2-min and 30-min exposures to chlorhexidine was observed (0.157 ± 0.047 and 0.077 ± 0.015 μg/mg tissue within the top 100 μm), and levels of chlorhexidine were minimal at deeper skin depths (less than 0.002 μg/mg tissue below 300 μm). After 24 h of exposure, there was more chlorhexidine within the upper 100-μm sections (7.88 ± 1.37 μg/mg tissue); however, the levels remained low (less than 1 μg/mg tissue) at depths below 300 μm. There was no detectable penetration through the full-thickness skin. The model presented in this study can be used to assess the permeation of antiseptic agents through various layers of skin in vitro. Aqueous chlorhexidine demonstrated poor permeation into the deeper layers of the skin, which may restrict the efficacy of skin antisepsis with this agent. This study lays the foundation for further research in adopting alternative strategies for enhanced skin antisepsis in clinical practice.
Effective skin antisepsis is essential in preventing infections associated with invasive procedures, such as intravascular catheter insertion or surgery. A range of skin antiseptic agents are available in the clinical setting, such as povidone-iodine and chlorhexidine compounds at various concentrations with alcoholic or aqueous solutions. However, a 2% (wt/vol) chlorhexidine solution is the recommended agent to be used prior to invasive procedures according to the EPIC (evidence-based practice in infection control) and CDC guidelines (18, 19). Two percent chlorhexidine digluconate (CHG) has been shown to significantly reduce intravascular catheter-related infections (14), yet 2% (wt/vol) CHG in 70% (vol/vol) isopropyl alcohol demonstrates activity superior to that of aqueous CHG solution in a preoperative skin preparation (9) and in vitro carrier tests (1). However, little is known about the kinetics of chlorhexidine skin permeation from either of these solutions (11, 25). Microorganisms colonizing the skin not only reside on the skin surface but are also found to inhabit hair follicles and lower skin depths (8). Many antimicrobial agents exhibit restricted permeation of the skin (8) and fail to reach the deeper layers, including the hair follicles, which harbor coagulase-negative staphylococci (2, 7, 8, 13, 15) and propionibacteria (13). Commensal microorganisms may therefore persist at the site of incision following skin antisepsis (4, 22), and such resident organisms may cause infection when the protective skin barrier is breached during surgical procedures (12, 20, 26). Therefore, effective and rapid permeation of the applied antiseptic agent into the deeper layers of the skin is essential in preventing infections associated with invasive procedures.
The aim of the current study was to use the Franz-cell skin model (6) to determine the penetration profile for CHG through excised human skin and to evaluate the skin permeation of 2% (wt/vol) aqueous CHG into the skin using this model.
MATERIALS AND METHODS
Materials.
CHG, diethylamine (high-performance-liquid-chromatography [HPLC] grade), dimethyl sulfoxide, phosphate-buffered saline (PBS), sodium heptane sulfonate (HPLC grade), and Tween 80 were purchased from Sigma-Aldrich (Dorset, United Kingdom). Acetic acid and methanol (both HPLC grade) were purchased from Fisher Scientific (Leicestershire, United Kingdom).
Skin samples.
Full-thickness human skin samples were obtained from patients undergoing breast reduction surgery, and full ethical committee approval was obtained prior to this study (REC 2002/169). The full-thickness human skin was frozen on the day of excision and stored at −70°C until required.
Quantification of CHG.
HPLC was used to measure the amounts of CHG in the skin samples obtained during the permeation studies. The analyses were performed using an Agilent 1200 series HPLC system (Agilent Technologies, United Kingdom). The samples were run at a flow rate of 1.2 ml/min at room temperature through a reverse-phase chromatography column (CPS-2 Hypersil 5-μm column; dimension, 150 by 4.6 mm [Thermo Electron Corporation, United Kingdom]), with UV detection at 254 nm. The isocratic mobile phase consisted of a methanol:water mixture (75:25) with 0.005 M sodium heptane sulfonate and 0.1% (vol/vol) diethylamine adjusted to pH 4 with glacial acetic acid. The HPLC method was validated by repeating a series of standardized CHG concentrations five times and plotting a graph of peak area versus CHG concentration. The level of detection (LOD) and level of quantification (LOQ) were calculated from the standard curve according to the following equations: LOD = (3 × standard deviation)/slope; LOQ = (10 × standard deviation)/slope.
Skin permeation studies.
Skin permeation studies were performed with vertical Franz diffusion cells (Fig. 1). The receptor compartment was filled with 29 ml of PBS, maintained at 37°C by using a circulating water jacket, and agitated by stirring with a magnetic bar. Skin samples were thawed in PBS at room temperature, dried with an absorbent towel, and mounted on Franz diffusion cells with the stratum corneum (SC) uppermost, facing the donor compartment. The surface area exposed to the test compound was 3.14 cm2 (2 cm in diameter). All entrapped air between the skin and receptor fluid was removed, and the skin was left to equilibrate for 30 min to reach the skin surface temperature of 32°C.
FIG. 1.
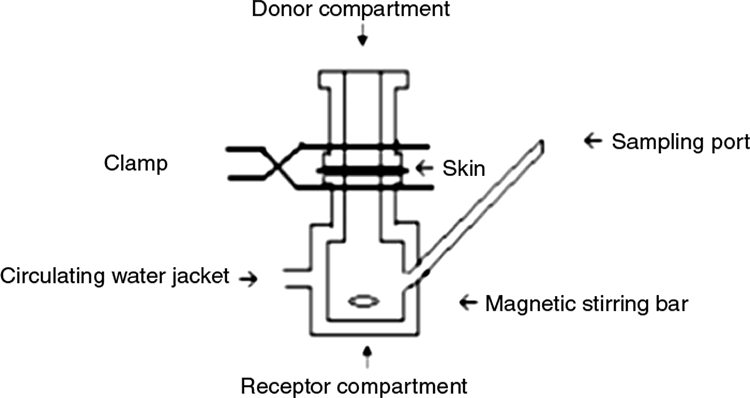
Diagram of Franz diffusion cell. The receptor compartment was filled with PBS, which was kept at 37°C by circulating water jacket. The skin was mounted between the receptor and donor compartments and clamped. The test drug was aliquoted into the donor compartment. The drug diffused through the skin was sampled by removing receptor fluid via the sampling port.
Twenty percent (wt/vol) aqueous CHG was diluted with distilled water and 0.1% (vol/vol) Tween 80 to obtain the final test solution of 2% (wt/vol) CHG. One milliliter of test solution was spread over the skin surface in the donor compartment, and the compartment was sealed with a moisture-resistant film (Parafilm M, Alcan packaging) to prevent evaporation. One milliliter of receptor fluid was removed every 30 min for 2 h, every hour between 2 to 6 h, and at 8 h, 12 h, and 24 h. Fluid removed from the receptor compartment was immediately replaced with an equal volume of fresh PBS solution. All samples were filtered through a 0.45-μm nylon filter (Kinesis, United Kingdom) and analyzed by HPLC. The assay was performed in triplicate and on two different donor skin samples.
CHG penetration profile studies.
Excised full-thickness human skin samples were mounted on the Franz diffusion cells as described above and exposed to 2% (wt/vol) CHG for 2 min, 30 min, and 24 h. Following exposure, the skin samples were removed, washed with PBS, and dried with an absorbent towel. The skin samples were immediately sprayed with a cryospray (Bright Instruments) and frozen at −20°C. Punch biopsy samples (7 mm in diameter) were cut from each frozen sample in triplicate and placed on a cork disc in embedding compound (Bright Instruments, Cambs, United Kingdom). The frozen samples were sectioned horizontally with a microtome (Bright Instruments) into 20-μm sections (from the surface to a depth of 600 μm) and 30-μm sections (from depths of 600 to 1,500 μm). Each section was placed in an Eppendorf tube and the total weight of each skin sample determined. Chlorhexidine was extracted from the skin by placing 1 ml of HPLC mobile-phase solution in each tube, followed by incubation of the sealed tubes at 60°C for 1 h. Following this, the samples were analyzed by HPLC and the concentration of CHG (μg/mg of skin) determined. Control skin (skin without treatment) was analyzed parallel to the test samples. Effective elution and recovery of CHG from the skin by this method were confirmed prior to the experiment by injecting a standardized quantity of CHG (128 μg) into 10 skin samples, extracting the CHG, and determining the recovered amount (94.4 ± 1.82%; data not shown).
RESULTS
HPLC validation.
The mean retention time for CHG was 3.6 min. There were no intervening peaks from endogenous contaminating compounds within skin samples. The HPLC method gave a linear response (r2 = 0.999) over the concentration range of 0.0039 μg/ml to 128 μg/ml. The level of detection and level of quantification were calculated at 0.016 μg/ml and 0.052 μg/ml, respectively.
Skin permeation studies.
No CHG was detected in the receptor compartment during the 24-h exposure of excised full-thickness human skin to 2% (wt/vol) aqueous CHG.
CHG retention studies.
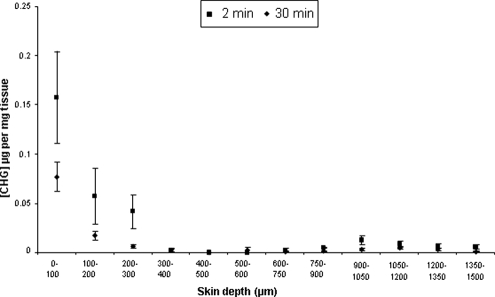
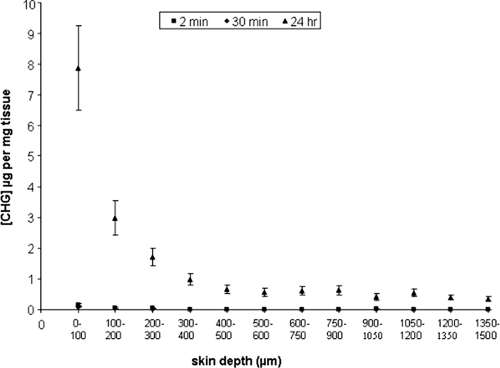
After 2 min, 30 min, and 24 h, concentrations of chlorhexidine within the skin were highest in the surface 100-μm sections and reduced below depths of 300 μm (Fig. 2 and 3). The concentrations of CHG within the top 100-μm sections of skin were 0.157 (± 0.047) μg/mg tissue and 0.077 (± 0.015) μg/mg tissue after 2-min and 30-min exposures to 2% (wt/vol) CHG, respectively (Fig. 2). The concentration of CHG within deeper layers (below 300 μm) fell to less than 0.002 μg/mg tissue following both 2-min and 30-min exposures. The difference between the amounts of chlorhexidine within the top layers between 2 min and 30 min of exposure was not significant (P > 0.05) (Student's t test, INSTAT2; Graphpad, San Diego, CA). The concentration of CHG was significantly higher within all skin sections following 24 h of exposure to CHG than with the shorter exposure times. The concentration of CHG was 7.88 (± 1.37) μg/mg tissue within the upper 100-μm sections and less than 1 μg/mg of tissue at depths of 300 μm and below.
FIG. 2.
Penetration profile showing the concentration and location of chlorhexidine (μg/mg tissue) in excised human skin after 2 min or 30 min of exposure to aqueous 2% (wt/vol) chlorhexidine digluconate (mean ± standard error; n = 15).
FIG. 3.
Penetration profile showing the concentration and location of chlorhexidine (μg/mg tissue) in excised human skin after 2 min or 30 min (n = 15) or 24 h (n = 30) of exposure to aqueous 2% (wt/vol) CHG (mean ± standard error).
DISCUSSION
This study demonstrates that 2% (wt/vol) chlorhexidine, the antiseptic agent recommended within EPIC and CDC guidelines for skin antisepsis prior to central venous catheter insertion, poorly permeates into deeper layers of the skin after 2 min and 30 min of exposure to the antiseptic. The concentrations of CHG within the upper 100-μm sections of skin were 0.157 (± 0.047) μg/mg tissue and 0.077 (± 0.015) μg/mg tissue after 2 min and 30 min, respectively. If 1 g of tissue is estimated to equal 1 ml, these levels are higher than the concentrations required to kill many common skin microorganisms, such as Staphylococcus epidermidis, under in vitro conditions (10). Below 300 μm, the CHG concentration remained less than 0.002 μg/mg tissue, which may not be effective for eradicating microorganisms on the skin (17), especially microorganisms residing deep in the hair follicles. Furthermore, chlorhexidine activity is reduced in the presence of organic compounds, such as fatty acids, and at lower pHs (16) and therefore may reduce the efficacy of skin antisepsis with CHG. An exposure time of 2 min was used to reflect the clinical conditions used prior to surgery (5). Although the 2-min study appears to show a larger amount of bound chlorhexidine than the 30-min study, there is variability in concentrations measured in the top layers, as is expected with the shorter exposure period (24), and the difference between 2 min and 30 min of exposure is not significant (P > 0.05). It is likely that a steady state has not yet been reached at 2 min. A similar phenomenon was reported by Wagner et al. (23). Skin was also exposed to 2% (wt/vol) CHG for 24 h, and the concentration of CHG in the deeper sections, i.e., beyond 300 μm, was less than 1 μg/mg tissue. These levels of CHG are more than the minimum bactericidal concentrations for many skin commensals (10); however, this level of CHG was obtained only after a prolonged time of contact of the skin with CHG. In this study, no detectable levels of CHG were recovered from the receptor compartment, suggesting that aqueous CHG does not permeate through the full thickness of excised skin and is retained within the tissue. These results support previous research on another CHG-based compound, chlorhexidine phosphanilate, which was also shown not to permeate through full-thickness skin samples (25).
In this study, a model for studying the delivery of CHG into excised full-thickness human skin was evaluated. Skin permeation studies are commonly performed in vitro with vertical or horizontal diffusion cells using skin or artificial membranes. This study was performed using vertical diffusion cells (Franz-type diffusion cells) to evaluate the delivery of CHG through excised full-thickness human skin. Such conditions mimic the in vivo environment by maintaining the physiological receptor fluid at body temperature and the skin surface temperature at 32°C (6, 23). Skin permeation studies generally evaluate drug delivery through the skin by measuring drug diffusion into the receptor fluid through the SC or epidermis, which are the main barriers for skin permeation. However, the use of stripped skin layers, such as isolated SC or epidermal layers, for drug permeation studies may influence the results, with possible retention of the drug in the dermal layers of the skin. Full-thickness skin was used in this study to determine the location of CHG throughout the skin, rather than studying the flux of the drug through the barrier layers. Following exposure to CHG, the full-thickness human skin was sectioned to a depth of 1,500 μm by sequential sectioning with a microtome, producing a total of 60 sections per skin sample. Skin sectioning has been used in many previous studies (21); however, the SC is often removed by tape stripping prior to sectioning of the skin. In this study, the full-thickness skin samples were sectioned throughout the sample without prior removal of the surface layers. This study demonstrates that the CHG permeation through the full-thickness skin was not linear, which was expected due to the variation in structure at various layers. The top 100-μm layer of the skin, which contains SC (average of 10 to 20 μm thick) and other epidermal layers (50 to 100 μm thick), contained the largest amount of CHG following exposure to 2% (wt/vol) CHG over all time points studied. Previous research has shown that the main permeation barrier for skin absorption is the SC (3, 11, 25), which is thought to be due to its high-lipid matrix and packed layers of keratinized epithelial cells. Furthermore, this study found that below 300 μm, at the dermal layer, the level of CHG remained constantly low. Depending on the body site, dermis contains hair follicles and other skin appendages, including sebaceous glands and sudoriferous glands (sweat-producing glands), which are of interest in skin antisepsis since they may be niches for microbial colonization of the skin following skin antisepsis (7, 8). It is generally recognized that skin antisepsis does not sterilize the skin; our study confirms this and demonstrates that it may be due to poor permeation of chlorhexidine into the deeper layers of the skin.
In conclusion, this study showed poor permeation of chlorhexidine through excised full-thickness human skin after 2 min and 30 min of exposure to aqueous 2% (wt/vol) CHG. The levels of CHG were highest within the top 100-μm sections of skin and remained consistently low within the deeper layers. Furthermore, the model presented in this study is a valuable tool in determining a permeation profile for chlorhexidine through human skin in vitro. This study lays the foundation for further research within this area with a view to potentially adopting alternative strategies for enhanced skin antisepsis in clinical practice.
Acknowledgments
This work was supported by EPSRC CASE grant CNA/05/09 with funding from Insight Health Ltd., United Kingdom.
Footnotes
Published ahead of print on 1 August 2008.
REFERENCES
- 1.Adams, D., M. Quayum, T. Worthington, P. Lambert, and T. Elliott. 2005. Evaluation of a 2% chlorhexidine gluconate in 70% isopropyl alcohol skin disinfectant. J. Hosp. Infect. 61:287-290. [DOI] [PubMed] [Google Scholar]
- 2.Brown, E., R. P. Wenzel, and J. O. Hendley. 1989. Exploration of the microbial anatomy of normal human skin by using plasmid profiles of coagulase-negative staphylococci: search for the reservoir of resident skin flora. J. Infect. Dis. 160:644-650. [DOI] [PubMed] [Google Scholar]
- 3.Cal, K., S. Janicki, and M. Sznitowska. 2001. In vitro studies on permeation of terpenes from matrix-type transdermal systems through human skin. Int. J. Pharm. 224:81-88. [DOI] [PubMed] [Google Scholar]
- 4.Elliott, T. S., H. A. Moss, S. E. Tebbs, I. C. Wilson, R. S. Bonser, T. R. Graham, L. P. Burke, and M. H. Faroqui. 1997. Novel approach to investigate a source of microbial contamination of central venous catheters. Eur. J. Clin. Microbiol. Infect. Dis. 16:210-213. [DOI] [PubMed] [Google Scholar]
- 5.Elliott, T. S., M. H. Faroqui, R. F. Amstrong, and G. C. Hanson. 1994. Guidelines for good practice in central venous catheterization. J. Hosp. Infect. 28:163-176. [DOI] [PubMed] [Google Scholar]
- 6.Franz, T. J. 1975. Percutaneous absorption—on the relevance of in vitro data. J. Investig. Dermatol. 64:190-195. [DOI] [PubMed] [Google Scholar]
- 7.Hendley, J. O., and K. M. Ashe. 2003. Eradication of resident bacteria of normal human skin by antimicrobial ointment. Antimicrob. Agents Chemother. 47:1988-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendley, J. O., and K. M. Ashe. 1991. Effect of topical antimicrobial treatment on aerobic bacteria in the stratum corneum of human skin. Antimicrob. Agents Chemother. 35:627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hibbard, J. S., G. K. Mulberry, and A. R. Brady. 2002. A clinical study comparing the skin antisepsis and safety of ChloraPrep, 70% isopropyl alcohol, and 2% aqueous chlorhexidine. J. Infus. Nurs. 25:244-249. [DOI] [PubMed] [Google Scholar]
- 10.Karpanen, T. J., T. Worthington, D. Rathbone, and P. A. Lambert. 2006. Activity of thiosemicarbazone and carboxamidrazone compounds and essential oils against microorganisms associated with intravascular device related infections. J. Hosp. Infect. 64(Suppl. 1):S37. [Google Scholar]
- 11.Lafforgue, C., L. Carret, F. Falson, M. E. Reverdy, and J. Freney. 1997. Percutaneous absorption of a chlorhexidine digluconate solution. Int. J. Pharm. 147:243-246. [Google Scholar]
- 12.Langgartner, J., H. J. Linde, N. Lehn, M. Reng, J. Schölmerich, and T. Glück. 2004. Combined skin disinfection with chlorhexidine/propanol and aqueous povidone-iodine reduces bacterial colonisation of central venous catheters. Intensive Care Med. 30:1081-1088. [DOI] [PubMed] [Google Scholar]
- 13.Leeming, J. P., K. T. Holland, and W. J. Cunliffe. 1984. The microbial ecology of pilosebaceous units isolated from human skin. J. Gen. Microb. 130:803-807. [DOI] [PubMed] [Google Scholar]
- 14.Maki, D. G., M. Ringer, and C. J. Alvarado. 1991. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet 338:339-343. [DOI] [PubMed] [Google Scholar]
- 15.Malcolm, S. A., and T. C. Hughes. 1980. The demonstration of bacteria on and within the stratum corneum using scanning electron microscopy. Br. J. Dermatol. 102:267-275. [DOI] [PubMed] [Google Scholar]
- 16.McDonnell, G., and A. D. Russell. 1999. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 12:147-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messager, S., P. A. Goddard, P. W. Dettmar, and J.-Y. Maillard. 2001. Determination of the antibacterial efficacy of several antiseptics tested on skin by an ‘ex-vivo’ test. J. Med. Microbiol. 50:284-292. [DOI] [PubMed] [Google Scholar]
- 18.O'Grady, N. P., M. Alexander, E. P. Dellinger, J. L. Gerberding, S. O. Heard, D. G. Maki, H. Masur, R. D. McCormick, L. A. Mermel, M. L. Pearson, I. I. Raad, A. Randolph, and R. A. Weinstein. 2002. Guidelines for the prevention of intravascular catheter-related infections. MMWR Recommend. Rep. 51(RR-10):1-29. [PubMed] [Google Scholar]
- 19.Pratt, R. J., C. M. Pellowe, J. A. Wilson, H. P. Loveday, P. J. Harper, S. R. L. J. Jones, C. McDougall, and M. H. Wilcox. 2007. epic2: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J. Hosp. Infect. 65(Suppl. 1):S1-S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safdar, N., and D. G. Maki. 2004. The pathogenesis of catheter-related bloodstream infection with noncuffed short-term central venous catheters. Intensive Care Med. 30:62-67. [DOI] [PubMed] [Google Scholar]
- 21.Touitou, E., V. M. Meidan, and E. Horwitz. 1998. Methods for quantitative determination of drug localized in the skin. J. Control. Release 56:7-21. [DOI] [PubMed] [Google Scholar]
- 22.Traoré, O., F. A. Allaert, S. Fournet-Fayard, J. L. Verrière, and H. Laveran. 2000. Comparison of in-vivo antibacterial activity of two skin disinfection procedures for insertion of peripheral catheters: povidone iodine versus chlorhexidine. J. Hosp. Infect. 44:147-150. [DOI] [PubMed] [Google Scholar]
- 23.Wagner, H., K.-H. Kostka, C.-M. Lehr, and U. F. Schaefer. 2002. Human skin penetration of flufenamic acid: in vivo/in vitro correlation (deeper skin layers) for skin samples from the same subject. J. Investig. Dermatol. 118:540-544. [DOI] [PubMed] [Google Scholar]
- 24.Wagner, H., K.-H. Kostka, C.-M. Lehr, and U. F. Schaefer. 2000. Drug distribution in human skin using two different in vitro test systems: comparison with in vivo data. Pharm. Res. 17:1475-1481. [DOI] [PubMed] [Google Scholar]
- 25.Wang, J. C. T., R. R. Williams, L. Wang, and J. Loder. 1990. In vitro skin permeation and bioassay of chlorhexidine phosphanilate, a new antimicrobial agent. Pharm. Res. 7:995-1002. [DOI] [PubMed] [Google Scholar]
- 26.Worthington, T., and T. S. J. Elliott. 2005. Diagnosis of central venous catheter related infection in adult patients. J. Infect. 51:267-280. [DOI] [PubMed] [Google Scholar]