Abstract
Oritavancin is a semisynthetic lipoglycopeptide in clinical development for serious gram-positive infections. This study describes the synergistic activity of oritavancin in combination with gentamicin, linezolid, moxifloxacin, or rifampin in time-kill studies against methicillin-susceptible, vancomycin-intermediate, and vancomycin-resistant Staphylococcus aureus.
Oritavancin is a semisynthetic lipoglycopeptide in clinical development that has activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. It differs from other glycopeptides such as vancomycin and teicoplanin in that its bactericidal activity in vitro is rapid and concentration dependent (1). Recent work demonstrated that oritavancin binds avidly to glass and plastic labware surfaces, causing its potency to be significantly underestimated during susceptibility testing and other microbiological assays (3). The Clinical Laboratory Standards Institute (CLSI) recent update to include polysorbate-80 at 0.002% throughout oritavancin broth microdilution testing (9), which limits binding of oritavancin to vessel surfaces, has prompted reevaluation of oritavancin activity in a range of in vitro microbiological assays.
The potential benefits of combination antimicrobial chemotherapy over monotherapy include decreased resistance development, synergistic antibacterial activity, and a broadened antibacterial spectrum (10, 12). Previous studies examining the activity of oritavancin in combinations were performed in the absence of polysorbate-80, which may have affected assay results (5, 15, 17, 20, 22). We have thus revisited oritavancin combination testing using time-kill methodology in the presence of 0.002% polysorbate-80 to determine whether combinations of oritavancin and other antimicrobial agents exhibit synergistic antibacterial activity against methicillin-susceptible S. aureus (MSSA), vancomycin-intermediate S. aureus (VISA), and vancomycin-resistant S. aureus (VRSA).
(Part of this work was presented at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 17 to 20 September 2007 [6].)
Oritavancin diphosphate powder (Targanta Therapeutics, Cambridge, MA) was dissolved in water containing 0.002% polysorbate-80 (9), and polysorbate-80 was maintained at this concentration to minimize oritavancin loss to the surface of vessels during in vitro testing (3). VISA isolate NRS402 and VRSA isolate VRS5 (both obtained from Network on Antimicrobial Resistance in S. aureus) were grown overnight in brain heart infusion broth containing 4 μg/ml vancomycin (to ensure the VISA and VRSA phenotypes). For time-kill assays, bacteria were subcultured in cation-adjusted Mueller-Hinton broth (CAMHB) until exponential phase (optical density at 600 of approximately 0.25), diluted to approximately 5 × 105 CFU/ml in CAMHB containing antimicrobial agents alone or in combination, and exposed for 24 h at 37°C (21). Inclusion of polysorbate-80 did not substantially affect killing kinetics for comparator agents compared to assays performed in its absence (data not shown). To prevent drug carryover during serial dilution plating, aliquots of the drug-challenged culture were added to an equal volume of a 25-mg/ml activated charcoal suspension. Synergy was defined as a ≥2-log10 decrease in CFU/ml between the combination and its most active constituent after 24 h (at least one of the drugs must be present at a concentration that does not affect the growth curve of the test organism), and the number of surviving organisms in the presence of the combination must be ≥ 2 log10 CFU/ml below the starting inoculum (2). Bacteriostatic and bactericidal activities were defined as <3-log10 and ≥3-log10 reductions in CFU/ml at 24 h, respectively, relative to the starting inoculum (21). All experiments were repeated at least three times, and results of a representative experiment are presented; data points are averages from duplicate CFU/ml determinations within an experiment.
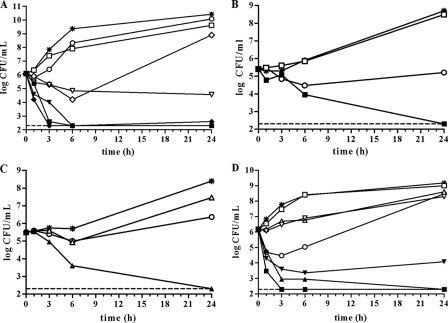
Oritavancin concentrations in the combination time-kill studies were selected to allow for assessment of synergy: oritavancin at concentrations below its MIC exerted transient antibacterial activity against the S. aureus isolates such that either an initial lag in growth or decrease in CFU was observed following addition of oritavancin (Fig. 1). In all cases, regrowth occurred to various levels by 24 h (Fig. 1).
FIG. 1.
Time-kill curves of synergistic combinations of oritavancin with gentamicin, linezolid, moxifloxacin, or rifampin against MSSA, VISA and VRSA. Exponential-phase MSSA ATCC 29213, VISA NRS402, or VRSA VRS5 was diluted in CAMHB containing 0.002% polysorbate-80 and challenged with the test agents at the indicated concentrations. The single-agent drug concentrations were maintained during combination testing with oritavancin. Viability was enumerated at the indicated time points by serial dilution plating. Each point represents the mean of duplicate determinations. Whereas experiments were performed in triplicate, the time-kill curves presented here are from a single experiment. The limit of detection (200 CFU/ml) is indicated by the dashed lines. (A) Oritavancin combinations versus MSSA ATCC 29213. Note that the points for oritavancin plus rifampin overlap those for oritavancin plus gentamicin at 6 and 24 h. *, growth control; ○, 0.031 μg/ml oritavancin; □, 0.25 μg/ml gentamicin; ⋄, 0.063 μg/ml moxifloxacin; ▿, 0.008 μg/ml rifampin; ▪, oritavancin plus gentamicin; ⧫, oritavancin plus moxifloxacin; ▾, oritavancin plus rifampin. (B and C) Oritavancin combinations versus VISA NRS402. *, growth control; ○, 0.50 μg/ml oritavancin; □, 0.13 μg/ml gentamicin; ▵, 0.25 μg/ml linezolid; ▪, oritavancin plus gentamicin; ▴, oritavancin plus linezolid. (D) Oritavancin combinations versus VRSA VRS5. *, growth control; ○, 0.13 μg/ml oritavancin; □, 0.25 μg/ml gentamicin; ▵, 1 μg/ml linezolid; ▿, 0.002 μg/ml rifampin; ▪, oritavancin plus gentamicin; ▴, oritavancin plus linezolid; ▾, oritavancin plus rifampin.
Against the MSSA reference strain S. aureus ATCC 29213, combinations of oritavancin with either gentamicin, moxifloxacin, or rifampin were synergistic and bactericidal at 24 h (Fig. 1A; Table 1). Knowledge of whether antimicrobial combinations exert bacteriostatic or bactericidal effects could be important for treatment outcomes in certain infections (14). Synergy was not observed with the combination of oritavancin and linezolid, a protein synthesis inhibitor (Table 1).
TABLE 1.
Summary of in vitro time-kill assays of oritavancin combinations against S. aureus isolates
| Agenta | MSSA ATCC 29213
|
VISA NRS402
|
VRSA VRS5
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concn (μg/ml)b | Fold MIC in combinationc | Δlog CFU at 24 h vsd:
|
Concn (μg/ml) | Fold MIC in combination | Δlog CFU at 24 h vs:
|
Concn (μg/ml) | Fold MIC in combination | Δlog CFU at 24 h vs:
|
|||||||
| C | A | I | C | A | I | C | A | I | |||||||
| ORI | 0.031 | 0.5 | −0.34 | NAe | NA | 0.50 | 0.5 | −3.5/−2.5g | NA | NA | 0.13 | 0.5 | −0.70 | NA | NA |
| GEN | 0.25 | 0.5 | −0.81 | −7.3 | −3.8 | 0.13 | 0.25 | −0.21 | −2.9 | −3.1 | 0.25 | 0.5 | −0.18 | −6.2 | −3.9 |
| LZD | 1 | 0.5 | −0.64 | −1.2 | 2.5 | 0.25 | 0.25 | −0.94 | −4.1 | −3.2 | 1 | 0.5 | −0.57 | −6.2 | −3.9 |
| MOX | 0.063 | 1 | −1.5 | −6.3 | −3.5 | NDf | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| RIF | 0.008 | 1 | −5.9 | −2.3 | −3.8 | ND | ND | ND | ND | ND | 0.002 | 0.5 | −0.88 | −4.2 | −2.1 |
Abbreviations: ORI, oritavancin; GEN, gentamicin; LZD, linezolid; MOX, moxifloxacin; RIF, rifampin.
Concentration of the antimicrobial agent tested as a single agent and used in combination in the time-kill assay.
Multiple of the broth microdilution MIC (determined as per the CLSI guidelines) of oritavancin or other agent used in combination.
Values presented are the log10 change in CFU (a negative value indicates a decrease) at 24 h for the single agent relative to the growth control (C) or for the combination of the indicated agent with oritavancin relative to the most active agent of the combination (A) or the inoculum (I). Values shown are the means of duplicate determinations from a representative experiment repeated at least three times.
NA, not applicable.
ND, not determined since inherent resistance or nonsusceptibility to the combination agent precluded testing in combination with oritavancin.
The value after the slash is for oritavancin used in combination with linezolid.
The combination of oritavancin and gentamicin was previously shown to be synergistic against two VISA isolates by time-kill methodology without polysorbate-80 (15). These findings were confirmed and extended in the current study: against the VISA isolate S. aureus NRS402, oritavancin with gentamicin or linezolid was synergistic (Fig. 1B and C; Table 1); these combinations were bactericidal at the 24-h time point. Oritavancin in combination with gentamicin or linezolid was also synergistic and bactericidal against the VRSA isolate VRS5 (Fig. 1D; Table 1). Conceivably, the ability of oritavancin to increase membrane permeability (19) may facilitate entry of gentamicin into the cell, as has been shown with sesquiterpenoids, agents that increase S. aureus membrane permeability and increase susceptibility to gentamicin (7). The combination of oritavancin and rifampin was synergistic and bacteriostatic against VRS5 at the 24-h time point (Fig. 1D; Table 1). That this combination was also synergistic against MSSA suggests a common killing mechanism of these two strains. We have observed that oritavancin inhibits RNA synthesis in S. aureus RN4220, a methicillin-susceptible laboratory strain (4). Loss of the permeability barrier function has been linked to inhibition of macromolecular synthesis, including RNA synthesis (25). Putative leakage of RNA precursors from the cell due to perturbation of cell membrane barrier function by oritavancin, coupled with inhibition of RNA polymerase by rifampin, may explain the synergy between these two agents.
Despite the synergy exhibited by certain combinations of antimicrobial agents in vitro, the overall benefit of combinations in clinical practice remains controversial (12, 16). For example, a recent meta-analysis examining inclusion of an aminoglycoside with a β-lactam for the treatment of endocarditis demonstrated no benefit in clinical outcome over β-lactam monotherapy and increased the frequency of nephrotoxicity (13). However, combination therapy may be beneficial for treatment of certain infections that harbor bacteria either in a tolerant state or in a biofilm, such as those associated with indwelling devices (8, 11, 18, 26), or for tuberculosis (24). Recent in vitro findings that the combination of a β-lactam with vancomycin evokes synergistic activity against methicillin-resistant VRSA (23) highlight the potential of antimicrobial combination therapy and thus the importance of in vitro synergy testing.
In conclusion, using newly approved methodology that for broth microdilution assays maintain oritavancin at its intended concentration, we have demonstrated in vitro synergy between oritavancin and representative, clinically used antimicrobial agents against drug-susceptible and -resistant S. aureus strains. Future studies in in vivo infection models should provide a better understanding of the therapeutic potential of oritavancin combinations.
Acknowledgments
We thank the scientific support personnel at Targanta Therapeutics for their contributions to this research. We also thank Norris Allen for helpful discussions and critical review of the manuscript.
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Allen, N. E., and T. I. Nicas. 2003. Mechanism of action of oritavancin and related glycopeptide antibiotics. FEMS Microbiol. Rev. 26:511-532. [DOI] [PubMed] [Google Scholar]
- 2.American Society for Microbiology. 2008. Instructions to authors. Antimicrob. Agents Chemother. 52:i-xxiii. [Google Scholar]
- 3.Arhin, F. F., I. Sarmiento, A. Belley, G. A. McKay, D. C. Draghi, P. Grover, D. F. Sahm, T. R. Parr, Jr., and G. Moeck. 2008. Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob. Agents Chemother. 52:1597-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arhin, F. F., I. Sarmiento, T. R. Parr, Jr., and G. Moeck. 2007. Mechanisms of action of oritavancin in Staphylococcus aureus, abstr. C1-1471. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17 to 20 September 2007.
- 5.Baltch, A. L., R. P. Smith, W. J. Ritz, and L. H. Bopp. 1998. Comparison of inhibitory and bactericidal activities and postantibiotic effects of LY333328 and ampicillin used singly and in combination against vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 42:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belley, A., E. Neesham-Grenon, S. Beaulieu, G. McKay, F. F. Arhin, T. R. Parr, Jr., and G. Moeck. 2007. Synergistic effects of oritavancin tested in combination with other agents, abstr. E-1619. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL, 17 to 20 September 2007.
- 7.Brehm-Stecher, B. F., and E. A. Johnson. 2003. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 47:3357-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuard, C., M. Herrmann, P. Vaudaux, F. A. Waldvogel, and D. P. Lew. 1991. Successful therapy of experimental chronic foreign-body infection due to methicillin-resistant Staphylococcus aureus by antimicrobial combinations. Antimicrob. Agents Chemother. 35:2611-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CLSI. 2008. Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement, CLSI document M100-S18, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.den Hollander, J., and J. Mouton. 2007. The predictive value of laboratory tests for efifcacy of antibiotic combination therapy, p. 103-127. In C. Nightingale, P. Ambrose, G. Drusano, and T. Murakawa (ed.), Antimicrobial pharmacodynamics in theory and clinical practice, 2nd ed. Informa Healthcare, New York, NY.
- 11.Drinkovic, D., A. J. Morris, S. Pottumarthy, D. MacCulloch, and T. West. 2003. Bacteriological outcome of combination versus single-agent treatment for staphylococcal endocarditis. J. Antimicrob. Chemother. 52:820-825. [DOI] [PubMed] [Google Scholar]
- 12.Eliopoulos, G. M., and C. T. Eliopoulos. 1988. Antibiotic combinations: should they be tested? Clin. Microbiol. Rev. 1:139-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falagas, M. E., D. K. Matthaiou, and I. A. Bliziotis. 2006. The role of aminoglycosides in combination with a beta-lactam for the treatment of bacterial endocarditis: a meta-analysis of comparative trials. J. Antimicrob. Chemother. 57:639-647. [DOI] [PubMed] [Google Scholar]
- 14.Finberg, R. W., R. C. Moellering, F. P. Tally, W. A. Craig, G. A. Pankey, E. P. Dellinger, M. A. West, M. Joshi, P. K. Linden, K. V. Rolston, J. C. Rotschafer, and M. J. Rybak. 2004. The importance of bactericidal drugs: future directions in infectious disease. Clin. Infect. Dis. 39:1314-1320. [DOI] [PubMed] [Google Scholar]
- 15.Hershberger, E., J. R. Aeschlimann, T. Moldovan, and M. J. Rybak. 1999. Evaluation of bactericidal activities of LY333328, vancomycin, teicoplanin, ampicillin-sulbactam, trovafloxacin, and RP59500 alone or in combination with rifampin or gentamicin against different strains of vancomycin-intermediate Staphylococcus aureus by time-kill curve methods. Antimicrob. Agents Chemother. 43:717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le, T., and A. S. Bayer. 2003. Combination antibiotic therapy for infective endocarditis. Clin. Infect. Dis. 36:615-621. [DOI] [PubMed] [Google Scholar]
- 17.Lefort, A., A. Saleh-Mghir, L. Garry, C. Carbon, and B. Fantin. 2000. Activity of LY333328 combined with gentamicin in vitro and in rabbit experimental endocarditis due to vancomycin-susceptible or -resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 44:3017-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucet, J. C., M. Herrmann, P. Rohner, R. Auckenthaler, F. A. Waldvogel, and D. P. Lew. 1990. Treatment of experimental foreign body infection caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 34:2312-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKay, G. A., I. Fadhil, S. Beaulieu, S. Ciblat, A. R. Far, G. Moeck, and T. R. Parr, Jr. 2006. Oritavancin disrupts transmembrane potential and membrane integrity concomitantly with cell killing in Staphylococcus aureus and vancomycin-resistant enterococci, abstr. C1-682. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 27 to 30 September 2006.
- 20.Mercier, R. C., S. R. Penzak, and M. J. Rybak. 1997. In vitro activities of an investigational quinolone, glycylcycline, glycopeptide, streptogramin, and oxazolidinone tested alone and in combinations against vancomycin-resistant Enterococcus faecium. Antimicrob. Agents Chemother. 41:2573-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NCCLS. 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline, NCCLS document M26-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 22.Noviello, S., F. Ianniello, and S. Esposito. 2001. In vitro activity of LY333328 (oritavancin) against Gram-positive aerobic cocci and synergy with ciprofloxacin against enterococci. J. Antimicrob. Chemother. 48:283-286. [DOI] [PubMed] [Google Scholar]
- 23.Perichon, B., and P. Courvalin. 2006. Synergism between beta-lactams and glycopeptides against VanA-type methicillin-resistant Staphylococcus aureus and heterologous expression of the vanA operon. Antimicrob. Agents Chemother. 50:3622-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spyridis, N. P., P. G. Spyridis, A. Gelesme, V. Sypsa, M. Valianatou, F. Metsou, D. Gourgiotis, and M. N. Tsolia. 2007. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin. Infect. Dis. 45:715-722. [DOI] [PubMed] [Google Scholar]
- 25.Xiong, Y. Q., A. S. Bayer, and M. R. Yeaman. 2002. Inhibition of intracellular macromolecular synthesis in Staphylococcus aureus by thrombin-induced platelet microbicidal proteins. J. Infect. Dis. 185:348-356. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerli, W., A. F. Widmer, M. Blatter, R. Frei, P. E. Ochsner, et al. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA 279:1537-1541. [DOI] [PubMed] [Google Scholar]