Abstract
The antimalarial activity and pharmacology of a series of phenylthiazolyl-bearing hydroxamate-based histone deacetylase inhibitors (HDACIs) was evaluated. In in vitro growth inhibition assays approximately 50 analogs were evaluated against four drug resistant strains of Plasmodium falciparum. The range of 50% inhibitory concentrations (IC50s) was 0.0005 to >1 μM. Five analogs exhibited IC50s of <3 nM, and three of these exhibited selectivity indices of >600. The most potent compound, WR301801 (YC-2-88) was shown to cause hyperacetylation of P. falciparum histones, which is a marker for HDAC inhibition in eukaryotic cells. The compound also inhibited malarial and mammalian HDAC activity in functional assays at low nanomolar concentrations. WR301801 did not exhibit cures in P. berghei-infected mice at oral doses as high as 640 mg/kg/day for 3 days or in P. falciparum-infected Aotus lemurinus lemurinus monkeys at oral doses of 32 mg/kg/day for 3 days, despite high relative bioavailability. The failure of monotherapy in mice may be due to a short half-life, since the compound was rapidly hydrolyzed to an inactive acid metabolite by loss of its hydroxamate group in vitro (half-life of 11 min in mouse microsomes) and in vivo (half-life in mice of 3.5 h after a single oral dose of 50 mg/kg). However, WR301801 exhibited cures in P. berghei-infected mice when combined at doses of 52 mg/kg/day orally with subcurative doses of chloroquine. Next-generation HDACIs with greater metabolic stability than WR301801 may be useful as antimalarials if combined appropriately with conventional antimalarial drugs.
Considerable research activity has focused on understanding the “histone code,” and in particular on the design of hydroxamate-based histone deacetylase inhibitors (HDACIs) as novel therapeutics for the treatment of a wide range of disorders, including cancer and neurodegenerative diseases (26). HDACIs owe their action to their ability to reactivate silenced genes by modulating the condensation status of DNA. The posttranslational acetylation status of chromatin is determined by the competing activities of two classes of enzymes, histone acetyltransferases (HATs) and histone deacetylases (HDACs) which control the acetylation of lysine residues on histone tails. In general, HATs function to acetylate lysine groups in nuclear histones, resulting in neutralization of the charges on the histones and a more open, transcriptionally active chromatin structure, while the HDACs function to deacetylate and suppress transcription. A shift in the balance of acetylation on chromatin may result in changes in the regulation of patterns of gene expression (16, 24, 37, 39). Since many cancers are associated with aberrant transcriptional activity, and HDACs can affect transcription factors and gene regulation, these enzymes have been identified as attractive targets for cancer therapy. Indeed, chemical inhibitors of HDACs have been shown to inhibit tumor cell growth and induce differentiation and cell death (28). Several such inhibitory agents, including suberoylanilide hydroxamic acid (SAHA) and depsipeptide (FR901228) have reached clinical trials (6, 20, 35), and SAHA has been approved by the U.S. Food and Drug Administration for use in treating cutaneous T-cell lymphoma. HDACIs are thought to function by allowing the transcription and expression of genes, including tumor suppressor genes. HDACIs also enhance the cytotoxic effects of therapeutic agents used in cancer treatment, including radiation and chemotherapeutic drugs (25, 27).
Initial studies suggest that HDAC may be an attractive target in P. falciparum. The antimalarial activity of HDACIs was first reported by Darkin-Rattray et al. (11). Apicidin, a fungal metabolite, was found to exhibit an MIC of 125 ng/ml against P. falciparum and exhibited a suppressive effect on blood-stage parasites when administered intraperitoneally at a dose rate of 50 mg/kg in mice. Functional studies suggested that the target of apicidin was indeed malarial HDAC. The compound did not appear to have much selectivity for malaria, since its 50% inhibitory concentration (IC50) against proliferating mammalian cells was 50 to 100 nM. Subsequently, Joshi et al. (18) cloned an HDAC directly from P. falciparum (HDAC-1) and showed that it localized in the parasite nucleus and was expressed primarily in mature asexual blood stages and gametocytes. A subsequent study (2) also demonstrated that other putative HDACIs were active in vitro and in vivo. These studies suggest that HDAC is a promising target. However, these initial findings have not yet been properly exploited in order to develop potential antimalarial drugs for clinical use. Recently, our research groups reported that certain triazolylphenyl (9)- and 2-aminosuberic acid (1)-based hydroxamates have nanomolar potency against drug-resistant strains of P. falciparum in vitro. We have also reported the synthesis and screening of phenylthiazolyl-based hydroxamate inhibitors as agents against pancreatic cancer (21). Here we report that this same group of compounds exhibits the most potent and selective antimalarial activity of all of the HDACIs tested thus far.
MATERIALS AND METHODS
Synthesis of HDACIs.
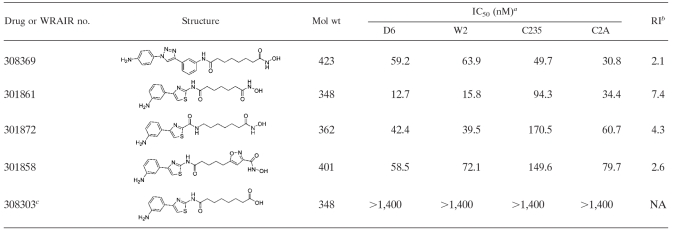
Approximately 50 phenylthiazolyl-hydroxamate-based HDACIs were synthesized. Those with IC50s < 10 nM (C2A strain) or with IC50s < 100 nM that revealed useful information about structure activity relationships are shown in Table 1. Synthetic schemes and characterization data are presented as Fig. S1 and S2 and Table S1 in the supplemental material, respectively. Compounds are referred to by their WR designations throughout (the original laboratory designations have been left associated with the characterization data in Table S1 in the supplemental material).
TABLE 1.
IC50s and RI values for selected HDACIs against four drug-resistant strains of P. falciparum
Means ± the standard deviation are given where applicable.
That is, the ratio of the highest to the lowest IC50. NA, not applicable.
A metabolite of WR301801.
In vitro susceptibility and toxicity assays.
The in vitro activities of HDACIs against P. falciparum strains W2, D6, TM91C235, and TM90C2A were evaluated by using the labeled hypoxanthine assay of Desjardins et al. (12) as modified by Milhous et al. (30) and described in one of our earlier reports (14). W2 is chloroquine resistant and mefloquine sensitive, D6 is chloroquine sensitive but naturally less susceptible to mefloquine, and TM91C235 is resistant to mefloquine, chloroquine, and pyrimethamine, as is TM90C2A; however, this latter parasite is a two pfmdr1 copy strain. The data for WR301801, WR308298, WR308291, WR308296, and WR308294 against the D6, W2, and C235 strains are reported as the averages of duplicate IC50s in Table 1. The remaining IC50s for other compounds against all strains are reported as single values for each compound in Table 1. We routinely run mefloquine and chloroquine as controls to ensure assay validity. The IC50s and standard deviations of chloroquine and mefloquine (for the last 15 assays) for each strain are reported in Table 1. Selectivity was defined at the cellular rather than enzymatic level (see Discussion). Selectivity was assessed by first determining the LC50s of three of the five most potent compounds against macrophages (RAW cell line) using the 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described in one of our earlier studies (7). Selectivity indices were determined by using the following formula: 50% lethal concentration (LC50) (RAW cells)/IC50 (P. falciparum).
Histone hyperacetylation assay.
The effect of compounds on P. falciparum histone acetylation status was determined as previously described (1). Approximately 3 × 109 late-trophozoite-stage 3D7-infected erythrocytes (a mefloquine-resistant clone of W2) were incubated with different concentrations of compounds or vehicle alone (0.01% dimethyl sulfoxide) for 3.5 h at 37°C, before histones were prepared. An additional untreated control sample was taken at the start of the assay. Histone extracts (ca. 6 × 108 parasites per lane) were separated by sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis (SDS-15% PAGE), and equal loading was confirmed using silver staining. To detect changes in acetylation patterns after treatment, SDS-PAGE separated samples were transferred to a polyvinylidene difluoride membrane (Roche) at 80 V for 1 h in 25 mM Tris-HCl-0.2 M glycine-20% methanol. Membranes were blocked in phosphate-buffered saline (PBS) containing 3% skim milk powder for at least 1 h before anti-(tetra)acetyl H4 histone antibody or anti-(di)acetyl-H3 antiserum (Upstate; 1:2,000 dilution in PBS-3% skim milk powder) was added, followed by incubation for 2 h at room temperature. After three washes in deionized water, the membranes were incubated for 1 h in goat anti-rabbit antibody conjugated to horseradish peroxidase (Zymed; 1:5,000 dilution in PBS-3% skim milk powder). The membranes were washed again, and signal was detected with enhanced chemiluminescence (GE Healthcare, United Kingdom) and autoradiography. The same membrane was then stripped and rehybridized with anti-(di)acetyl-H3 antisera (Upstate; 1:2,000 dilution in PBS-3% skim milk powder) using the conditions described above.
Functional HDAC assay.
The effect of WR301801 on malarial and mammalian HDAC was evaluated by using a commercially available fluorometric activity assay in 96-well plates according to the manufacturer's instructions (kit AK-503; Biomol International) as follows. For the mammalian assay, confluent Vero cells were used. Approximately 106 isolated parasites (W2, late trophozoites) were used for the malaria assay. Cells were incubated with a cell-permeable substrate (Fleur de Lys) for 3 to 4 h. During this time the substrate accumulates intracellularly and is deacetylated. After incubation, the cells were treated for 20 min with a developer. This generates a fluorescent signal from the deacetylated substrate and induces cell lysis. Plates were read in a Tecan fluorescence reader (excitation, 350 to 380 nm; emission, 440 to 460 nm). HDAC inhibition is manifested as a reduction in the arbitrary fluorescence units relative to drug-free controls. Each drug concentration was tested in triplicate at least twice. Trichostatin A was used as a control in the mammalian assay and exhibited the expected results (data not shown).
In vitro isobologram studies.
The in vitro drug combination activity of HDACIs with known antimalarial drugs against P. falciparum strains W2 and D6 were evaluated by using the malaria Sybr green I fluorescence-based assay of Smilkstein et al. (36) and as described in one of our earlier studies (17). Briefly, each antimalarial drug was tested alone and at fixed ratios with 12 twofold serial dilutions. The starting concentration (for serial dilutions across the microtiter plate) was assigned so that the IC50 of each drug would be in the center of the plate. The IC50s were calculated for each drug alone and for their respective fixed concentration ratios. The IC50s of drug A and drug B were standardized by allocating a value of 1 to each drug that was tested alone and pro rata values for each fixed concentration ratio. The individual and sum 50% fractional inhibitory concentrations (FIC50 and ∑FIC50, respectively) were determined as described by Berenbaum (3). Isobolograms were constructed from the FIC50s of drug A and drug B at different fixed concentration ratios: a straight line represents additivity (∑FIC = 1), a concave line denotes a trend toward synergy (∑FIC < 1) or synergy (∑FIC ≤ 0.5), and a convex curve represents a trend toward antagonism (∑FIC > 1) or antagonism (∑FIC ≥ 2). The data are reported here as the average ∑FIC (across all drug dilutions) or the average maximum ∑FIC at any dilution for at least two replicate experiments for each drug combination.
In vivo efficacy studies.
The antimalarial activity of WR301801 alone and in combination with chloroquine against blood stage Plasmodium berghei in mice and P. falciparum in Aotus lemurinus lemurinus monkeys was evaluated as described by us in an earlier publication (13). Briefly, in the mouse assay, all mice were inoculated intraperitoneally with 106 P. berghei-parasitized erythrocytes. WR301801 (0.32 to 640 mg/kg/day for 3 days) alone or in combination with chloroquine at subtherapeutic doses (2 to 64 mg/kg/day for 3 days) orally once per day on days 3, 4, and 5 postinfection in a hydroxethylcellulose-Tween 20 vehicle (n = 5 mice per dose group). Blood smears were obtained on study days 0, 3, 6, 10, 13, 17, 20, 24, 27, and 31. The efficacy was assessed by monitoring intermittently the effect of the drugs on parasitemia and cure rates. A cure was defined as the absence of microscopic parasites in blood smears without histopathological evidence of malaria infection on day 31 postinfection. In other experiments WR301801 was given subcutaneously or over 7 days. In the Aotus studies, the five monkeys selected for the study had active infections of the chloroquine-resistant FVO strain of P. falciparum. The animals had previously been treated unsuccessfully with a single 25-mg/kg dose of an experimental quinoline methanol. Once parasitemias reached 5,000/μl (or as deemed appropriate by the attending veterinarian), they were treated orally with WR30801 at 8, 16, or 32 mg/kg/day for 3 days. When these treatments failed they were rescued with a single 20-mg/kg dose of mefloquine orally). The Aotus monkeys were cared for and maintained at Gorgas Memorial Institute Aotus colony as previously described (34). All animal experiments were conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhere to principles stated in the Guide for the Care and Use of Laboratory Animals (32).
Metabolism-pharmacokinetic studies.
The half-life of WR30801 in mouse and human liver microsomes was determined as described in our earlier study (13). Drug interaction potential was evaluated in vitro by using BD Gentest (Franklin Lakes, NJ) CYP450 inhibition kits as recommended by the manufacturer. Plasma stability was determined by individually incubating the compounds for 0, 1, and 2 h at room temperature and 37°C using a lab rocker and a water bath, respectively, set at 90 rpm. The data were compared to similarly treated acetonitrile-water samples. Plasma samples were extracted and analyzed as described for the pharmacokinetic studies below. In the pharmacokinetic studies, WR301801 was administered as a single dose orally and subcutaneously at a dose of 50 mg/kg to three groups of six 8-week-old male ICR mice for each dosing route. At 15, 30, and 60 min and at 3, 6, and 24 h, one mouse from each group (six mice total) was sacrificed, and plasma was collected and frozen at −80°C. Plasma proteins were precipitated by adding 2 volumes of acetonitrile to 100-μl plasma samples, followed by high-speed vortexing for 10 to 12 s and centrifugation at 13,000 rpm for 10 min at 4°C. Then, 5 μl of the undisturbed supernatants was analyzed by liquid chromatography-tandem mass spectrometry (LEAP Technologies, Carrboro, NC, and ThermoFissher, Waltham, MA) based on standard curves of WR301801 and WR308301 in the range of 0 to 500 ng/ml and quality controls (10 and 100, n = 4 each) prepared by serial dilutions using blank plasma. Samples were chromatographically separated using a shallow acetonitrile-water gradient ran trough a 3.5-μm, 2.1-by-50-mm, C-18 XTerra MS column from Waters (Milford, MA) at a flow rate of 0.3 ml/min. Quantification was done by using the Excalibur (ThermoFisher) software. For the purposes of determining pharmacokinetic parameters, each group of six mice was considered to be a single “animal.” Plasma concentration-time data for WR301801 for each animal were fitted to a two-compartment open model using a nonlinear, extended least-squares fitting procedure (WinNonlin 5.1; Scientific Consulting, Inc., Apex, NC) using weighted (1/concentration) nonlinear regression. The area under the curve (AUC) was determined by the linear trapezoidal rule with extrapolation to infinity based on the concentration of the last time point divided by the terminal rate constant. Extrapolations to time zero were done using zero concentration for oral or subcutaneous dosing and using C0 values determined from the two-compartment model equation at time zero. The elimination half-life (t1/2) was calculated as ln(2)Vss/Vmax[Km+C]. Cmax and Tmax were determined individually for each “animal.” Cmax and Tmax were determined individually for each “animal.” For WR308303, the AUC was determined after fitting plasma-concentration time curves to a noncompartmental model. Significant inter-“animal” variability prevented the determination of other pharmacokinetic parameters.
RESULTS
In vitro antimalarial activity and SAR.
The IC50s of the 50 phenylthiazolyl hydroxamates against four strains of P. falciparum spanned a range of 0.5 to >1,000 nM. The data for the analogs with IC50s of <10 nM and the analogs that reveal useful information about structure-activity relationships (SAR) are presented in Table 1. The most potent compounds (IC50s < 3 nM) were all substituted at the meta position of the phenyl ring with an amino or hydroxyl group. Using these analogs as a baseline, one can gauge a general impression of the SAR. Introduction of an amino group at the meta position of the terminal phenyl ring imparts greater potency than if the same substitution is made at the ortho or para position (compare WR301801 to WR301871, WR301826, and WR301839, Table 1). Potency is lost if the meta substituent is a tertiary amine, methyl, or methoxy group rather than an amino or hydroxyl group (compare WR301801 and WR308295 with WR301803, WR308292, and WR308294, Table 1). Other terminal ring systems could probably be substituted without loss of potency (compare WR308293 and WR308288 to WR301839). In the linker region, reduction of the linker length, substitution or rearrangement of the central amide bond reduces potency (compare WR301801 to WR301861, WR301858, and WR301872). Finally, substitution of nonthiazole ring systems generally reduces potency (compare WR301801 with WR301849 and WR301871 with WR308369, Table 1), although some of these alternative compounds are still more active than SAHA or other antimalarials. Different HDACIs appear to have different patterns of activity across the four malaria strains tested (e.g., compare WR301801 with WR308294), and there does not appear to be a shared pattern of cross-susceptibility with mefloquine and chloroquine (Table 1). The average resistance index (RI, highest IC50/lowest IC50) for the HDACI in Table 1 was 2.4 (range, 1.3 to 7.4), whereas those of mefloquine and chloroquine were 7.4 and 27, respectively.
In vitro cytotoxicity and selectivity indices.
The cytotoxicity of three of the five most potent compounds was evaluated against RAW macrophage cells. As outlined in Table 2, the SI was >600 in all cases. A similar LC50 (950 nM) was observed against Vero cells (data not shown). For WR301801 we observed similar trends in Vero cells (data not shown) and with a variety of other cell lines in our earlier studies (21, 23). The LC50s of WR301801 (and corresponding SI values relative to P. falciparum) are lower against the specific pancreatic cancer cells against which this series was originally optimized (21, 23).
TABLE 2.
Cytotoxicity and selectivity of selected HDACIs
| Compound | Lowest IC50 (nM) for P. falciparum | Mean RAW cell LD50 (nM) (range, n)a | SI (RAW cells) |
|---|---|---|---|
| SAHA (WR308364) | 110 | 2,200 (1,700-2,700, 2) | 22 |
| WR301801 | 0.59 | 600 (170-840, 4) | 1,080 |
| WR308298 | 0.81 | 540 (460-620, 2) | 670 |
| WR308291 | 0.9 | 3,200 (2,500-3,800, 2) | 3,560 |
n, number of experiments.
WR301801 (YC-2-88) alters histone acetylation in P. falciparum parasites.
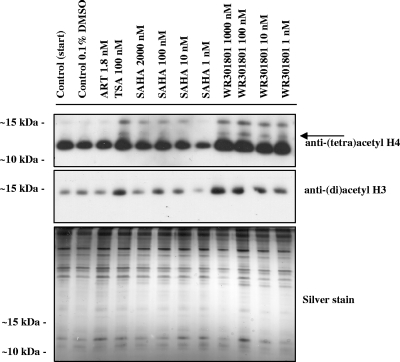
Histone hyperacetylation is the marker of HDAC inhibition in eukaryotic cells, including Plasmodium spp. (1, 11, 15, 19). The natural product derivative apicidin and synthetic hydroxamic acid-based HDACIs based on 2-amino suberic acid cause hyperacetylation of P. falciparum histones (1, 11) and, conversely, the HAT inhibitor curcumin hypoacetylates P. falciparum histone H3 (10). Here we demonstrate that WR301801 also alters P. falciparum histone acetylation profiles (Fig. 1). After treatment with WR301801 for 3.5 h, an increase in acetylated histone-H3 and -H4 was detected using anti-(di)acetyl H3 and anti-(tetra)acetyl H4 antibodies, respectively. The anti-(tetra)acetyl H4 antibody recognizes a major species at ca. 12 to 13 kDa in control sample lanes, which corresponds to histone H4. In samples treated with WR301801 an increase in the intensity of this band is observed, as well as additional higher-molecular-weight species, one of which likely corresponds to H2A.Z since the anti-acetyl H4 antibody is known to cross-react with histone H2 (Fig. 1, indicated by an arrow) (29). The anti-(di)acetyl H3 antibody recognizes a single band at ∼15 kDa in all samples, with increased intensity in samples treated with WR301801. The altered acetylation profiles observed after WR301801 treatment occurred at concentrations ranging from the approximate IC50 (1 nM) to 1,000 times the IC50 (1,000 nM). Although WR301801 concentrations of 10 nM or higher resulted in complete killing of P. falciparum parasites, cultures treated with 1 nM WR301801 for 3.5 h that were washed and returned to culture showed ∼40% growth compared to matched controls after 48 h (data not shown). No change in the histone acetylation profile was observed for infected erythrocytes treated with the ∼IC50 of artemisinin (1.8 nM; Fig. 3) or 1,000 nM (data not shown). Altered histone acetylation profiles were obtained for the HDACI control trichostatin A (100 nM) and for another HDACI SAHA at 10, 100, and 2000 nM (SAHA IC50 of ∼100 nM), although at 1 nM the acetylation state returned to that of controls. These observations were confirmed in an independent functional assay in which WR301801 was found to inhibit parasite HDAC activity in a concentration-dependent manner with an IC50 in the range of 1 to 10 nM (Fig. 2). WR301801 exhibited an IC50 of 10 nM in the mammalian HDAC inhibition assay (Fig. 2), confirming the results of earlier studies (9, 23).
FIG. 1.
Hyperacetylation of P. falciparum histones. P. falciparum-infected erythrocytes (3D7) were cultured in vitro with different concentrations of control HDACIs (trichostatin A [TSA] and SAHA), the antimalarial control artemisinin (ART), or WR301801. Controls were taken at the start of the experiment (Control [Start]), and a sample was matched to the treatment groups that received the vehicle alone (Control, 0.1% dimethyl sulfoxide). Infected erythrocytes were treated with the indicated concentration of compound for 3.5 h before extraction of histones and separation via SDS-15% PAGE. Histones were detected by Western blotting using membranes probed with anti-(tetra)acetyl-H4 and anti-(di)acetyl-H3 antisera. Silver staining was carried out as a loading control. The arrow indicates histone H2A.Z that is recognized by the anti-(tetra)acetyl-H4 antibody.
FIG. 3.
Dose-related parasite killing by WR301801 in vivo. When administered in a once daily oral regimen, the fraction of parasites remaining relative to controls declines linearly with increasing dose (40 to 640 mg/kg/day) on a log scale. A further increase of the dose to 1,280 mg/kg/day would probably result in a decline in parasitemia below the limit of detection but is impractical.
FIG. 2.
Activity of WR301801 and artemsinin on malarial (A) and mammalian (B) HDAC activity. The vertical axes represent arbitrary fluorescence units (AFU) reflecting the degree of substrate deacetylation. Drug treatments and/or concentrations are indicated on the horizontal axes. WR301801 but not artemisinin exhibited dose-related inhibition of HDAC activity in malaria (A), with an IC50 of between 1 and 10 nM. WR301801 exhibited a similar effect, with an IC50 of 10 nM in mammalian cells (B).
In vivo efficacy of WR301801 as monotherapy in mice and A. lemurinus lemurinus.
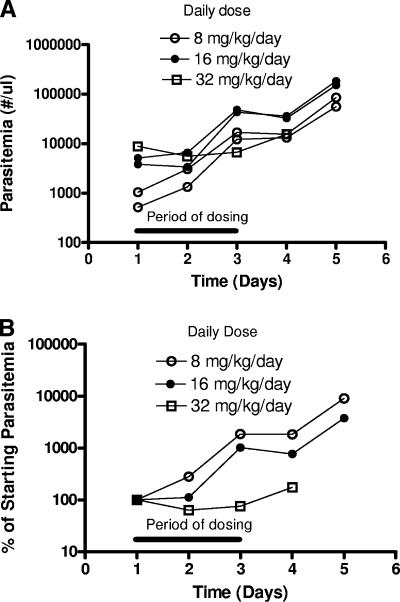
WR301801 as the most potent and selective compound was selected for in vivo studies in the P. berghei-mouse blood schizonticidal model. The compound was tested from doses of 40 to 640 mg/kg/day for 3 days given once per day by oral gavage (Table 3). Control animals typically died or were euthanized at the moribund early endpoint between day 6 to 10 postinfection. No animals were cured using this regimen, although posttreatment parasitemia relative to controls declined with dose. We attempted to induce cures through split-dosing regimes, longer periods of administration, subcutaneous dosing, and administration at the time of parasite inoculation. None of these attempts were successful. Split dosing actually decreased the efficacy marginally, whereas subcutaneous dosing was associated with toxicity (Table 3). The induction of clearance with the once-a-day regimen is theoretically possible at the next highest dose of 1,280 mg/kg/day for 3 days based on the observed dose response (Fig. 3) but not practical. Toxicity was observed only after subcutaneous dosing (Table 3) and in one of five mice given a split oral dose of 130 mg/kg/day for 3 days in preliminary experiments (data not shown). Given that the metabolic instability of WR301801 was greater in rodent than in primate microsomes (see below) and toxicity was not observed after once daily oral administration, we considered a focused set of Aotus experiments to be reasonable. Five Aotus monkeys infected with P. falciparum FVO, in which an earlier course of experimental quinoline methanols had failed to induce cures, were given WR301801 at once daily oral doses of 8, 16, or 32 mg/kg/day for 3 days. Although WR301801 was not effective, there did appear to be a dose-related effect on parasitemia (Fig. 4). The 32-mg/kg/day dose stabilized parasitemia during the treatment period despite a relatively high starting level. In contrast, the parasitemia of animals given lower doses increased. This is evident when parasitemias are averaged for each dose group and normalized relative to starting parasitemias (Fig. 4B). The 32-mg/kg/day dosing regimen is probably equivalent to a 160-mg/kg/day dose in mice (based on similar starting and finishing parasitemias; see Table 6 and Fig. 4).
TABLE 3.
Efficacy and toxicity of WR301801 in P. berghei-infected mice
| Daily dose (mg/kg/day) | Route | No. of days | Single (S) or split (D) daily dose | Fraction of posttreatment control parasitemia on day 6a | No. of animals/total no. in group
|
|
|---|---|---|---|---|---|---|
| No. of cures | Toxic deaths | |||||
| 40 | Oral | 3 | D | 0.32 | 0/5 | 0/5 |
| 80 | Oral | 3 | D | 0.05 | 0/5 | 0/5 |
| 160 | Oral | 3 | D | 0.03 | 0/5 | 0/5 |
| 320 | Oral | 3 | D | 0.004 | 0/5 | 0/5 |
| 640 | Oral | 3 | D | 0.001 | 0/5 | 0/5 |
| 20 | Oral | 3 | S | 0.57 | 0/5 | 0/5 |
| 40 | Oral | 3 | S | 0.12 | 0/5 | 0/5 |
| 80 | Oral | 3 | S | 0.096 | 0/5 | 0/5 |
| 130 | Oral | 7 | D | 0.01 | 0/5 | 0/5 |
| 130 | Subcutaneous | 3 | D | NA | 0/5 | 5/5 (day 5) |
Calculated as the parasitemia in the treatment group on day 6/the parasitemia in control group on day 6. NA, not applicable.
FIG. 4.
Effect of WR301801 on P. falciparum FVO in Aotus. (A) Five Aotus monkeys previously administered a failed regimen of an experimental quinoline methanol were given WR301801 at doses of 8, 16, or 32 mg/kg/day orally for 3 days as indicated by the black bar. Two days after the third dose the animals were rescued with a single 20-mg/kg dose of mefloquine, since the WR301801 treatments failed. However, WR301801 did exhibit a dose-related effect on parasitemia. (B) This is evident when parasitemias are averaged for each dose group and normalized relative to the starting values.
TABLE 6.
Efficacy of WR301801-chloroquine combinations on P. berghei in mice
| Dose (mg/kg/day ×3)
|
Parasitemia (%)
|
Curesa | ||
|---|---|---|---|---|
| WR301801 | Chloroquine | Day 3 | Day 6 | |
| Control | Control | 0.46 | 37 | 0/5 |
| None | 1 | 0.52 | 41 | 0/5 |
| None | 2 | 0.43 | 5.0 | 0/5 |
| None | 4 | 0.36 | 0.004 | 0/5 |
| None | 8 | 0.21 | 0.001 | 0/5 |
| None | 16 | 0.26 | 0.000 | 0/5 |
| None | 64 | 0.38 | 0.002 | 0/5 |
| 320 | None | 0.52 | 0.107 | 0/5 |
| 160 | None | 0.64 | 8.8 | 0/5 |
| 32 | None | 0.38 | 21 | 0/5 |
| 3.2 | None | 0.64 | 40 | 0/5 |
| 0.32 | None | 0.62 | 45 | 0/5 |
| 160 | 2 | 0.5 | 0.36 | 0/5 |
| 32 | 3.6 | 0.44 | 0.08 | 0/5 |
| 3.2 | 4 | 0.6 | 0.029 | 0/5 |
| 0.32 | 4 | 0.72 | 0.024 | 0/5 |
| 12.8 | 16 | 0.52 | 0.000 | 0/5 |
| 52 | 64 | 0.48 | 0.000 | 3/5 |
That is, the number of animals cured/total number of animals tested.
WR301801 metabolism-pharmacokinetic studies.
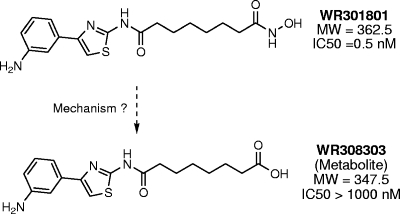
The half-lives of WR301801 in mouse and human liver microsomes were 11 and >60 min, respectively. A metabolic product was identified with a mass that is 15 atomic mass units less than WR301801. The putative, inactive metabolite of WR301801 is WR308301 (Fig. 5). This was later confirmed to be WR308303 by nuclear magnetic resonance analysis. This metabolite was produced in mouse and human microsomes. This metabolite was observed in the absence of microsomal cofactors, suggesting that its metabolism was not mediated by cytochrome P450. It was not a heat degradation product since it was not observed after incubation in buffer at 37°C. In addition, subsequent analyses with rhesus monkey plasma at room temperature and at 37°C failed to show significant decay of either compound. When evaluated for drug interaction potential in vitro, WR301801 showed low to moderate inhibition of 1A2, 2C9, 2C19, and 2D6 (IC50s of >40, 14, 26, and >40 μM, respectively). The potential was more marked for 3A4 (IC50 < 5.7 μM using two substrates). The pharmacokinetics of WR301801 administered orally and subcutaneously at 50 mg/kg was evaluated in mice. The plasma levels of WR301801 and its putative metabolite WR308303 were monitored prospectively in the same sample by using liquid chromatography-tandem mass spectrometry. The standard curves of drug concentration versus peak area were linear within the analytical range with an r2 value of 0.99 or better. Quality control samples at 10 and 100 ng/ml were within 15 and 10% (respectively) of their predicted values with a percent coefficient of variation (%CV) that is less than 10%. Extracts placed in a refrigerated autosampler were stable throughout the course of the analysis. Plasma concentration-time curves are presented in Fig. 6, and the pharmacokinetic parameters are given in Table 4. WR301801 was apparently adequately bioavailable since plasma levels markedly exceeded the in vitro IC90, and the AUC and Cmax values were higher than after subcutaneous dosing. The terminal half-lives of WR301801 were approximately 3.5 and 8.5 h after oral and subcutaneous dosing. The putative acid metabolite WR308303 was present after both oral and subcutaneous dosing (Fig. 6), but only AUC values were calculable due to variability in the concentration-time curves, particularly after subcutaneous dosing.
FIG. 5.
Putative metabolism of WR301801 to the inactive metabolite WR308303. The metabolite was observed in in vitro microsome experiments and in vivo in mice.
FIG. 6.
Plasma concentration-time curves for WR301801 (A) and its metabolite WR308303 (B) after the administration of WR301801 (50 mg/kg) orally and subcutaneously to mice.
TABLE 4.
Pharmacokinetic parameters of WR301801 and its metabolite WR308303 after oral and subcutaneous dosinga
| Parameter | Mean ± SD (%CV)
|
|||
|---|---|---|---|---|
| WR301801
|
Metabolite (WR308303)
|
|||
| Oral | Subcutaneous | Oral | Subcutaneous | |
| Cmax (ng/ml) | 2,500 ± 1,100 (48) | 403 ± 431 (110) | NC | NC |
| Tmax (h) | 0.83 ± 0.29 (35) | 1.3 ± 1.5 (58) | NC | NC |
| AUC (ng·h/ml) | 3,690 ± 1,274 (35) | 3,603 ± 2,597 (72) | 478 ± 229 (48) | 400 ± 590 (150) |
| t1/2 (h) | 3.5 ± 0.71 (20) | 8.5 ± 0.51 (6.0) | NC | NC |
CV, coefficient of variation. Cmax refers to the maximum plasma concentration, and Tmax is the time at which the maximum plasma concentration occurs.
In vitro and in vivo drug combination studies.
For other treatment indications, HDACIs are being evaluated as combination agents. Using isobologram analysis, we investigated the interaction of WR301801 with the following representative members of several important antimalarial drug classes: mefloquine, chloroquine, artemisinin, and azithromyicin. Parasites were continuously exposed to both drugs over 72 h to mimic the likely clinical scenario where parasites are continuously exposed to effective plasma concentrations of the agents for this length of time. As can be see from Table 5, WR301801 exhibited generally antagonistic effects when combined with these agents. In preliminary studies a similar trend toward antagonism with atovaquone was also seen (data not shown). WR301801 was then combined with chloroquine in an in vivo study (Table 6). The drugs were tested alone at several different doses and combined in two different experimental paradigms. First, the fractional inhibitory dose of WR301801 was diluted 1:1, 1:10, 1:100, and 1:1,000 relative to chloroquine where the equi-active doses of the drugs alone were assumed to be 320 mg/kg (WR301801) and 4 mg/kg (chloroquine) based on preliminary data. Second, the mg/kg dose ratio of 1.25 at the 1:100 dilution was maintained, but the actual doses of both drugs given was higher. The first of these combination approaches was attempted since it is possible that only small amounts of HDACI might be required for synergism in vivo. The second approach was attempted because in a normal clinical dosing situation it is more likely that the dose of a deployed HDACI would be closer in absolute mg/kg terms to that of the combination partner (see Discussion). In the dilution mode, the combination of WR301801 and chloroquine is consistent with an additive to antagonistic effect. For example, when chloroquine at 2 mg/kg was combined with WR301801 at 160 mg/kg, the suppression of parasitemia was less than that induced by chloroquine at 4 mg/kg alone. In a more normal clinical dosing mode, cures were actually observed when both agents were combined at a dose of 64 mg/kg chloroquine and 54 mg/kg WR301801, whereas doses of WR301801 as high as 640 mg/kg/day did not cure mice. Chloroquine doses as high as 90 mg/kg/day for 3 days are required to induce cures. Chloroquine is toxic in this model at doses of 160 mg/kg/day for 3 days (data not shown).
TABLE 5.
Sum of FICs for WR301801 combined with conventional antimalarials
| Partner drug | ΣFIC (n, range)a
|
|||
|---|---|---|---|---|
| W2
|
D6
|
|||
| Avg for all dilutions | Maximum avg | Avg for all dilutions | Maximum avg | |
| Mefloquine | 1.7 (3, 1.3-2.5) | 2.1 (3, 1.5-3.2) | 1.8 (3, 1.3-2.1) | 2.2 (3, 1.7-2.5) |
| Chloroquine | 1.5 (2, 1.2-1.7) | 1.9 (2, 1.7-2.1) | 1.5 (2, 1.5-1.5) | 2.2 (2, 2.2-2.2) |
| Azithromycin | 1.2 (3, 1.0-1.3) | 1.5 (3, 1.2-1.7) | 1.3 (3, 1.2-1.4) | 1.7 (3, 1.5-1.9) |
| Artemisinin | 1.7 (3, 1.7-1.8) | 2.1 (3, 2.0-2.1) | 1.8 (3, 1.7-2.0) | 2.1 (3, 1.9-2.3) |
FICs of <0.5, 1, and >2 are normally considered to be synergistic, additive, and synergistic, respectively. n, number of experiments.
DISCUSSION
In this study we profiled the antimalarial activity and pharmacology of a group of phenylthiazolyl hydroxamate-based histone deacetylase inhibitors, with a focus on the most potent compound, WR301801. This compound exhibited an IC50 of 0.5 to 1.5 nM against several drug-resistant strains of P. falciparum. Prior studies demonstrated WR301801 to be a mammalian HDACI (23), an observation confirmed here (Fig. 2). Here we show that WR301801, like SAHA, trichostatin A, and 2-aminosuberic acid analogs (1), induces hyperacetylation of P. falciparum histones (Fig. 1) and inhibits parasite HDAC activity (Fig. 2). WR301801 has a key advantage over these other compounds: much greater selectivity at the cellular level (Table 7). In vivo, WR301801 was orally bioavailable, suppressed parasitemia when given as monotherapy, and cured infections when administered with subtherapeutic doses of chloroquine. WR301801 exhibited a short half-life in both in vivo and in vitro microsomal studies and appeared to be metabolized via hydrolysis of its hydroxamate group. As outlined in Table 7, WR301801 represents an improvement on previously described P. falciparum HDACIs. WR301801 is by an order of magnitude the most potent HDACI tested to date. It exhibits markedly greater selectivity than other compounds. The in vivo potency of WR301801 relative to apicidin and SBHA is difficult to assess; these compounds have been tested in different ways in each respective study (Table 7). However, WR301801 is probably more active in vivo than apicidin, since both drugs exhibited similar suppression of parasitemia on day 6 postinfection (>95%), and yet apicidin was administered in a prophylactic mode (starting 2 h postinfection) and given intraperitoneally (neither was the case for WR301801; Table 7). WR301801 may therefore serve as a useful scaffold from which to develop a new class of antimalarial drugs.
TABLE 7.
Potency, selectivity, and in vivo efficacy of WR301801 compared to other HDACIs
| Compound | Potency (nM)a | SI (cells) | In vivo activity; 50% curative dose (CD50) in mg/kg/day | Source or reference |
|---|---|---|---|---|
| WR301801 | 0.6 | >1,000 | CD50 > 640 mg/kg/day for 3 days perorally (monotherapy treatment); CD50 ∼ 52 mg/kg/day for 3 days perorally (combination treatment) | This study |
| 2ASA analogs | 13 | 5-118 | NTd | 1 |
| YC-V-40 | 17 | >60 | NT | 9 |
| Trichostatin A | 11 | 25 | NT | 1, 2 |
| SAHA | 110 | 22 | NT | This study |
| Apicidinb | 125 | ∼1 | CD50 > 100 mg/kg/day for 3 days intraperitoneally (split daily dose, monotherapy prophylactic mode) | 11 |
| SBHA | 1,300 | >230c | CD50 > 400 mg/kg/day for 3 days intraperitoneally (split dose, monotherapy treatment) | 2, 4 |
That is, the lowest IC50.
The MIC was reported as 125 mg/ml. The SI is approximately 1 since the reported activity against mammalian HDAC is 50 to 100 nM.
Based on a reported IC50 of >300 μM against mammalian cells.
NT, not tested.
There is not yet a consensus on how HDACIs might hypothetically be deployed as antimalarial agents. The development of antimalarial drugs is entering a new phase. Effective artemisinin-based combination therapies (ACTs) have been deployed (33), so the research and development focus is broadening to include a wider array of malaria indications. Medicines for Malaria Venture (31) now describes seven target product profiles, including uncomplicated malaria, acute/severe malaria, P. vivax radical cure, intermittent preventive treatment (IPT) in children, IPT in pregnancy, malaria prevention and standby treatment for travelers. Based on the mechanisms of action of HDACIs in mammalian cells, use for IPT is probably not appropriate. The prophylaxis and severe malaria indications require specific technical attributes (rapidity of action/excellent tolerability, etc.) for which there are as yet insufficient data to determine whether HDACIs would be a logical match. The uncomplicated malaria and traveler standby treatment indications seem the most appropriate at this stage given the results we and others have shown. If the currently deployed ACTs fail en mass, it will likely be due to the development of artemisinin resistance. It therefore seems appropriate to explore the possibility that HDACIs might serve as a functional replacement for artemisinin compounds in future drug combinations regimens.
What kind of HDACI drug combination might be used in light of the in vitro and in vivo drug combination data? In the in vitro isobologram studies, WR301801 exhibited apparent antagonism with chloroquine, atovaquone, azithromycin, and mefloquine. This also appeared to be the case in vivo when the fractional inhibitory dose of the WR301801 component of the combination was diluted relative to choroquine. In this respect, the in vitro data can be seen as predictive of some of the in vivo data. If this trend remains true, it would indicate that combination of small dose of an HDACI with a clinically effective dose of a conventional antimalarial might have limited utility. However, there was a clear difference in outcomes when the dose combination used approached mg/kg parity; the “64/52 mg/kg/day for 3 days” chloroquine-WR301801 combination resulted in cures where 10-fold-higher doses of WR301801 were ineffective and chloroquine doses of 90 mg/kg/day for 3 days were required to induce cures. These data are generally supportive of the use of HDACIs in future drug combinations. We do not know how to interpret these data in the context of the antagonism observed in vitro. The in vivo situation is complicated by metabolism, species differences, and changing ratios of WR301801 and chloroquine plasma concentrations over time. The utility of the isobologram approach for predicting in vivo outcomes may, perhaps unsurprisingly, be limited. Our future combination studies will focus on mouse studies in order to provide the rationale for a more definitive set of Aotus experiments (discussed below).
A drug deployed for treatment of uncomplicated malaria must be effective in an oral administration route, in a once-daily regimen, for three consecutive days (31). The expected cure rate is >99% (31). However, this is not the case for the artemisinin component of ACTs administered as monotherapy. Artemisinin compounds administered even as a 7-day regimen result in a 10% failure rate (33), and this failure rate is higher with shorter regimens. However, the rapidity of action of artemisinins allows dramatic reductions in the parasite burden (33). When combined, this enhances the clinical efficacy of their combination partner even against a background of resistance (e.g., mefloquine). How then might we test the utility of this approach for an HDACI? Experimentally, the most appropriate model would be evaluation of a drug combination against drug-resistant P. falciparum strain in Aotus monkeys. Strains resistant to antifolates and chloroquine, but not the currently used ACTs, have been adapted to Aotus. Therefore, chloroquine is a logical partner drug with which to test the hypothesis in a proof-of-principle experiment. The combination experiments described here represent the first in a series required to establish a rationale for future Aotus testing. However, WR301801 is not the compound with which this proof of principle should be demonstrated since the drug does not clear parasitemia like artemisinin when used alone as monotherapy. We would expect that WR301801 would clear parasitemias at a dose of 1,280 mg/kg/day in mice (Fig. 4), and we know that a dose of 32 mg/kg/day for 3 days is tolerated in Aotus (Fig. 5) and is equivalent to 160 mg/kg/day for 3 days in mice (Table 6). A new HDACI therefore would need to be at least eightfold more potent than WR301801 in vivo and similarly well tolerated to be considered for progression to Aotus studies.
From a medicinal chemistry perspective, much can be done to meet this objective. HDACIs are generally considered to have a cap group, linker region, and zinc binding group (ZBG; see Fig. 7). WR301801 is already as potent or more potent than existing antimalarials and is relatively selective at a whole-cell level (discussed in more detail below). Therefore, further derivatization of the head or linker regions to yield these properties may not be productive. WR301801 appears to be bioavailable. Thus, our hypothesis is that metabolic instability, mediated through hydrolysis of the hydroxamate group, is one of the reasons for the lack of desired efficacy in vivo. There are two logical approaches to address this deficiency. First, the half-life of WR301801 in mouse microsomes in vitro is markedly shorter than that of SAHA (data not shown), despite the fact that both compounds contain a hydroxamate ZBG. Therefore, a spectrum of metabolic stability may exist within this class of compounds, so WR308298, WR308291, WR308296, and WR308294 may be worth testing in vivo if it turns out they have greater metabolic stability in vitro than WR301801. Alternatively, it may be worth attempting to replace the hydroxamate moiety with an alternative ZBG. This approach has been used successfully by our group for alternate indications (8, 22).
FIG. 7.
Structure of YC-2-88 in the context of a typical HDACI. HDACIs typically incorporate a ZBG and cap groups separated by a linker region.
We have not yet considered the issue of the mechanism of action, toxicity, and selectivity in detail. Earlier studies have shown that WR301801 is a nM inhibitor of mammalian HDAC (9, 23). This was confirmed in a functional assay here (Fig. 2). Putatively, this will also be the case in P. falciparum, as suggested indirectly by the hyperacetylation and functional data reported here (Fig. 1 and 2). As with most antimalarial drugs, it is likely that WR301801 will also exhibit additional mechanisms of action. The issue of the selectivity of HDACIs is complicated. There are 11 zinc-based isoforms of mammalian HDAC and at least two class I/II HDACs are expressed in blood-stage P. falciparum parasites (5, 18, 38). Development of an HDACI selective for malaria parasites at the enzymatic level could therefore be a daunting task, although recent in silico modeling studies have provided useful information for rational development of new compounds designed to target P. falciparum HDAC-1 (1). The HDACIs in clinical development do not target the known mammalian isozymes with any great selectivity (38). Rather, their selectivity is based on the biological context; cancer cells are thought to be more vulnerable to intermittent HDAC inhibition than normal cells. Similarly, in our view, selectivity of HDACIs for infectious diseases should be determined at the cellular level rather than enzyme level. In this respect, WR301801 exhibits suitable selectivity. Our in vivo and pharmacokinetic studies offer some insights into the toxicology of the compound in vivo. Substantial toxicity was observed only after subcutaneous dosing. The half-lives of WR301801 after oral and subcutaneous doses of 50 mg/kg were 3.5 and 8.5 h, respectively (Table 4). This suggests that the duration of exposure may contribute to toxicity. It will be important to determine whether this is the case in any new series of more metabolically stable HDACIs.
Supplementary Material
Acknowledgments
This manuscript was reviewed by the Walter Reed Army Institute of Research and the U.S. Army Medical Research and Materiel Command, and there is no objection to its publication or dissemination. The opinions expressed herein are those of the authors and do not reflect the views of opinions of the Department of the Army and the Department of Defense. All animal experiments were conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhere to the principles stated in the Guide for the Care and Use of Laboratory Animals (32).
Y.C. synthesized the compounds designated with the prefix YC.
We gratefully acknowledge the technical assistance of Jason Sousa, Arsen Gaysin, Subhasish Tapadar, Miriam Lopez Sanchez, Norma Roncal, Ricky Denull, Drew Reinbold, Anchalee Tungtaeng, and Constance Asher, without whose efforts the execution of the work contained herein would not have been feasible. We thank William McCalmont and Rong He for helpful review of the manuscript.
K.T.A. was supported by Griffith University (GURG) and the Queensland Institute of Medical Research (Directors Initiative Funding). T.N.T. was supported by an ANZ Trustees Medical Research Scholarship. The WRAIR, AFRIMS, and Gorgas Institute component of this effort was supported by the U.S. Military Infectious Diseases Research Program through the funding of core screening capabilities.
Footnotes
Published ahead of print on 21 July 2008.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Andrews, K. T., T. N. Tran, A. J. Lucke, P. Kahnberg, G. T. Le, G. M. Boyle, D. L. Gardiner, T. S. Skinner-Adams, and D. P. Fairlie. 2008. Potent antimalarial activity of histone deacetylase inhibitor analogues. Antimicrob. Agents Chemother. 52:1454-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, K. T., A. Walduck, M. J. Kelso, D. P. Fairlie, A. Saul, and P. G. Parsons. 2000. Anti-malarial effect of histone deacetylation inhibitors and mammalian tumour cytodifferentiating agents. Int. J. Parasitol. 30:761-768. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122-130. [DOI] [PubMed] [Google Scholar]
- 4.Brinkmann, H., A. L. Dahler, C. Popa, M. M. Serewko, P. G. Parsons, B. G. Gabrielli, A. J. Burgess, and N. A. Saunders. 2001. Histone hyperacetylation induced by histone deacetylase inhibitors is not sufficient to cause growth inhibition in human dermal fibroblasts. J. Biol. Chem. 276:22491-22499. [DOI] [PubMed] [Google Scholar]
- 5.Butler, K. V., and A. P. Kozikowski. 2008. Chemical origins of isoform selectivity in histone deacetylase inhibitors. Curr. Pharm. Des. 14:505-528. [DOI] [PubMed] [Google Scholar]
- 6.Carducci, M. A., J. Gilbert, M. K. Bowling, D. Noe, M. A. Eisenberger, V. Sinibaldi, Y. Zabelina, T. L. Chen, L. B. Grochow, and R. C. Donehower. 2001. A phase I clinical and pharmacological evaluation of sodium phenylbutyrate on an 120-h infusion schedule. Clin. Cancer Res. 7:3047-3055. [PubMed] [Google Scholar]
- 7.Caridha, D., D. Yourick, M. Cabezas, L. Wolf, T. H. Hudson, and G. S. Dow. 2008. Mefloquine-induced disruption of calcium homeostasis in mammalian cells is similar to that induced by ionomycin. Antimicrob. Agents Chemother. 52:684-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, B., P. A. Petukhov, M. Jung, A. Velena, E. Eliseeva, A. Dritschilo, and A. P. Kozikowski. 2005. Chemistry and biology of mercaptoacetamides as novel histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 15:1389-1392. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Y., M. Lopez-Sanchez, D. N. Savoy, D. D. Billadeau, G. S. Dow, and A. P. Kozikowski. 2008. A series of potent and selective, triazolylphenyl-based histone deacetylases inhibitors with activity against pancreatic cancer cells and Plasmodium falciparum. J. Med. Chem. 51:3437-3448. [DOI] [PubMed] [Google Scholar]
- 10.Cui, L., and J. Miao. 2007. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob. Agents Chemother. 51:488-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darkin-Rattray, S. J., A. M. Gurnett, R. W. Myers, P. M. Dulski, T. M. Crumley, J. J. Allocco, C. Cannova, P. T. Meinke, S. L. Colletti, M. A. Bednarek, S. B. Singh, M. A. Goetz, A. W. Dombrowski, J. D. Polishook, and D. M. Schmatz. 1996. Apicidin: a novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 93:13143-13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dow, G. S., T. N. Heady, A. K. Bhattacharjee, D. Caridha, L. Gerena, M. Gettayacamin, C. A. Lanteri, N. Obaldia III, N. Roncal, T. Shearer, P. L. Smith, A. Tungtaeng, L. Wolf, M. Cabezas, D. Yourick, and K. S. Smith. 2006. Utility of alkylaminoquinolinyl methanols as new antimalarial drugs. Antimicrob. Agents Chemother. 50:4132-4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dow, G. S., M. L. Koenig, L. Wolf, L. Gerena, M. Lopez-Sanchez, T. H. Hudson, and A. K. Bhattacharjee. 2004. The antimalarial potential of 4-quinolinecarbinolamines may be limited due to neurotoxicity and cross-resistance in mefloquine-resistant Plasmodium falciparum strains. Antimicrob. Agents Chemother. 48:2624-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glenn, M. P., P. Kahnberg, G. M. Boyle, K. A. Hansford, D. Hans, A. C. Martyn, P. G. Parsons, and D. P. Fairlie. 2004. Antiproliferative and phenotype-transforming antitumor agents derived from cysteine. J. Med. Chem. 47:2984-2994. [DOI] [PubMed] [Google Scholar]
- 16.Grunstein, M. 1997. Histone acetylation in chromatin structure and transcription. Nature 389:349-352. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, J. D., R. A. Dennull, L. Gerena, M. Lopez-Sanchez, N. E. Roncal, and N. C. Waters. 2007. Assessment and continued validation of the malaria Sybr green I-based fluorescence assay for use in malaria drug screening. Antimicrob. Agents Chemother. 51:1926-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi, M. B., D. T. Lin, P. H. Chiang, N. D. Goldman, H. Fujioka, M. Aikawa, and C. Syin. 1999. Molecular cloning and nuclear localization of a histone deacetylase homologue in Plasmodium falciparum. Mol. Biochem. Parasitol. 99:11-19. [DOI] [PubMed] [Google Scholar]
- 19.Kahnberg, P., A. J. Lucke, M. P. Glenn, G. M. Boyle, J. D. Tyndall, P. G. Parsons, and D. P. Fairlie. 2006. Design, synthesis, potency, and cytoselectivity of anticancer agents derived by parallel synthesis from alpha-aminosuberic acid. J. Med. Chem. 49:7611-7622. [DOI] [PubMed] [Google Scholar]
- 20.Kelly, W. K., V. M. Richon, O. O'Connor, T. Curley, B. MacGregor-Curtelli, W. Tong, M. Klang, L. Schwartz, S. Richardson, E. Rosa, M. Drobnjak, C. Cordon-Cordo, J. H. Chiao, R. Rifkind, P. A. Marks, and H. Scher. 2003. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin. Cancer Res. 9:3578-3588. [PubMed] [Google Scholar]
- 21.Kozikowski, A. P., Y. Chen, A. Gaysin, D. D. Billadeau, and K. H. Kim. 2008. Chemistry, biology, and QSAR studies of substituted biaryl hydroxamates and mercaptoacetamides as HDAC inhibitors: nanomolar potency inhibitors of pancreatic cancer cell growth. ChemMedChem 3:487-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozikowski, A. P., Y. Chen, A. Gaysin, B. Chen, M. A. D'Annibale, C. M. Suto, and B. C. Langley. 2007. Functional differences in epigenetic modulators-superiority of mercaptoacetamide-based histone deacetylase inhibitors relative to hydroxamates in cortical neuron neuroprotection studies. J. Med. Chem. 50:3054-3061. [DOI] [PubMed] [Google Scholar]
- 23.Kozikowski, A. P., S. Tapadar, D. N. Luchini, K. H. Kim, and D. D. Billadeau. 2008. Use of the nitric oxide cycloaddition (NOC) reaction for molecular probe generation: a new class of enzyme selective histone deacetylase inhibitors (HDACIs) showing picomolar activity at HDAC6. J. Med. Chem. 51:4370-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurdistani, S. K., and M. Grunstein. 2003. Histone acetylation and deacetylation in yeast. Nat. Rev. Mol. Cell. Biol. 4:276-284. [DOI] [PubMed] [Google Scholar]
- 25.Marks, P., R. A. Rifkind, V. M. Richon, R. Breslow, T. Miller, and W. K. Kelly. 2001. Histone deacetylases and cancer: causes and therapies. Nat. Rev. Cancer 1:194-202. [DOI] [PubMed] [Google Scholar]
- 26.Marks, P. A., and M. Dokmanovic. 2005. Histone deacetylase inhibitors: discovery and development as anticancer agents. Expert Opin. Investig. Drugs 14:1497-1511. [DOI] [PubMed] [Google Scholar]
- 27.Marks, P. A., V. M. Richon, T. Miller, and W. K. Kelly. 2004. Histone deacetylase inhibitors. Adv. Cancer Res. 91:137-168. [DOI] [PubMed] [Google Scholar]
- 28.Marks, P. A., V. M. Richon, and R. A. Rifkind. 2000. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J. Natl. Cancer Inst. 92:1210-1216. [DOI] [PubMed] [Google Scholar]
- 29.Miao, J., Q. Fan, L. Cui, and J. Li. 2006. The malaria parasite Plasmodium falciparum histones: organization, expression, and acetylation. Gene 369:53-65. [DOI] [PubMed] [Google Scholar]
- 30.Milhous, W. K., N. F. Weatherly, J. H. Bowdre, and R. E. Desjardins. 1985. In vitro activities of and mechanisms of resistance to antifol antimalarial drugs. Antimicrob. Agents Chemother. 27:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medicines for Malaria Venture. 2008, posting date. Medicines for malaria venture (MMV) product profiles for antimalarial drugs. Medicines for Malaria Venture, Geneva, Switzerland. http://www.mmu.org/IMG/pdf/PRODUCT_PROFILE_with_logo.pdf/.
- 32.National Academy Press. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 33.Nosten, F., and N. J. White. 2007. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 77:181-192. [PubMed] [Google Scholar]
- 34.Obaldia, N., III. 1991. Detection of Klebsiella pneumoniae antibodies in Aotus l. lemurinus (Panamanian owl monkey) using an enzyme linked immunosorbent assay (ELISA) test. Lab. Anim. 25:133-141. [DOI] [PubMed] [Google Scholar]
- 35.Sasakawa, Y., Y. Naoe, T. Inoue, T. Sasakawa, M. Matsuo, T. Manda, and S. Mutoh. 2002. Effects of FK228, a novel histone deacetylase inhibitor, on human lymphoma U-937 cells in vitro and in vivo. Biochem. Pharmacol. 64:1079-1090. [DOI] [PubMed] [Google Scholar]
- 36.Smilkstein, M., N. Sriwilaijaroen, J. X. Kelly, P. Wilairat, and M. Riscoe. 2004. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 48:1803-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Struhl, K., and Z. Moqtaderi. 1998. The TAFs in the HAT. Cell 94:1-4. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, T., and N. Miyata. 2005. Non-hydroxamate histone deacetylase inhibitors. Curr. Med. Chem. 12:2867-2880. [DOI] [PubMed] [Google Scholar]
- 39.Wolffe, A. P., and D. Guschin. 2000. Review: chromatin structural features and targets that regulate transcription. J. Struct. Biol. 129:102-122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.