Abstract
Ceftobiprole medocaril is the parenteral prodrug of ceftobiprole, a novel pyrrolidinone broad-spectrum cephalosporin with in vitro and in vivo bactericidal activities against methicillin-resistant Staphylococcus aureus (MRSA) and penicillin-resistant Streptococcus pneumoniae (PRSP). We have used murine thigh and lung infection models in neutropenic and normal mice to characterize the in vivo pharmacokinetic (PK)-pharmacodynamic (PD) activities of ceftobiprole against multiple strains of S. aureus (including MRSA), S. pneumoniae (including PRSP), and gram-negative bacilli. Serum levels of ceftobiprole following the administration of multiple doses were determined by a microbiological assay. In vivo bactericidal activities and postantibiotic effects (PAEs) of ceftobiprole against MRSA and PRSP strains were determined from serial CFU/thigh values following single doses of ceftobiprole (40 and 160 mg/kg of body weight). Dose fractionation studies were used to determine which PK-PD index correlated best with activity. Magnitudes of the PK-PD indices were calculated from MICs and PK parameters. A sigmoid dose-response model was used to estimate the dose (mg/kg/24 h) required to achieve a static and 2-log10 kill effects over 24 h. PK results showed area under the concentration-time curve/dose values of 1.8 to 2.8 and half-lives of 0.29 to 0.51 h. MICs ranged from 0.015 to 2 μg/ml. Ceftobiprole demonstrated time-dependent killing; its in vivo PAEs varied from 3.8 h to 4.8 h for MRSA and from 0 to 0.8 h for PRSP. The time above MIC (T > MIC) correlated best with efficacy for both MRSA and PRSP. The T > MIC values required for the static doses were significantly longer (P < 0.001) for Enterobacteriaceae (36 to 45%) than for S. aureus (14 to 28%) and S. pneumoniae (15 to 22%). The drug showed activities in the lung model similar to those in the thigh model. The presence of neutrophils significantly enhanced the activity of ceftobiprole against S. pneumoniae but only slightly against Klebsiella pneumoniae. Based on its PD profile, ceftobiprole is a promising new β-lactam agent with activity against gram-negative and gram-positive organisms including MRSA and PRSP.
Ceftobiprole medocaril is the water-soluble prodrug of ceftobiprole, a novel cephalosporin with potent activity in vitro against resistant pathogens including methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis, and penicillin-resistant Streptococcus pneumoniae (PRSP) (14). Ceftobiprole has been shown to be effective in animal models of abscess, pneumonia, and aortic valve endocarditis caused by multidrug-resistant pathogens including MRSA and PRSP (5, 13, 14, 16). In vitro and in vivo animal studies have also demonstrated its good activity against clinical isolates of gram-negative bacteria (11, 12, 14, 15).
The goals of the present experiments were to (i) characterize killing and postantibiotic effects (PAEs) of ceftobiprole, (ii) determine which pharmacokinetic (PK)-pharmacodynamic (PD) index best correlates with efficacy, (iii) determine whether the magnitude of the PK-PD index needed for efficacy varies among different pathogens and at different sites of infection, and (iv) determine whether the activity of ceftobiprole is enhanced by the presence of neutrophils.
(Part of this work was presented at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 2000.)
MATERIALS AND METHODS
Bacterial strains and antibiotic.
A total of 19 organisms, including 6 strains of S. pneumoniae (4 penicillin-resistant strains and 1 ciprofloxacin-resistant strain), 8 strains of S. aureus (5 methicillin-resistant and 3 methicillin-sensitive strains), 1 strain of Escherichia coli, 1 strain of Klebsiella pneumoniae, 2 strains of Enterobacter cloacae, and 1 strain of Pseudomonas aeruginosa, were used in the study. Ceftobiprole was provided by F. Hoffman, La Roche Ltd., Basel, Switzerland, as a standard powder for assays and as ceftobiprole medocaril, along with the appropriate buffers for administration to mice.
In vitro antibiotic susceptibility testing.
The MICs of ceftobiprole for the various strains were determined by broth microdilution methods approved by the Clinical and Laboratory Standards Institute (formerly NCCLS) (21).
Murine infection models.
The neutropenic mouse thigh infection model has been used extensively and has facilitated the quantitative determination of PK-PD indices useful for the prediction of antibiotic efficacy in patients (9). Animals for the present studies were maintained in accordance with criteria of the American Association for Accreditation of Laboratory Animal Care (1). All animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial Veterans Hospital.
Six-week-old, specific-pathogen-free, female CD1 (ICR/Swiss) mice weighing 23 to 27 g were used for all studies. Mice were rendered neutropenic (<100 neutrophils/mm3) by intraperitoneal injections of cyclophosphamide given 4 days (at 150 mg/kg of body weight) and 1 day (at 100 mg/kg) before thigh infection. Studies have shown that this regimen produces neutropenia in this model for 4 to 5 days (19, 25). Thigh infections with each of the strains were produced by injecting 0.1 ml of a 1:10 dilution of a 108-CFU/ml inoculum into thighs 2 h prior to starting treatment with ceftobiprole (2, 3). Lung infection with K. pneumoniae ATCC 43816 was produced by a 45-min exposure to an aerosol of 109 CFU/ml generated with a Colison nebulizer (18); treatment was started 10 h later. Lung infection with S. pneumoniae ATCC 10813 was produced by the intranasal instillation of about 108 CFU of bacteria in 50 μl 4 h before starting therapy.
Ceftobiprole PK.
Single-dose serum PK studies of ceftobiprole were performed in neutropenic thigh-infected mice given subcutaneous doses (0.2 ml/dose) of ceftobiprole medocaril (10, 40, and 160 mg/kg). For each of the doses examined, two groups of three mice were sampled by retro-orbital puncture at 1.5- to 2-h intervals over 6 h (sample times included 0.5, 1, 2, 3, 4, 5, and 6 h). Individual animals were sampled three or four times.
Bioassay.
Serum ceftobiprole concentrations were determined by an agar diffusion microbiological assay using Escherichia coli NIH-J as the test organism. The assay was linear from 0.25 to 256 μg/ml. The lower and upper limits of detection of the assay were 0.17 and 256 μg/ml, respectively. Intraday and interday variation was less than 15%. Protein binding in the serum of infected neutropenic mice was performed with ultrafiltration methods (3).
Treatment protocols.
Two hours to 10 h after infection with the study organisms, neutropenic mice were treated for 24 h with subcutaneous doses of ceftobiprole medocaril (0.010 to 160 mg/kg) administered every 3, 4, 6, 8, 12, and/or 24 h. Untreated controls were sacrificed at the start of treatment and after 24 h. Thighs and lungs were removed and homogenized in iced saline. Aliquots of serial 10-fold dilutions were plated onto Mueller-Hinton agar or blood agar for CFU determinations.
Data analysis.
PK constants, including elimination half-life (t1/2), area under the concentration-time curve (AUC), and peak concentration (Cmax), were calculated using a one-compartmental model. The PAE was calculated by subtracting the time it took the infecting organisms to increase by 1 log10 CFU/thigh in untreated animals from the time it took the pathogens to increase by the same amount in treated animals after serum drug levels fell below the MIC of the drug for the pathogens (PAE = T − C, where T is the time for a 1-log10 increase in growth after treatment after serum levels have fallen below the MIC and C is the time for a 1-log10 increase in control growth) (2). A sigmoid dose-response model derived from the four-parameter Hill equation was used to calculate the dose of ceftobiprole producing a net bacteriostatic effect and 2-log10 kill over 24 h (static and 2-log kill doses) (3, 17). The time above MIC (T > MIC) values for each static and 2-log kill dose were estimated from PK and MICs. The correlation between efficacy and each of the three PK-PD indices (T > MIC, AUC/MIC, and peak/MIC) studied was determined by nonlinear least-squares multivariate regression performed with Sigma Stat software (Jandel Scientific Software, San Rafael, CA). The coefficient of determination (R2) was used to estimate the percentage of the total variance in efficacy that could be attributed to its regression with each PK-PD index. Combined data for all eight staphylococci, six pneumococci, and five gram-negative bacilli using the T > MIC for each of the doses studied were also determined by nonlinear least-squares multivariate regression. Data among groups (staphylococci, pneumococci, and Enterobacteriaceae) were compared by analysis of variance.
RESULTS
In vitro antibiotic susceptibility.
The MICs of ceftobiprole for the 19 strains used in the study ranged from 0.015 μg/ml to 2.0 μg/ml, as shown in Table 1.
TABLE 1.
Comparative efficacies of ceftobiprole against various pathogensa
| Organism | MIC (μg/ml) | Static dose (mg/kg) and dosing interval | Static-dose T > MIC (% of dosing interval) | 2-Log kill dose (mg/kg) and dosing interval | 2-Log kill dose T > MIC (% of dosing interval) |
|---|---|---|---|---|---|
| S. pneumoniae strains | |||||
| ATCC 10813 | 0.03 | 0.180 every 6 h | 19.4 | 0.552 every 6 h | 24.8 |
| 0.307 every 6 h | 0.450 every 6 h | ||||
| MNO-418 | 0.015 | 0.238 every 6 h | 21.3 | 0.624 every 6 h | 31.8 |
| CDC 145 | 0.25 | 0.956 every 6 h | 15.2 | 3.27 every 6 h | 23.0 |
| CDC 1293 | 0.5 | 2.72 every 6 h | 16.3 | 3.55 every 6 h | 18.4 |
| CDC 1329 | 0.25 | 2.93 every 6 h | 22.2 | 5.51 every 6 h | 27.0 |
| CDC 673 | 1.0 | 6.68 every 6 h | 18.5 | 29.0 every 6 h | 29.7 |
| Total (mean ± SD) | 18.8 ± 2.7 | 25.8 ± 4.8 | |||
| S. aureus strains | |||||
| ATCC 33591 | 1.0 | 7.91 every 6 h | 19.1 | 15.3 every 6 h | 24.4 |
| MRSA WIS-1 | 1.0 | 16.1 every 6 h | 25.0 | 53.9 every 6 h | 39.1 |
| MRSA 11888 | 2.0 | 22.6 every 6 h | 22.5 | 40.2 every 6 h | 27.4 |
| MRSA 12248 | 2.0 | 29.2 every 6 h | 24.5 | 50.5 every 6 h | 31.6 |
| MRSA 22115 | 1.0 | 16.9 every 6 h | 25.4 | 34.4 every 6 h | 29.6 |
| ATCC 22923 | 0.5 | 2.03 every 6 h | 14.1 | 10.3 every 6 h | 26.5 |
| ATCC 29213 | 0.5 | 3.78 every 6 h | 18.9 | 15.4 every 6 h | 29.9 |
| ATCC Smith | 0.5 | 4.21 every 6 h | 19.6 | 9.60 every 6 h | 25.9 |
| Total (mean ± SD) | 21.1 ± 3.9 | 29.3 ± 4.6 | |||
| Enterobacteriaceae | |||||
| E. coli ATCC 25922 | 0.06 | 8.25 every 6 h | 41.9 | 42.4 every 6 h | 57.8 |
| 10.4 every 6h | 44.7 every 6 h | ||||
| K. pneumoniae ATCC 43816 | 0.06 | 6.61 every 6 h | 41.2 | 43.8 every 6 h | 59.2 |
| 8.57 every 6 h | 47.5 every 6 h | ||||
| E. cloacae 2249 | 2.0 | 22.2 every 3 h | 44.6 | 155 every 3 h | 100 |
| E. cloacae 4567 | 0.5 | 3.28 every 3 h | 35.6 | 4.67 every 3 h | 40.9 |
| Total (mean ± SD) | 40.8 ± 3.8 | 64.5 ± 25.1 | |||
| P. aeruginosa ATCC 27853 | 2.0 | 25.2 every 3 h | 46.7 | 110 every 3 h | 98.8 |
The R2 for the individual organisms varied from 0.932 to 0.999 (mean of 0.983).
PK.
The PK characteristics of ceftobiprole in infected neutropenic mice following single subcutaneous doses of 10, 40, or 160 mg/kg ceftobiprole medocaril are shown in Table 2. Over the dose range studied, PK parameters were dose dependent, with the t1/2 increasing approximately 1.5-fold, from 20 min to 31 min, as the dose rose from 40 mg/kg to 160 mg/kg. The AUC/dose values for the escalating single doses ranged from 0.585 to 1.33, and the Cmax/dose values decreased from 1.08 to 0.90. Because protein binding of ceftobiprole in mouse serum was less than 10%, PK calculations throughout the report incorporate total drug concentrations.
TABLE 2.
Single-dose PK of ceftobiprole in mice infected by thigh
| Dose (mg/kg) | Mean Cmax (mg/liter) ± SD | t1/2 (min) | AUC (mg·h/liter) |
|---|---|---|---|
| 100 | 10.8 ± 3.6 | 19.0 | 5.85 |
| 40 | 31.2 ± 6.7 | 19.8 | 27.8 |
| 160 | 144 ± 31 | 31.7 | 213 |
In vivo PAE.
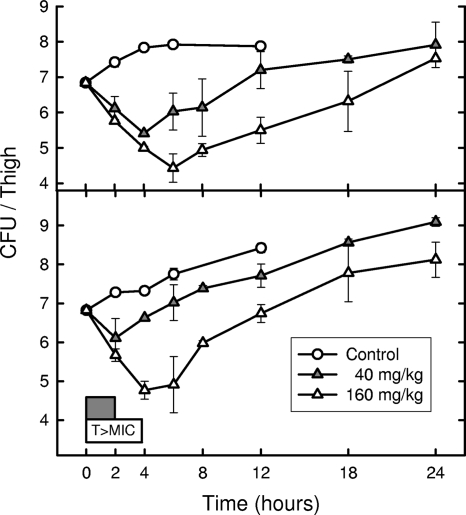
The time course of antimicrobial activity with S. aureus ATCC 33591 and S. pneumoniae CDC 673 following single doses of ceftobiprole at 40 and 160 mg/kg are shown in Fig. 1. The killing of both organisms by ceftobiprole showed minimal enhancement with higher drug concentrations. Untreated controls of the MRSA and PRSP strains required 3.9 and 4.5 h, respectively, to grow 1 log10 CFU/thigh. Escalating doses of ceftobiprole produced in vivo PAEs of 3.8 to 4.8 h against S. aureus ATCC 33591. In a similar study with S. pneumoniae CDC 673 in vivo, PAEs of only 0 to 0.8 h were demonstrated.
FIG. 1.
Killing and regrowth of S. aureus ATCC 33591 and S. pneumoniae CDC 673 over time in the thighs of neutropenic mice after exposure to 2 doses of ceftobiprole. Each point represents the mean of data for four thighs. The widths of the boxes at the bottom of the graph represent the duration of time that serum concentrations were above the MIC (1.0 μg/ml for both strains) for each of the 2 doses.
PK-PD index determination.
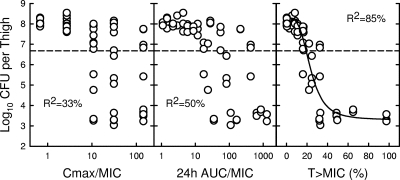
At baseline (the start of therapy), mice had between 6.5 and 6.8 log10 CFU/thigh of S. aureus ATCC 33591. The relationships between microbiological effect and each of the PD parameters, peak/MIC, 24-h AUC/MIC, and percent T > MIC for S. aureus ATCC 33591, are shown in Fig. 2. The PK-PD index that best correlated with in vivo efficacy was the percentage of time that serum concentrations were above the MIC, with an R2 value of 85% (compared with R2 values of 33% for peak/MIC and 50% for 24-h AUC/MIC). A similar analysis of a study with S. pneumoniae CDC 673 (starting inoculum of 6.2 to 7.2 log10 CFU/thigh) confirmed the good correlation of the percent T > MIC with in vivo efficacy (R2 values of 67% for T > MIC compared to 8% for peak/MIC and 18% for 24-h AUC/MIC).
FIG. 2.
Correlation of ceftobiprole PK-PD indices with efficacy against S. aureus ATCC 33591. Each circle represents the mean data per mouse from two thighs.
Twenty-four-hour static dose determination.
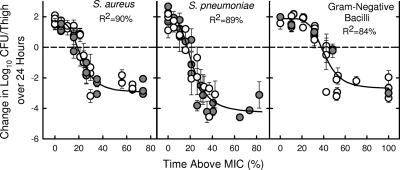
The dose-response curves for the various pathogens plotted as T > MIC versus efficacy are shown in Fig. 3. The starting inoculums in these studies ranged from 5.7 to 7.5 log10 CFU/thigh, and growth in control mice over 24 h varied from 1.4 to 3.9 log10 CFU/thigh. The coefficients of determination for the combined data were 90% for staphylococci, 89% for pneumococci, and 84% for gram-negative bacilli. The T > MIC for a static effect and a 2-log kill were 19.8 and 31.9%, respectively, for staphylococci; 15.9 and 25.1%, respectively, for pneumococci; and 36.5 and 54.3%, respectively, for gram-negative bacilli.
FIG. 3.
Relationship between T > MIC of ceftobiprole and efficacy against multiple strains of S. aureus (open circles, MSSA; solid circles, MRSA) (left), multiple strains of S. pneumoniae (open circles, penicillin-susceptible S. pneumoniae; solid circles, PRSP) (center), and multiple strains of gram-negative bacilli (open circles, Enterobacteriaceae; solid circles, P. aeruginosa) (right). Each point is the mean and standard deviation from two to three mice. The dotted line is the starting inoculum.
The dose and dosing interval of ceftobiprole necessary to achieve a static effect and a 2-log10 kill, along with the MICs and T > MIC for each static and 2-log10 kill dose against the 19 individual organisms tested, are shown in Table 1. Static and 2-log kill doses are shown for studies repeated on different days; however, the average value for these two studies is shown for T > MIC. The static doses ranged from 0.180 mg/kg every 6 h for S. pneumoniae ATCC 10813 to 25.2 mg/kg every 3 h for P. aeruginosa ATCC 27853, while T > MIC varied from 14.1% to 46.7% of the dosing interval. The T > MIC for the four strains of Enterobacteriaceae (40.8% ± 3.8% of the dosing interval) was significantly longer than that for the six strains of S. pneumoniae or the eight strains of S. aureus (18.8% ± 2.7% and 21.1% ± 3.9% of the dosing interval, respectively; P < 0.001). The T > MIC for the 2-log kill dose for strains of Enterobacteriaceae (64.5% ± 25.1% of the dosing interval) was also significantly longer than those for the strains of S. pneumoniae and S. aureus (25.8% ± 4.8% and 29.3% ± 4.6% of the dosing interval, respectively; P < 0.001). The mean T > MIC required for the static and 2-log kill doses in the three groups of organisms from individual mice were similar to values obtained by combining all the data and plotting it against T > MIC.
Impact of different sites of infection and presence of neutrophils.
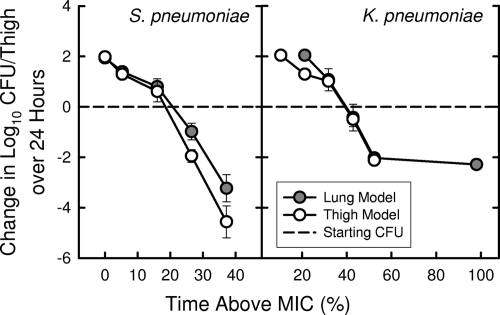
S. pneumoniae ATCC 10813 and K. pneumoniae ATCC 43816 are strains capable of growing well in the lungs of neutropenic mice and in the thighs of normal, nonneutropenic mice (10, 18). The static dose in the lung model for the S. pneumoniae strain was 0.268 mg/kg every 6 h, which was quite similar to the values for the thigh shown in Table 1. Similarly, the static dose for the K. pneumoniae strain in the lung model (6.47 mg/kg every 6 h) was very close to the value of 6.61 mg/kg every 6 h observed in the thigh model. Figure 4 illustrates the relationship between T > MIC and the activity of ceftobiprole against these two organisms at both sites of infection.
FIG. 4.
Relationship of T > MIC for 6-h dosing of increasing doses of ceftobiprole with its efficacy against S. pneumoniae ATCC 10813 and K. pneumoniae ATCC 43816 in the thighs and lungs of neutropenic mice.
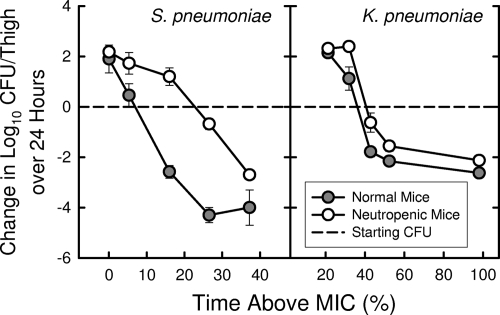
The activity of ceftobiprole against the S. pneumoniae strain was markedly enhanced by the presence of neutrophils. Static doses determined on the same day were 0.0486 mg/kg every 6 h in normal mice and 0.332 mg/kg every 6 h in neutropenic mice. This represented a 6.8-fold reduction in the static dose with the presence of neutrophils. For the strain of K. pneumoniae, the presence of neutrophils reduced the static dose only twofold (1.06 mg/kg every 6 h in normal mice and 2.11 mg/kg every 6 h in neutropenic mice). Figure 5 illustrates the relationship between T > MIC and the activity of ceftobiprole against these two organisms in thighs of mice with and without neutropenia.
FIG. 5.
Relationship of T > MIC for 6-h dosing of increasing doses of ceftobiprole with its efficacy against S. pneumoniae ATCC 10813 and K. pneumoniae ATCC 43816 in the thighs of normal (nonneutropenic) and neutropenic mice.
For S. pneumoniae, the presence of neutrophils reduced the T > MIC for a static effect to less than 10% of the 6-h dosing interval from a value just above 20% in neutropenic mice.
DISCUSSION
The present studies were designed to characterize the PK-PD characteristics of ceftobiprole, a new pyrrolidinone extended-spectrum cephalosporin. PK-PD indices, such as Cmax/MIC ratio, 24-h AUC/MIC ratio, and the T > MIC, are good indicators of the interaction between the dose of drug and the organism. T > MIC is an important determinant of the activity of β-lactam antibiotics, with the absence of major concentration-dependent killing and minimal or no persistent effects except for staphylococci, common characteristics of β-lactams (7). In these studies, escalating doses of ceftobiprole produced moderate in vivo PAEs of 3.8 to 4.8 h against S. aureus ATCC 33591, a standard strain of MRSA. Minimal or no in vivo PAEs from 0 to 0.8 h were demonstrated against S. pneumoniae CDC 673, a highly penicillin-resistant strain.
The in vitro and in vivo efficacies of β-lactam antibiotics were previously correlated with T > MIC (6-8, 10). The maximal efficacy of cephalosporins against Enterobacteriaceae and streptococci in several animal infection models was approached when serum levels were above the MIC for 55% to 70% of the dosing interval; the maximal efficacy of cephalosporins against S. aureus was approached when serum levels were above the MIC for 40% to 50% of the dosing interval. Results from the present studies of multiple dosing regimens indicate that the T > MIC is the PK-PD index that best predicts the efficacy of ceftobiprole. The T > the MIC required to produce a static effect varied from 14% to 48% of the dosing interval. It was about twice as long for the Enterobacteriaceae strains and Pseudomonas aeruginosa than for the S. aureus and S. pneumoniae strains. The higher percentage of T > MIC required for ceftobiprole against Enterobacteriaceae than that required for ceftobiprole against S. aureus demonstrated in these studies supports many previously reported observations (8, 10). However, previous studies and a recent study with ceftaroline demonstrated similar T > MIC values required for stasis for Enterobacteriaceae strains and S. pneumoniae strains (4, 8, 10). Lower requirements for T > MIC with β-lactams have been associated with the presence of modest in vivo PAEs as with S. aureus strains or increased rates of killing as seen with the carbapenems (10). Ceftobiprole did not produce a prolonged in vivo PAE with a penicillin-resistant pneumococcus in this study. It is not known if this enhanced activity against S. pneumoniae represents a higher rate of killing or a greater affinity of ceftobiprole for pneumococcal penicillin-binding proteins.
The other interesting observation with ceftobiprole is that its activity against S. pneumoniae was markedly enhanced by the presence of neutrophils. This has not been observed with older cephalosporins and penicillins (10). We hypothesize that ceftobiprole markedly enhances the intracellular killing of pneumococci by neutrophils. Previous in vitro studies with other β-lactams showed a more modest enhancement in the intracellular killing of gram-positive cocci by neutrophils (20, 23). The twofold enhancement in activity of ceftobiprole against K. pneumoniae by the presence of neutrophils is similar to data reported previously for other β-lactams (10).
In the two pneumonia models, ceftobiprole was similarly as potent in the lung as in the thigh. In other pneumonia studies, drug doses that produced serum concentrations that were above the MIC for at least 35% of the dosing interval in nonneutropenic mice were effective against non-extended-spectrum β-lactamase-producing strains of K. pneumoniae and E. cloacae (22). In neutropenic mice, Azoulay-Dupuis et al. (5) previously observed that the T > MIC required for the efficacy of ceftobiprole against various pneumococci, including some resistant strains, was only 9 to 18% of the dosing interval. In the same model, ceftriaxone required a longer T > MIC of about 30 to 50% for efficacy. The differences in PK-PD requirements for ceftobiprole with these different organisms are similar to those observed in our studies. The PD profile of ceftobiprole, demonstrated here and in other studies, supports its potential for clinical value against a wide range of gram-negative and gram-positive pathogens, including MRSA and PRSP.
Footnotes
Published ahead of print on 1 August 2008.
REFERENCES
- 1.AAALAC. 2006, posting date. Guide to the Care and Use of Laboratory Animals. AAALAC, Frederick, MD. http://www.aaalac.org.
- 2.Andes, D., and W. A. Craig. 2003. Pharmacodynamics of the new des-F(6)-quinolone garenoxacin in a murine thigh infection model. Antimicrob. Agents Chemother. 47:3935-3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andes, D., and W. A. Craig. 2002. Pharmacodynamics of the new fluoroquinolone gatifloxacin in murine thigh and lung infection models. Antimicrob. Agents Chemother. 46:1665-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andes, D., and W. A. Craig. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob. Agents Chemother. 50:1376-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azoulay-Dupuis, E., J. P. Bedos, J. Mohler, A. Schmitt-Hoffmann, M. Schleimer, and S. Shapiro. 2004. Efficacy of BAL5788, a prodrug of cephalosporin BAL9141, in a mouse model of acute pneumococcal pneumonia. Antimicrob. Agents Chemother. 48:1105-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cars, O. 1997. Efficacy of beta-lactam antibiotics: integration of pharmacokinetics and pharmacodynamics. Diagn. Microbiol. Infect. Dis. 27:29-33. [DOI] [PubMed] [Google Scholar]
- 7.Craig, W. A. 2001. Does the dose matter? Clin. Infect. Dis. 33(Suppl. 3):S233-S237. [DOI] [PubMed] [Google Scholar]
- 8.Craig, W. A. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89-96. [DOI] [PubMed] [Google Scholar]
- 9.Craig, W. A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed]
- 10.Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 17:479-501. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande, L., P. R. Rhomberg, T. R. Fritsche, H. S. Sader, and R. N. Jones. 2004. Bactericidal activity of BAL9141, a novel parenteral cephalosporin against contemporary gram-positive and gram-negative isolates. Diagn. Microbiol. Infect. Dis. 50:73-75. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande, L. M., and R. N. Jones. 2003. Bactericidal activity and synergy studies of BAL9141, a novel pyrrolidinone-3-ylidenemethyl cephem, tested against streptococci, enterococci and methicillin-resistant staphylococci. Clin. Microbiol. Infect. 9:1120-1124. [DOI] [PubMed] [Google Scholar]
- 13.Entenza, J. M., P. Hohl, I. Heinze-Krauss, M. P. Glauser, and P. Moreillon. 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob. Agents Chemother. 46:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Issa, N. C., M. S. Rouse, K. E. Piper, W. R. Wilson, J. M. Steckelberg, and R. Patel. 2004. In vitro activity of BAL9141 against clinical isolates of gram-negative bacteria. Diagn. Microbiol. Infect. Dis. 48:73-75. [DOI] [PubMed] [Google Scholar]
- 16.Jones, R. N., L. M. Deshpande, A. H. Mutnick, and D. J. Biedenbach. 2002. In vitro evaluation of BAL9141, a novel parenteral cephalosporin active against oxacillin-resistant staphylococci. J. Antimicrob. Chemother. 50:915-932. [DOI] [PubMed] [Google Scholar]
- 17.Lalonde, R. L. 1992. Pharmacodynamics, p. 4.1-4.33. In W. E. Evans, J. J. Schentag, and W. J. Jusko (ed.), Applied pharmacokinetics, 3rd ed. Applied Therapeutics, Inc., Vancouver, WA.
- 18.Leggett, J. E., B. Fantin, S. Ebert, K. Totsuka, B. Vogelman, W. Calame, H. Mattie, and W. A. Craig. 1989. Comparative antibiotic dose-effect relationships at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159:281-292. [DOI] [PubMed] [Google Scholar]
- 19.McDonald, P. J., B. L. Wetherall, and H. Pruul. 1981. Postantibiotic leukocyte enhancement: increased susceptibility of bacteria pretreated with antibiotics to activity of leukocytes. Rev. Infect. Dis. 3:38-44. [DOI] [PubMed] [Google Scholar]
- 20.Meinen, J. B., J. T. McClure, and E. Rosin. 1995. Pharmacokinetics of enrofloxacin in clinically normal dogs and mice and drug pharmacodynamics in neutropenic mice with Escherichia coli and staphylococcal infections. Am. J. Vet. Res. 56:1219-1224. [PubMed] [Google Scholar]
- 21.NCCLS. 2000. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A7, 7th ed. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 22.Perea, S., G. J. Pennick, A. Modak, A. W. Fothergill, D. A. Sutton, D. J. Sheehan, and M. G. Rinaldi. 2000. Comparison of high-performance liquid chromatographic and microbiological methods for determination of voriconazole levels in plasma. Antimicrob. Agents Chemother. 44:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruul, H., and P. J. McDonald. 1996. Uptake of cefepime by phagocytosing polymorphonuclear neutrophils and subsequent intracellular killing. Antimicrob. Agents Chemother. 40:1870-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouse, M. S., M. M. Hein, P. Anguita-Alonso, J. E. Steckelberg, and R. Patel. 2006. Ceftobiprole medocaril (BAL5788) treatment of experimental Haemophilus influenzae, Enterobacter cloacae, and Klebsiella pneumoniae murine pneumonia. Diagn. Microbiol. Infect. Dis. 55:333-336. [DOI] [PubMed] [Google Scholar]
- 25.Zuluaga, A. F., B. E. Salazar, C. A. Rodriguez, A. X. Zapata, M. Agudelo, and O. Vesga. 2006. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect. Dis. 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]