Abstract
In a murine model of disseminated zygomycosis, low doses of amphotericin B (0.3 mg/kg body weight/day) combined with posaconazole (40 mg/kg/day) prolonged survival and reduced tissue burden with respect to that of controls and that of both drugs administered alone. Results were similar to those obtained with amphotericin B given alone at 0.8 mg/kg/day.
Zygomycosis is an opportunistic fungal infection that causes a high degree of morbidity and mortality in immunocompromised patients, particularly those with hematologic malignancy or diabetes mellitus. Rhyzopus oryzae infection is the most common cause of zygomycosis in humans (10). Optimal management of zygomycosis has not been defined. Rapid diagnosis, mitigation or reduction of risk factors, administration of antifungal therapy, surgical debridement where possible, and the use of adjunctive therapies all contribute to the successful management of zygomycosis (11). Amphotericin B (AMB) deoxycholate and lipid formulations of AMB are the drugs of choice for treating zygomycoses (10, 11, 12). Posaconazole (PSC) is safer than AMB and shows good in vitro activity against some zygomycetes (1, 14). Clinical data seem to confirm that in two compassionate use trials, outcomes obtained with PSC therapy were similar to those obtained with AMB, supporting the use of this azole in the management of refractory zygomycosis or in cases of patient intolerance to AMB therapy (6, 15). Because animal studies showed limited activity of PSC against R. oryzae infection (2, 4), we hypothesized that the combined use of two drugs with different targets and with proven activities against zygomycetes may act synergistically against R. oryzae infection or, at least, would allow the use of reduced therapeutic doses. Therefore, we evaluated the efficacy of PSC alone and of PSC combined with AMB in a model of disseminated R. oryzae infection in neutropenic mice.
Two clinical isolates of R. oryzae, FMR 6485 and FMR 8542, were used. The in vitro antifungal susceptibility of each strain was tested using a reference microdilution method (8), and the in vitro interactions were assessed using a checkerboard method (5, 7). For both of the drugs and their combinations, we used a MIC of 0 as the endpoint criterion.
Male OF1 mice weighing 30 g were used. All animal care procedures were supervised and approved by the Universitat Rovira i Virgili animal welfare committee. Animals were immunosuppressed by intraperitoneal administration of a single dose of cyclophosphamide, 200 mg/kg of body weight, plus a single intravenous administration of 5-fluorouracil, 150 mg/kg, 1 day before the infection (3). Mice were injected with 1 × 105 CFU in 0.2 ml in the lateral tail vein. Preliminary experiments with both strains (data not shown) showed that this concentration was the optimal dose for killing 100% of the animals within 6 days of infection.
AMB (Fungizona) and PSC (Noxafil) were tested. AMB was administered intravenously at 0.3 mg/kg/day or 0.8 mg/kg/day, once daily; PSC was administered orally at 20 mg/kg/day, 40 mg/kg/day, or 80 mg/kg/day, once daily. In addition, AMB at 0.3 mg/kg/day or 0.8 mg/kg/day was combined with PSC at 40 mg/kg/day. Treatments were started 24 h after the challenge and lasted for 7 days. We evaluated the efficacy of the different regimens in prolonging survival and in reducing tissue burden in the brain and kidneys.
For survival studies, groups of 10 mice used with each strain and from each treatment group were randomly established and checked daily for 20 days. For tissue burden studies, groups of 10 mice were also established, and the animals were sacrificed on day 4 after they were infected. Kidneys and brains were removed aseptically and gently homogenized in 2 ml of sterile saline. Serial 10-fold dilutions of the homogenates were plated on Sabouraud dextrose agar and incubated for 24 h at 35°C. Data were expressed as log10 CFU per gram of tissue. Mean survival time was estimated by the Kaplan-Meier method and compared among groups by using the log rank test. Colony counts from tissue burden studies were analyzed by using the Mann-Whitney U test.
The in vitro results are shown in Table 1. The combination of AMB plus PSC was synergistic against the strain FMR 6485 but showed an indifferent interaction against the strain FMR 8542.
TABLE 1.
In vitro antifungal activities and interactions among antifungal drugs against two strains of R. oryzae
| Strain | MIC (μg/ml)a
|
|||
|---|---|---|---|---|
| AMB | PSC | AMB/PSC | FICI | |
| FMR 6485 | 0.5 | 1 | 0.125/0.25 | 0.50 |
| FMR 8542 | 0.5 | 2 | 0.125/1 | 0.625 |
Values greater than or equal to 0.5, synergistic activity; values ranging from >0.5 to ≤4, indifferent activity; values of more than 4, antagonistic activity (8). FICI, fractional inhibitory concentration index.
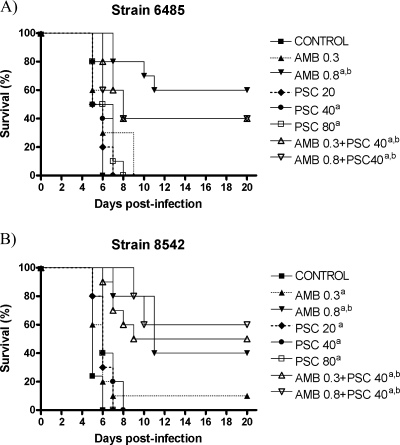
For both strains, the results of two studies are pooled; thus, there were 20 control mice for each strain. For strain FMR 6485, all treatment regimens, with the exception of AMB at 0.3 mg/kg/day and PSC at 20 mg/kg/day, significantly prolonged survival compared with that for the control group (Fig. 1). For strain FMR 8542, all treatments prolonged survival compared with those for the control group. In addition, for both strains, AMB at 0.8 mg/kg/day and with the two combinations were the most effective regimens for prolonging survival. We observed no statistical differences between AMB at 0.8 mg/kg/day and the two combinations of AMB and PSC.
FIG. 1.
Cumulative mortality of mice infected with R. oryzae FMR 6485 (A) or R. oryzae FMR 8542 (B) and treated with AMB and PSC. a, P < 0.05 versus control; b, P < 0.05 versus PSC (20 mg/kg), PSC (40 mg/kg), PSC (80 mg/kg), and AMB (0.3 mg/kg).
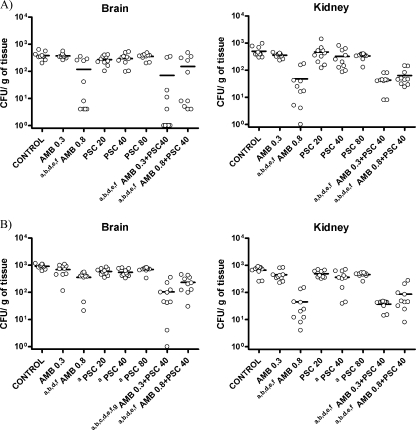
AMB at 0.8 mg/kg/day and the two combinations of AMB plus PSC significantly reduced the fungal load of strain FMR 6485 in the brain and the kidney with respect to that of the other groups (Fig. 2). For strain FMR 8542, the same results were seen with the kidneys. However, in brain the combination of AMB at 0.3 mg/kg plus PSC at 40 mg/kg was the most effective therapy. The combination of AMB at 0.8 mg/kg plus PSC at 40 mg/kg and AMB at 0.8 mg/kg also significantly reduced fungal load in brain tissue compared with those of the monotherapies and the control group. Finally, PSC 40 and 80 at mg/kg/day significantly reduced fungal load in both the brain and the kidney compared with that of the control group, whereas PSC at 20 mg/kg/day did so only in brain.
FIG. 2.
Effects of the antifungal treatment on colony counts of the R. oryzae strains FMR 6485 (A) and FMR 8542 (B) in brain and kidney of mice. a, P < 0.05 versus control; b, P < 0.05 versus AMB (0.3 mg/kg); c, P < 0.05 versus AMB (0.8 mg/kg); d, P < 0.05 versus PSC (20 mg/kg); e, P < 0.05 versus PSC (40 mg/kg); f, P < 0.05 versus PSC (80 mg/kg); g, P < 0.05 versus AMB (0.8 mg/kg) plus PSC (40 mg/kg). Horizontal lines indicate mean values.
As in other studies, AMB at 0.8 mg/kg/day was generally effective against both isolates (4, 13). In an animal infection model, PSC showed poor efficacy against a strain of R. oryzae (4). Similarly, prophylactic administration of PSC to neutropenic mice prolonged survival but did not reduce the tissue burden in a disseminated infection caused by R. oryzae (2). The current study partly confirmed these results.
Although the combination of PSC plus AMB has not been extensively tested in patients with infections caused by R. oryzae, recently, a patient with disseminated infection caused by Rhizomucor sp. (a genus close to Rhizopus) was cured with combined therapy of liposomal AMB and PSC, without surgical intervention (9).
In the current study, although the combination of PSC with AMB was not superior to high-dose AMB alone, the combination did allow a reduction of the AMB dose with no loss of efficacy. In addition, for one fungal strain, the combination reduced the fungal burden in the brain. Further controlled studies are needed to ascertain the clinical relevance of this combination.
Acknowledgments
This work was supported by a grant from Fondo de Investigaciones Sanitarias from the Ministerio de Sanidad y Consumo of Spain (PI 050031).
Footnotes
Published ahead of print on 11 August 2008.
REFERENCES
- 1.Almyroudis, N. G., D. A. Sutton, A. W. Fothergill, M. G. Rinaldi, and S. Kusne. 2007. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob. Agents Chemother. 51:2587-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barchiesi, F., E. Spreghini, A. Santinelli, A. W. Fothergill, E. Pisa, D. Giannini, M. G. Rinaldi, and G. Scalise. 2007. Posaconazole prophylaxis in experimental systemic zygomycosis. Antimicrob. Agents Chemother. 51:73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capilla, J., C. Serena, F. J. Pastor, M. Ortoneda, and J. Guarro. 2003. Efficacy of voriconazole in treatment of systemic scedosporiosis in neutropenic mice. Antimicrob. Agents Chemother. 47:3976-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dannaoui, E., J. F. G. M. Meis, D. Loebenberg, and P. E. Verweij. 2003. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob. Agents Chemother. 47:3647-3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliopoulos, G. M., and R. C. Moellering. 1991. Antimicrobial combinations, p. 432-492. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. Williams & Wilkins, Baltimore, MD.
- 6.Greenberg, R. N., K. Mullane, J. A. van Burik, I. Raad, M. J. Abzug, G. Anstead, R. Herbrecht, A. Langston, K. A. Marr, G. Schiller, M. Schuster, J. R. Wingard, C. E. Gonzalez, S. G. Revankar, G. Corcoran, R. J. Kryscio, and R. Hare. 2006. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 50:126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson, M. D., C. MacDougall, L. Ostrosky-Zeichner, J. R. Perfect, and J. H. Rex. 2004. Combination antifungal therapy. Antimicrob. Agents Chemother. 48:693-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 9.Rickerts, V., J. Atta, S. Herrmann, V. Jacobi, E. Lambrecht, R. Bialek, and G. Just-Nübling. 2006. Successful treatment of disseminated mucormycosis with a combination of liposomal amphotericin B and posaconazole in a patient with acute myeloid leukaemia. Mycoses 49:27-30. [DOI] [PubMed] [Google Scholar]
- 10.Roden, M. M., T. E. Zaoutis, W. L. Buchanan, T. A. Knudsen, T. A. Sarkisova, R. L. Schaufele, M. Sein, T. Sein, C. C. Chiou, J. H. Chu, D. P. Kontoyiannis, and J. Walsh. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634-653. [DOI] [PubMed] [Google Scholar]
- 11.Rogers, T. R. 2008. Treatment of zygomycosis: current and new options. J. Antimicrob. Chemother. 61:35-39. [DOI] [PubMed] [Google Scholar]
- 12.Spellberg, B., J. Edwards, Jr., and A. Ibrahim. 2005. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin. Microbiol. Rev. 18:556-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugar, A. M., and X. Liu. 2000. Combination antifungal therapy in treatment of murine pulmonary mucormycosis: roles of quinolones and azoles. Antimicrob. Agents Chemother. 44:2004-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun, Q. N., A. W. Fothergill, D. I. McCarthy, M. G. Rinaldi, and J. R. Graybill. 2002. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob. Agents Chemother. 46:1581-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Burik, J., R. S. Hare, H. F. Solomon, M. L. Corrado, and D. P. Kontoyiannis. 2006. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin. Infect. Dis. 42:61-65. [DOI] [PubMed] [Google Scholar]