Abstract
Since levofloxacin at high doses was more active than levofloxacin at conventional doses and was the best therapy alone in a rat model of staphylococcal foreign-body infection, in this study we tested how these differences affect the activities of their respective combinations with rifampin in vitro and in vivo. In vitro studies were performed in the log and stationary phases. By using this model, rifampin at 25 mg/kg of body weight/12 h, levofloxacin at 100 mg/kg/day, levofloxacin at 100 mg/kg/day plus rifampin, levofloxacin at 50 mg/kg/day, levofloxacin at 50 mg/kg/day plus rifampin, or a control treatment was administered for 7 days; and therapy with for levofloxacin at 100 mg/kg/day alone and rifampin alone was prolonged to 14 days. We screened for the appearance of resistant strains. Killing curves in the log phase showed a clear antagonism with levofloxacin at concentrations ≥2× MIC and rifampin and tended to occur in the stationary phase. At the end of 7 days of therapy, levofloxacin at 100 mg/kg/day was the best treatment and decreased the bacterial counts from tissue cage fluid (P < 0.05 compared with the results for groups except those receiving rifampin alone). At the end of 14 days of therapy with levofloxacin at 100 mg/kg/day, levofloxacin at 100 mg/kg/day plus rifampin, and the control treatment, the bacterial counts on the coverslips were 2.24 (P < 0.05 compared with the results with the combined therapy), 3.36, and 5.4 log CFU/ml, respectively. No rifampin or levofloxacin resistance was detected in any group except that receiving rifampin alone. In conclusion, high-dose levofloxacin was the best treatment and no resistant strains appeared; the addition of rifampin showed an antagonistic effect. The efficacy of the rifampin-levofloxacin combination is not significantly improved by the dosage of levofloxacin.
Orthopedic prosthetic infections are difficult to treat because of the presence of bacterial biofilms. The definitive therapy for such infections requires a combination of surgical and medical approaches and the use of selected antibiotics active against the microorganisms involved (11, 40, 48).
On the basis of previous experimental and clinical studies, rifampin plays a main role in the treatment of staphylococcal foreign-body infections (4, 45, 47, 49), while fluoroquinolones are considered the best drugs for use in combination with rifampin (14, 15, 46). Recent work recommended the use of a combination of high doses of levofloxacin (750 to 1,000 mg/day) plus rifampin for the treatment of staphylococcal prosthetic infections (48), even though the information available from this setting is limited (3, 34, 39).
The rat model of staphylococcal tissue cage infection is a well-standardized model of chronic foreign-body infection that has provided relevant information in this regard (8, 9, 32, 44). Using this model, we previously reported that high-dose levofloxacin (equivalent to 750 to 1,000 mg/day) was more active than the conventional levofloxacin dose (500 mg/day) and that it was the best therapy for use alone in comparison to therapy with other antistaphylococcal drugs (34). The aim of the present study was to test the extent to which these differences in activity between conventional and high doses of levofloxacin affect the efficacies of their respective combinations with rifampin in vitro and in vivo.
MATERIALS AND METHODS
Microorganism and antimicrobial agents.
Methicillin-susceptible strain Staphylococcus aureus ATCC 29213 was used for all experiments.
The antimicrobial agents (levofloxacin and rifampin) were kindly provided by Sanofi-Aventis (Madrid, Spain).
In vitro studies. (i) Determination of MICs and MBCs.
The MICs and the minimal bactericidal concentrations (MBCs) were determined in the log phase by the macrodilution method and by the methodology recommended previously (10). The MICs were defined as the minimal concentration of antibiotic that was able to inhibit macroscopic growth. The MBCs were defined as the minimal concentration of antibiotic that was able to kill 99.9% of the bacteria from the initial inoculum.
The MBCs were also determined in the stationary phase of growth. The methodology used has been reported previously and proved to be a reliable method for correlating in vivo efficacy in the rat tissue cage model of foreign-body infection (34, 47). The MBCs were defined as described above.
(ii) Twenty-four-hour killing curve assays in log and stationary phases.
The methodology used for the killing curve assays in the log phase followed previous standardized recommendations (35), and that used for the 24-h killing curve assays in the stationary phase was previously described in detail (34).
The concentrations of antibiotics selected for the log-phase killing curve studies were those that represented subinhibitory and clinically achievable levels greater than the MIC, while the concentrations used for the stationary-phase studies were equivalent to peak and trough levels in tissue cage fluid (TCF).
For all experiments, bactericidal activity was defined as a ≥3-log10 decrease in the initial inoculum (in CFU/ml) at 24 h. The results of the combination treatments were compared with the results with the most active single drug; synergy, indifference, and antagonism were then defined as a ≥2-log increase in killing, a <2-log change (increase or decrease) in killing, and a ≥2-log decrease in killing, respectively.
To avoid carryover antimicrobial agent interference, the sample was placed on the plate in a single streak down the center and was allowed to absorb into the agar until the plate surface appeared dry; the inoculum was then spread over the plate.
Animal studies.
The animal model was approved by the Ethical Committee for Animal Experiments at the University of Barcelona.
The model was derived from that reported by Lucet et al. (32); we standardized this model using the methodology reported in our previous study (34). Briefly, two multiperforated Teflon tissue cages, each of which contained two polymethyl methacrylate coverslips, were subcutaneously implanted in the flank of a male Wistar rat. Three weeks later, a sample of TCF was obtained and checked for sterility; 0.1 ml of saline solution containing S. aureus (0.2 × 106 to 2 × 106 CFU/ml) was then inoculated into the tissue cage. Three weeks after inoculation (designated day 1), a sample of TCF was again recovered to quantify the bacterial counts. A minimum of 105 CFU/ml was required to include the sample in the therapeutic experiments; in all cases, each tissue cage was counted separately. Animals were killed only if both tissue cages were not valid for further use; thus, the rats used in the experiments had one or two valid tissue cages. The mean of the bacterial inoculum from the TCF included at the beginning of the experiments was 6.66 ± 0.88 log CFU/ml (mean ± standard deviation). The animals were treated intraperitoneally for 7 days, and 24 h after the last dose (designated day 8), a sample of TCF was recovered and the bacterial counts were quantified. To avoid carryover, we proceeded as indicated above.
The therapeutic groups received the following: rifampin at 25 mg/kg of body weight/12 h, levofloxacin at 100 mg/kg/day, levofloxacin at 50 mg/kg/day, levofloxacin at 100 mg/kg/day plus rifampin, levofloxacin at 50 mg/kg/day plus rifampin, and a control treatment.
To investigate further the antimicrobial efficacy of high doses of levofloxacin alone and in combination with rifampin, additional in vivo studies were performed and treatment was continued for 14 days. We had previously analyzed the spontaneous evolution of experimental foreign-body infection in our animal model and found a chronic and stable infection until day 35 after inoculation (34). Thus, for these specific 14-day treatment experiments, TCF was obtained on day 8, as indicated above, and then the animals received the next dose of antibiotic and were treated for a further 7 days; no dose was missed. Twenty-four hours after the last dose (designated day 15), TCF was again obtained to quantify the bacterial counts, and finally, the animals were killed and the coverslips were recovered from the tissue cages. The therapeutic groups included in these experiments for the double evaluation of efficacy were limited to the groups receiving levofloxacin at 100 mg/kg/day plus rifampin, levofloxacin at 100 mg/kg/day alone, and the control treatment.
The differences in the bacterial counts from TCF samples at the beginning and end of treatment were used as the criteria for therapeutic efficacy; thus, we determined differences between the beginning and day 8 for all therapeutic groups and also between the beginning and day 15 exclusively for the three groups mentioned above. Once the rats had been killed at the end of 14 days of therapy, the coverslips were removed and processed to quantify bacterial counts.
All the procedures used to process the TCFs and coverslips have been reported to be harmless to the bacteria (9, 32) and have previously been described in detail (34).
Pharmacokinetic studies.
Before the therapeutic experiments, the antibiotic dosages were selected according to pharmacokinetic and pharmacodynamic parameters by using the methodology described in detail in our previous work (34). On the basis of previous data, we selected the dose whose pharmacodynamic parameters in TCF were close to those in human serum (12, 43); area under the concentration-time curve (AUC) values were adjusted for each antibiotic to allow similar ratios of AUC/MIC in both animals and humans (7, 27, 37). Peak and trough levels in TCF were determined on day 4 for each drug to check the equilibrium test concentrations during treatment.
The levels of antibiotics were determined by a bioassay method (6) with antibiotic medium 1 (Difco); thus, the free drug concentrations were used in all cases to make the comparisons more accurate. The microorganisms used were Escherichia coli ATCC 35218 for levofloxacin (lower limit of detection, 0.5 μg/ml; for linearity of assay, r2 = 0.99) and Staphylococcus epidermidis ATCC 27626 for rifampin (lower limit of detection, 0.25 μg/ml; for linearity of assay, r2 = 0.98).
All main pharmacokinetic and pharmacodynamic parameters determined for the selected doses were published in our previous report (34). We noted that the peak levels in TCF, the trough levels in TCF, the AUC for serum, the AUC for TCF, the AUC/MIC in TCF, and the maximum concentration (Cmax)/MIC in TCF were 12 mg/liter, 1.1 mg/liter, 106 μg·h/ml, 117 μg·h/ml, 234, and 24, respectively, for levofloxacin at 100 mg//kg/day; 6 mg/liter, 0.6 mg/liter, 77 μg·h/ml, 68 μg·h/ml, 136, and 13, respectively, for levofloxacin at 50 mg/kg/day; and 6.6 mg/liter, 3.8 mg/liter, 277 μg·h/ml, 304 μg·h/ml, 20,260, and 440, respectively, for rifampin.
For the combination of levofloxacin at 100 mg/day and rifampin, we determined the Cmax of levofloxacin after 4 days of therapy in order to screen for potential interactions due to rifampin. The Cmax was 11 mg/liter; thus, it did not significantly differ from that obtained with levofloxacin at 100 mg/day alone.
Resistance to antimicrobial agents.
All therapeutic groups were screened for the development of levofloxacin and rifampin resistance at the end of therapy. In all cases, 100 μl of TCF obtained directly and processed TCF from the coverslips was cultured in plates containing 1 mg/liter of either levofloxacin or rifampin. The results were expressed qualitatively as positive (with any macroscopic growth) or negative (with no macroscopic growth) (limit of detection, 10 CFU/ml).
Statistical analysis.
All bacterial counts are presented as log CFU/ml (means ± standard deviations). The data were found to be normally distributed when the Kolmogorov-Smirnov test was applied. The differences in the bacterial counts for the treated and the untreated animals were analyzed for statistical significance by analysis of variance. An unpaired Student's t test with the Bonferroni correction was used to determine statistical significance. For all tests, differences were considered statistically significant when P values were <0.05.
RESULTS
In vitro studies.
The MICs of levofloxacin and rifampin in the log phase were 0.5 and 1 μg/ml, respectively, and the MBCs were 0.015 and 0.12 μg/ml, respectively. The MBCs of both antibiotics in the stationary phase were 4 and >8 μg/ml, respectively.
In the killing curve assays, levofloxacin proved to be bactericidal against both dividing and nondividing bacteria, although higher concentrations were required in the stationary-phase studies (8× MIC) than in the log-phase ones (2× MIC).
In vitro studies with rifampin alone yielded a wide range of results in killing activity that were related to differences in the inoculum size and the appearance of resistance during the studies. Rifampin showed bactericidal activity (at concentrations 8× MIC) in the log phase, although a strictly adjusted inoculum of 105 CFU/ml was required; in contrast, small increases in inocula were associated with notorious decreases in killing, which did not reach more than 3 log CFU/ml. Thus, the MBC in the stationary phase, in which high inocula were needed, was >8 μg/ml, yielding a high MBC/MIC ratio of 533.
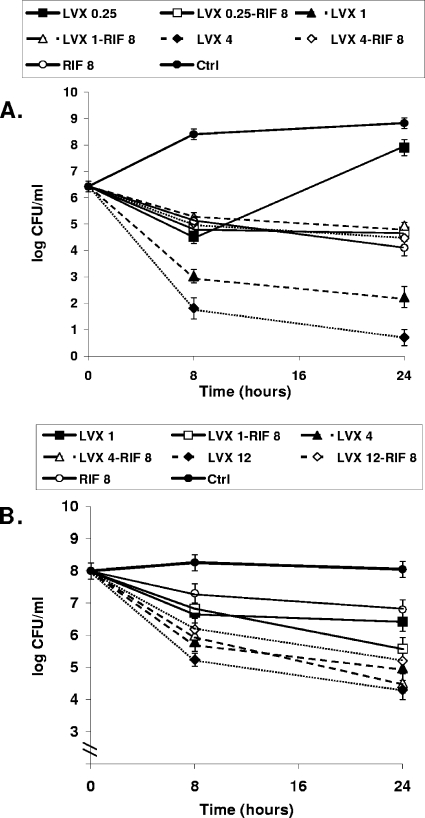
The addition of rifampin to levofloxacin in the log phase showed antagonism at levofloxacin concentrations as low as 2× MIC. When subinhibitory concentrations of levofloxacin were combined with rifampin, this antagonism was not seen. In contrast, the combination of levofloxacin and rifampin in the stationary phase showed an indifferent effect, although some decrease in the efficacy (tending toward antagonism) was observed when rifampin was added to levofloxacin at concentrations higher than its MBC. Figure 1 shows the killing curves in the log and stationary phases obtained with representative concentrations of levofloxacin plus rifampin. Note that the concentrations used in the stationary-phase studies were higher than those used in the log-phase studies.
FIG. 1.
Time-kill curves for strain in the log phase obtained with subinhibitory and clinically available concentrations (μg/ml) (A) and in the stationary phase obtained with the trough and peak concentrations (B) in TCF of rats treated with levofloxacin (LVX) alone and in combination with rifampin (RIF). Note that the levofloxacin concentrations used in the log-phase studies were lower than those used in the stationary-phase studies. In the stationary-phase studies, the trough concentrations of levofloxacin were equivalent to the subinhibitory ones in the log phase, whereas two peak concentrations of levofloxacin are represented as being equivalent to the peak levels of both dosages. The antagonistic effect obtained with the combination of levofloxacin and rifampin in the log phase was observed with levofloxacin at concentrations of 1 and 4 μg/ml (LVX 1 and LVX 4, respectively), whereas the combination of levofloxacin at 12 μg/ml (LVX 12) and rifampin in the stationary phase decreased the efficacy of levofloxacin at 12 μg/ml alone by more than 1 log CFU/ml. Errors bars indicate standard deviations; in some cases, the bars are smaller than the symbols and so cannot be seen.
Animal studies. (i) Seven-day therapy.
Ten rats were not included because of inadequate bacterial counts. A total of 85 rats (170 tissue cages) were used, and among these rats, 7 rats had only one valid tissue cage. There were no significant differences between the groups in the bacterial counts from TCF at the beginning of experiments; these counts (mean ± standard deviation) were as follows: 6.9 ± 0.8 log CFU/ml for rifampin, 6.74 ± 0.89 log CFU/ml for levofloxacin at 100 mg/kg/day, 6.4 ± 0.8 log CFU/ml for levofloxacin at 50 mg/kg/day, 6.74 ± 1 log CFU/ml for levofloxacin at 100 mg/kg/day plus rifampin, 6.7 ± 1 log CFU/ml for levofloxacin at 50 mg/kg/day plus rifampin, and 6.8 ± 0.8 log CFU/ml for the controls.
At the end of the therapy, the counts for all therapeutic groups were better than those for the controls. Levofloxacin at high doses was the best treatment; it was significantly better than levofloxacin at 50 mg/kg/day (P < 0.03) and the combinations of levofloxacin at 50 mg/kg/day plus rifampin and levofloxacin at 100 mg/kg/day plus rifampin (P < 0.05). The counts for the groups treated with rifampin alone did not show significant differences compared with those for the two groups treated with one of the levofloxacin doses plus rifampin.
Resistance to rifampin appeared in 90% of the rats treated with rifampin alone, whereas no rifampin- or levofloxacin-resistant strains appeared in the remaining groups.
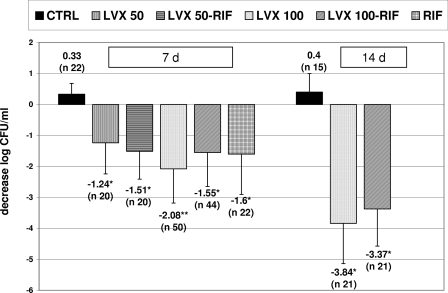
A comparison of the final decreases in the log numbers of CFU/ml between the groups is shown in Fig. 2.
FIG. 2.
Between-group comparisons of the decreases in bacterial counts from TCF (mean log CFU/ml) at the end of 7 days and 14 days of therapy. The n values represent the number of TCF samples and not the number of animals for each group. Note that the number of samples used for the two groups treated with levofloxacin at 100 mg/kg/day and levofloxacin at 100 mg/kg/day plus rifampin for the studies with 7 days of therapy was larger than that for the remaining therapeutic groups because the animals were maintained for a longer period. Differences in efficacy between the two therapeutic groups were maintained over time, although the number of samples included in the study with 14 days of therapy was less than that in the study with 7 days of therapy. Errors bars indicate standard deviations. Abbreviations and symbols: 7d, 7 days of therapy; 14d, 14 days of therapy; LVX, levofloxacin; RIF, rifampin; CTRL, control; *, P < 0.05 versus the results for the control; **, P < 0.05 versus the results for the groups treated with levofloxacin at 50 mg/kg/day (LVX 50) plus rifampin and levofloxacin at 100 mg/kg/day (LVX 100) plus rifampin and P < 0.03 versus the results for the groups treated with levofloxacin at 50 mg/kg/day and the controls.
(ii) Fourteen-day therapy.
At the end of 14 days of therapy, the bacterial counts were 2.9 ± 1.2 log CFU/ml for the group treated with levofloxacin at 100 mg/kg/day (n = 21), 3.37 ± 1.3 log CFU/ml for the group treated with levofloxacin at 100 mg/kg/day plus rifampin (n = 21), and 7.2 ± 0.6 log CFU/ml for the control group (n = 15). Levofloxacin alone was more active than levofloxacin in combination with rifampin, and the final efficacy for both groups was higher than that at the end of 7 days of therapy. The differences in the decrease in TCF bacterial counts (Fig. 2) between the two therapeutic groups that included levofloxacin alone and in combination with rifampin (0.5 log CFU/ml) were maintained over time, and levofloxacin alone was more active than levofloxacin in combination with rifampin, however; and probably because of the smaller number of animals, the differences in efficacy at the end of 14 days of therapy were not statistically significant. In contrast, analysis of the bacterial counts from the coverslips showed that the counts were significantly better for the group treated with levofloxacin alone (P < 0.05); these counts were 2.24 ± 1.3 log CFU/ml for the group treated with levofloxacin at 100 mg/kg/day, 3.3 ± 1.1 log CFU/ml for the group treated with levofloxacin at 100 mg/kg/day plus rifampin, and 5.4 log CFU/ml ± 1 for the control group.
At the end of 14 days of therapy, there were no rifampin- or levofloxacin-resistant strains in either TCF or coverslips.
DISCUSSION
The present study with a standardized rat model of S. aureus foreign-body infection provides information on the comparative efficacies of conventional and high doses of levofloxacin alone, and it is the first to test the differences in activity when both doses were combined with rifampin.
In our in vitro studies, we found that levofloxacin was bactericidal against S. aureus in both the log and the stationary phases, in agreement with the findings presented in previous reports (19, 28, 31, 34). In contrast, the efficacy of rifampin alone was greatly influenced by the inoculum size, as well as by the emergence of resistant strains. While rifampin has generally been considered an antimicrobial that is bactericidal for S. aureus (33, 38), contradictory results in this regard can often be found in the literature (1, 9, 18, 25). In our stationary-phase studies, where the presence of a high inoculum was needed, rifampin showed a moderate killing effect, but it was not strictly bactericidal. The bactericidal activity of rifampin against stationary-phase bacteria has been considered the basis for its main role in the treatment of staphylococcal foreign-body infections, although the MBC reported in the previous studies was always relatively high (between 3 and 4 μg/ml), with a very high stationary-phase MBC/MIC ratio (42, 47).
The in vitro activity of the combination of fluoroquinolones plus rifampin, mainly in the log phase, is usually reported to be antagonistic (22, 24, 26). The inhibition of RNA synthesis by rifampin is responsible for abolishing the bactericidal killing activities of quinolones, which is related to the activity against DNA supercoiling (29, 30). However, the extent to which this activity of rifampin affects the activities of newer fluoroquinolones and its efficacy against nongrowing bacteria is not yet well known (21, 28). In this regard, Bahl et al. previously reported how the combination of ciprofloxacin and rifampin showed antagonism against growing S. aureus strains but only indifference against nongrowing strains (1). In our study, it should be noted that a decrease in the bactericidal activity of levofloxacin was also observed when rifampin was added to high concentrations of levofloxacin (>8× MIC) in the stationary phase. In contrast, levofloxacin did not antagonize the activity of rifampin.
The clinical significance of the in vitro antagonism observed for the combination of rifampin plus fluoroquinolones has been of great concern. In fact, the combination of either ciprofloxacin or levofloxacin plus rifampin in animal models of staphylococcal endocarditis has been reported to produce an antagonistic effect (5, 24), whereas clinical studies have reported that the combination of ciprofloxacin and rifampin has beneficial effects (17). The combination of rifampin and ciprofloxacin or ofloxacin has demonstrated good efficacy in abscess, osteomyelitis, and foreign-body infection models; and accordingly, these combinations have been considered reference treatments for these human infections (2, 15, 16, 47, 49).
Currently, the use of high doses (750 to 1,000 mg/day) of levofloxacin in combination with rifampin has been incorporated into clinical practice for the treatment of staphylococcal orthopedic prosthetic infections (48), although very limited information is available (3, 39). Thus, a recent study by Trampuz et al. described results similar to those previously reported with the combination of rifampin and classical fluoroquinolones when rifampin and levofloxacin were used at very low doses (Cmax, 0.97 μg/ml) in a guinea pig model of staphylococcal foreign-body infection (42).
In the present study, we confirmed the high level of efficacy of levofloxacin alone against staphylococci when it is used at high doses and that it is the best therapy, as we reported previously (34). The results obtained for the combined therapies showed that they have different activities, depending on the dose of levofloxacin used. While the addition of rifampin was beneficial with conventional doses of levofloxacin (Cmax, 6 μg/ml; AUC, 68 μg·h/ml), as reported in classical studies of fluoroquinolones plus rifampin (45, 47), the combination of rifampin with high doses of levofloxacin (Cmax, 12 μg/ml; AUC, 117 μg·h/ml) diminished the efficacy of the most active treatment, which was levofloxacin alone. The antagonistic effect of adding rifampin to high doses of levofloxacin was observed in TCF with treatment for the usual 7-day period and also in the coverslips with treatment for the prolonged 14-day period. It should be of interest that no differences in efficacy were found between the combination of rifampin with a conventional dose or a high dose of levofloxacin. With respect to the efficacy of rifampin alone, both combination therapies protected against the emergence of resistance, and no significant differences in final efficacies were noticed. Furthermore, no strains resistant to levofloxacin or rifampin were detected by use of this 14-day treatment period, supporting the good safety profile against resistance development achieved with levofloxacin (13, 20), in contrast to that observed for classical quinolones (i.e., ciprofloxacin) (23). While some interaction between moxifloxacin and rifampin has been reported anecdotally (36), quinolone concentrations are not significantly altered by the addition of rifampin (41), as we noticed for the particular case of levofloxacin.
In conclusion, because of treatment efficacy and the safety profile against staphylococcal resistance, our results provide strong support for the potential use of levofloxacin at high doses alone as treatment for foreign-body infections. The clinical relevance of the antagonistic effect of adding rifampin in this setting should be further evaluated in patients receiving long-term therapy because of osteoarticular device infections. When use of the rifampin-levofloxacin combination is considered, the use of a high dosage of levofloxacin seems to offer a nonsignificant contribution to the efficacy of the combined therapy.
Acknowledgments
We thank C. Masuet of the Hospital Universitari de Bellvitge for her assistance with the statistical analysis.
This work was supported by a research grant from Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (grant FIS 04/005), and grants from the Spanish Network for Research in Infectious Diseases (grants REIPI C03/14 and REIPI RD06/0008). O.M. was supported by a grant from the REIPI.
Footnotes
Published ahead of print on 1 August 2008.
REFERENCES
- 1.Bahl, D., D. A. Miller, I. Leviton, P. Gialanella, M. J. Wolin, W. Liu, R. Perkins, and M. H. Miller. 1997. In vitro activities of ciprofloxacin and rifampin alone and in combination against growing and nongrowing strains of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 41:1293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamberger, D. M., M. T. Fields, and B. L. Herndon. 1991. Efficacies of various antimicrobial agents in treatment of Staphylococcus aureus abscesses and correlation with in vitro tests of antimicrobial activity and neutrophil killing. Antimicrob. Agents Chemother. 35:2335-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barberan, J., L. Aguilar, G. Carroquino, M. J. Gimenez, B. Sanchez, D. Martinez, and J. Prieto. 2006. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am. J. Med. 119:993.e7-993.e10. [DOI] [PubMed] [Google Scholar]
- 4.Blaser, J., P. Vergeres, A. F. Widmer, and W. Zimmerli. 1995. In vivo verification of in vitro model of antibiotic treatment of device-related infection. Antimicrob. Agents Chemother. 39:1134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers, H. F., Q. X. Liu, L. L. Chow, and C. Hackbarth. 1999. Efficacy of levofloxacin for experimental aortic-valve endocarditis in rabbits infected with viridans group streptococcus or Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2742-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapin-Robertson, K., and S. C. Edberg. 1991. Measurements of antibiotics in human body fluids: techniques and significance, p. 295-366. In V. Lorian (ed.), Antibiotics in laboratory medicine. The Williams & Wilkins Co., New York, NY.
- 7.Chow, A. T., C. Fowler, R. R. Williams, N. Morgan, S. Kaminski, and J. Natarajan. 2001. Safety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob. Agents Chemother. 45:2122-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuard, C., M. Herrmann, P. Vaudaux, F. A. Waldvogel, and D. P. Lew. 1991. Successful therapy of experimental chronic foreign-body infection due to methicillin-resistant Staphylococcus aureus by antimicrobial combinations. Antimicrob. Agents Chemother. 35:2611-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chuard, C., J. C. Lucet, P. Rohner, M. Herrmann, R. Auckenthaler, F. A. Waldvogel, and D. P. Lew. 1991. Resistance of Staphylococcus aureus recovered from infected foreign body in vivo to killing by antimicrobials. J. Infect. Dis. 163:1369-1373. [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2005. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 6th ed. M7-A6. Clinical and Laboratory Standards Institute, Wayne, PA.
- 11.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 12.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 13.Cui, J., Y. Liu, R. Wang, W. Tong, K. Drlica, and X. Zhao. 2006. The mutant selection window in rabbits infected with Staphylococcus aureus. J. Infect. Dis. 194:1601-1608. [DOI] [PubMed] [Google Scholar]
- 14.Drancourt, M., A. Stein, J. N. Argenson, R. Roiron, P. Groulier, and D. Raoult. 1997. Oral treatment of Staphylococcus spp. infected orthopaedic implants with fusidic acid or ofloxacin in combination with rifampicin. J. Antimicrob. Chemother. 39:235-240. [DOI] [PubMed] [Google Scholar]
- 15.Drancourt, M., A. Stein, J. N. Argenson, A. Zannier, G. Curvale, and D. Raoult. 1993. Oral rifampin plus ofloxacin for treatment of Staphylococcus-infected orthopedic implants. Antimicrob. Agents Chemother. 37:1214-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dworkin, R., G. Modin, S. Kunz, R. Rich, O. Zak, and M. Sande. 1990. Comparative efficacies of ciprofloxacin, pefloxacin, and vancomycin in combination with rifampin in a rat model of methicillin-resistant Staphylococcus aureus chronic osteomyelitis. Antimicrob. Agents Chemother. 34:1014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dworkin, R. J., B. L. Lee, M. A. Sande, and H. F. Chambers. 1989. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet ii:1071-1073. [DOI] [PubMed] [Google Scholar]
- 18.Eng, R. H., F. T. Padberg, S. M. Smith, E. N. Tan, and C. E. Cherubin. 1991. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob. Agents Chemother. 35:1824-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Firsov, A. A., I. Y. Lubenko, S. N. Vostrov, Y. A. Portnoy, and S. H. Zinner. 2005. Antistaphylococcal effect related to the area under the curve/MIC ratio in an in vitro dynamic model: predicted breakpoints versus clinically achievable values for seven fluoroquinolones. Antimicrob. Agents Chemother. 49:2642-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firsov, A. A., S. N. Vostrov, I. Y. Lubenko, K. Drlica, Y. A. Portnoy, and S. H. Zinner. 2003. In vitro pharmacodynamic evaluation of the mutant selection window hypothesis using four fluoroquinolones against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1604-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu, K. P., S. C. Lafredo, B. Foleno, D. M. Isaacson, J. F. Barrett, A. J. Tobia, and M. E. Rosenthale. 1992. In vitro and in vivo antibacterial activities of levofloxacin (l-ofloxacin), an optically active ofloxacin. Antimicrob. Agents Chemother. 36:860-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hackbarth, C. J., H. F. Chambers, and M. A. Sande. 1986. Serum bactericidal activity of rifampin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob. Agents Chemother. 29:611-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooper, D. C., and J. S. Wolfson. 1991. Fluoroquinolone antimicrobial agents. N. Engl. J. Med. 324:384-394. [DOI] [PubMed] [Google Scholar]
- 24.Kaatz, G. W., S. M. Seo, S. L. Barriere, L. M. Albrecht, and M. J. Rybak. 1989. Ciprofloxacin and rifampin, alone and in combination, for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 33:1184-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaka, A. S., A. M. Rueda, S. A. Shelburne III, K. Hulten, R. J. Hamill, and D. M. Musher. 2006. Bactericidal activity of orally available agents against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 58:680-683. [DOI] [PubMed] [Google Scholar]
- 26.Kang, S. L., M. J. Rybak, B. J. McGrath, G. W. Kaatz, and S. M. Seo. 1994. Pharmacodynamics of levofloxacin, ofloxacin, and ciprofloxacin, alone and in combination with rifampin, against methicillin-susceptible and -resistant Staphylococcus aureus in an in vitro infection model. Antimicrob. Agents Chemother. 38:2702-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucers, A., S. Crowe, M. L. Grayson, and J. Hoy. 1997. Rifampicin (rifampin), p. 676-708. In A. Kucers, S. Crowe, M. L. Grayson, and J. Hoy (ed.), The use of antibiotics. A clinical review of antibacterial, antifungal and antiviral drugs, 5th ed. Butterworth, Heinemann, Oxford, United Kingdom.
- 28.Lewin, C. S., and S. G. Amyes. 1989. The bactericidal activity of DR-3355, an optically active isomer of ofloxacin. J. Med. Microbiol. 30:227-231. [DOI] [PubMed] [Google Scholar]
- 29.Lewin, C. S., I. Morrissey, and J. T. Smith. 1991. The mode of action of quinolones: the paradox in activity of low and high concentrations and activity in the anaerobic environment. Eur. J. Clin. Microbiol. Infect. Dis. 10:240-248. [DOI] [PubMed] [Google Scholar]
- 30.Lewin, C. S., and J. T. Smith. 1988. Bactericidal mechanisms of ofloxacin. J. Antimicrob. Chemother. 22(Suppl. C):1-8. [DOI] [PubMed] [Google Scholar]
- 31.Lister, P. D. 2001. Pharmacodynamics of moxifloxacin and levofloxacin against Staphylococcus aureus and Staphylococcus epidermidis in an in vitro pharmacodynamic model. Clin. Infect. Dis. 32(Suppl 1):S33-S38. [DOI] [PubMed] [Google Scholar]
- 32.Lucet, J. C., M. Herrmann, P. Rohner, R. Auckenthaler, F. A. Waldvogel, and D. P. Lew. 1990. Treatment of experimental foreign body infection caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 34:2312-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maduri, T. M., D. A. Goldmann, and P. Murphy. 1983. In vitro activity of rifampin in combination with oxacillin against Staphylococcus aureus. Antimicrob. Agents Chemother. 23:571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murillo, O., A. Domenech, A. Garcia, F. Tubau, C. Cabellos, F. Gudiol, and J. Ariza. 2006. Efficacy of high doses of levofloxacin in experimental foreign-body infection by methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 50:4011-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents. M26-A. National Committee for Clinical Laboratory Standards, Villanova, PA.
- 36.Nijland, H. M., R. Ruslami, A. J. Suroto, D. M. Burger, B. Alisjahbana, R. van Crevel, and R. E. Aarnoutse. 2007. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin. Infect. Dis. 45:1001-1007. [DOI] [PubMed] [Google Scholar]
- 37.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]
- 38.Sabath, L. D., C. Garner, C. Wilcox, and M. Finland. 1976. Susceptibility of Staphylococcus aureus and Staphylococcus epidermidis to 65 antibiotics. Antimicrob. Agents Chemother. 9:962-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soriano, A., S. Garcia, G. Bori, M. Almela, X. Gallart, F. Macule, J. Sierra, J. A. Martinez, S. Suso, and J. Mensa. 2006. Treatment of acute post-surgical infection of joint arthroplasty. Clin. Microbiol. Infect. 12:930-933. [DOI] [PubMed] [Google Scholar]
- 40.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 41.Temple, M. E., and M. C. Nahata. 1999. Interaction between ciprofloxacin and rifampin. Ann. Pharmacother. 33:868-870. [DOI] [PubMed] [Google Scholar]
- 42.Trampuz, A., C. K. Murphy, D. M. Rothstein, A. F. Widmer, R. Landmann, and W. Zimmerli. 2007. Efficacy of a novel rifamycin derivative, ABI-0043, against Staphylococcus aureus in an experimental model of foreign-body infection. Antimicrob. Agents Chemother. 51:2540-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trampuz, A., M. Wenk, Z. Rajacic, and W. Zimmerli. 2000. Pharmacokinetics and pharmacodynamics of levofloxacin against Streptococcus pneumoniae and Staphylococcus aureus in human skin blister fluid. Antimicrob. Agents Chemother. 44:1352-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaudaux, P., P. Francois, C. Bisognano, J. Schrenzel, and D. P. Lew. 2002. Comparison of levofloxacin, alatrofloxacin, and vancomycin for prophylaxis and treatment of experimental foreign-body-associated infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:1503-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Widmer, A. F., R. Frei, Z. Rajacic, and W. Zimmerli. 1990. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J. Infect. Dis. 162:96-102. [DOI] [PubMed] [Google Scholar]
- 46.Widmer, A. F., A. Gaechter, P. E. Ochsner, and W. Zimmerli. 1992. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin. Infect. Dis. 14:1251-1253. [DOI] [PubMed] [Google Scholar]
- 47.Zimmerli, W., R. Frei, A. F. Widmer, and Z. Rajacic. 1994. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J. Antimicrob. Chemother. 33:959-967. [DOI] [PubMed] [Google Scholar]
- 48.Zimmerli, W., A. Trampuz, and P. E. Ochsner. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645-1654. [DOI] [PubMed] [Google Scholar]
- 49.Zimmerli, W., A. F. Widmer, M. Blatter, R. Frei, P. E. Ochsner, et al. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA 279:1537-1541. [DOI] [PubMed] [Google Scholar]