Abstract
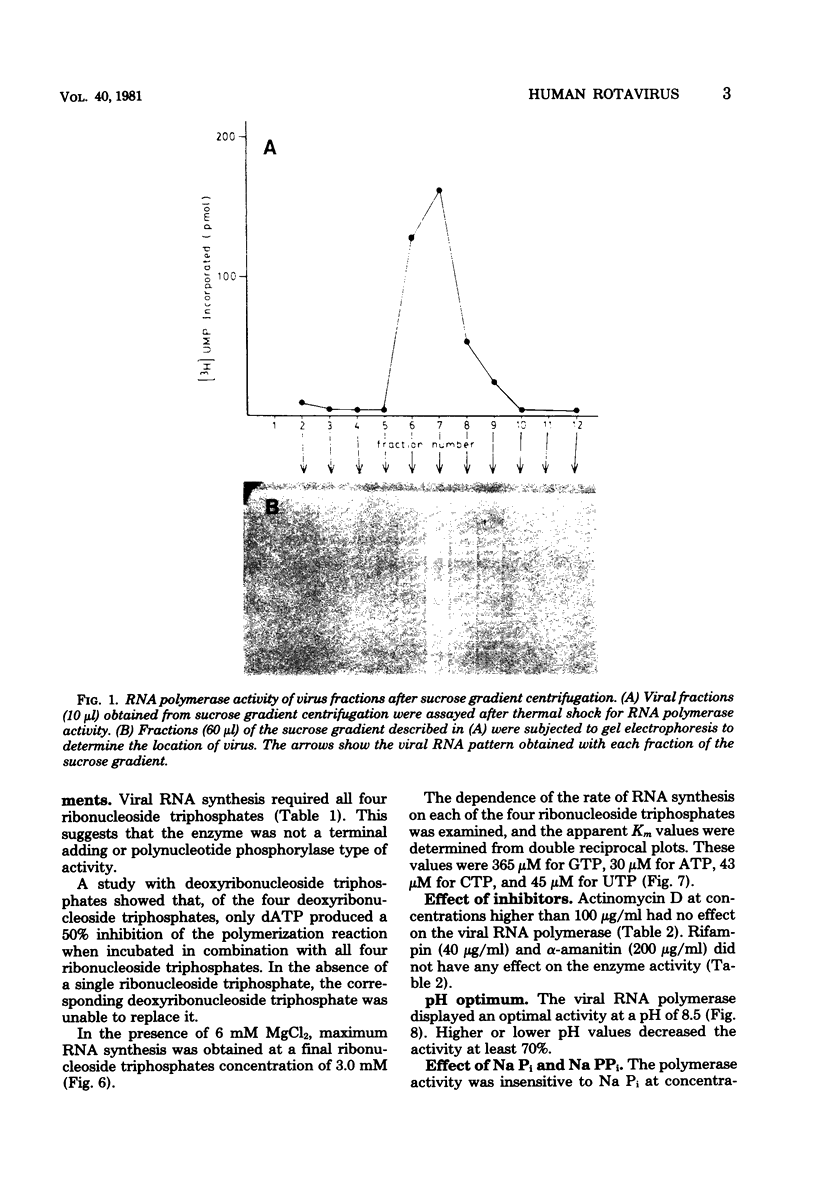
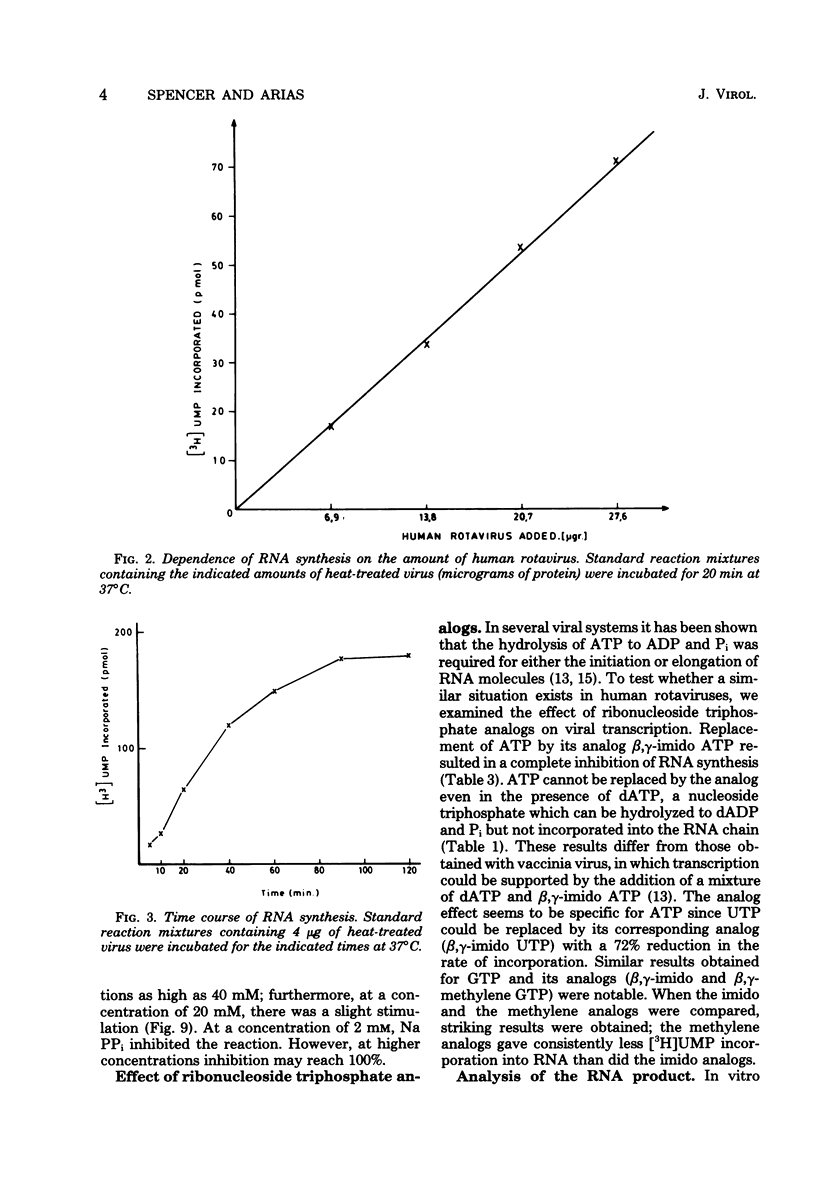
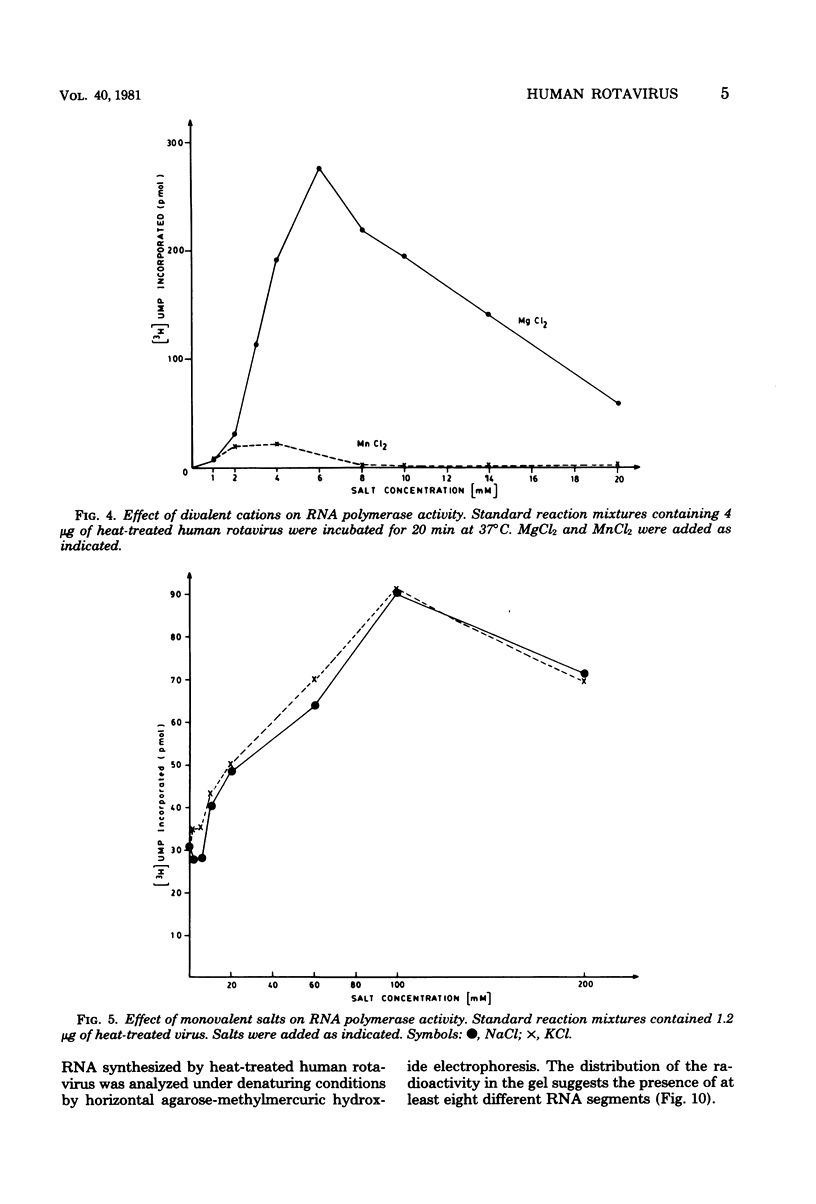
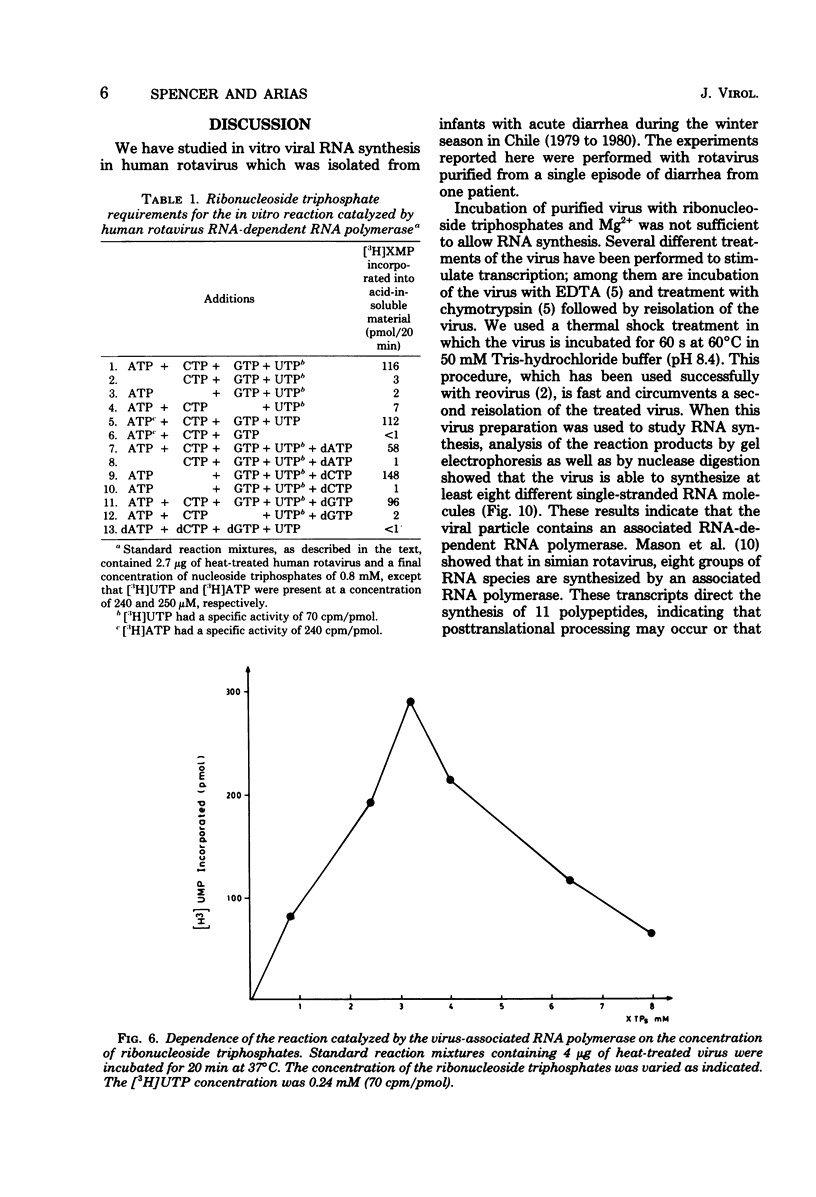
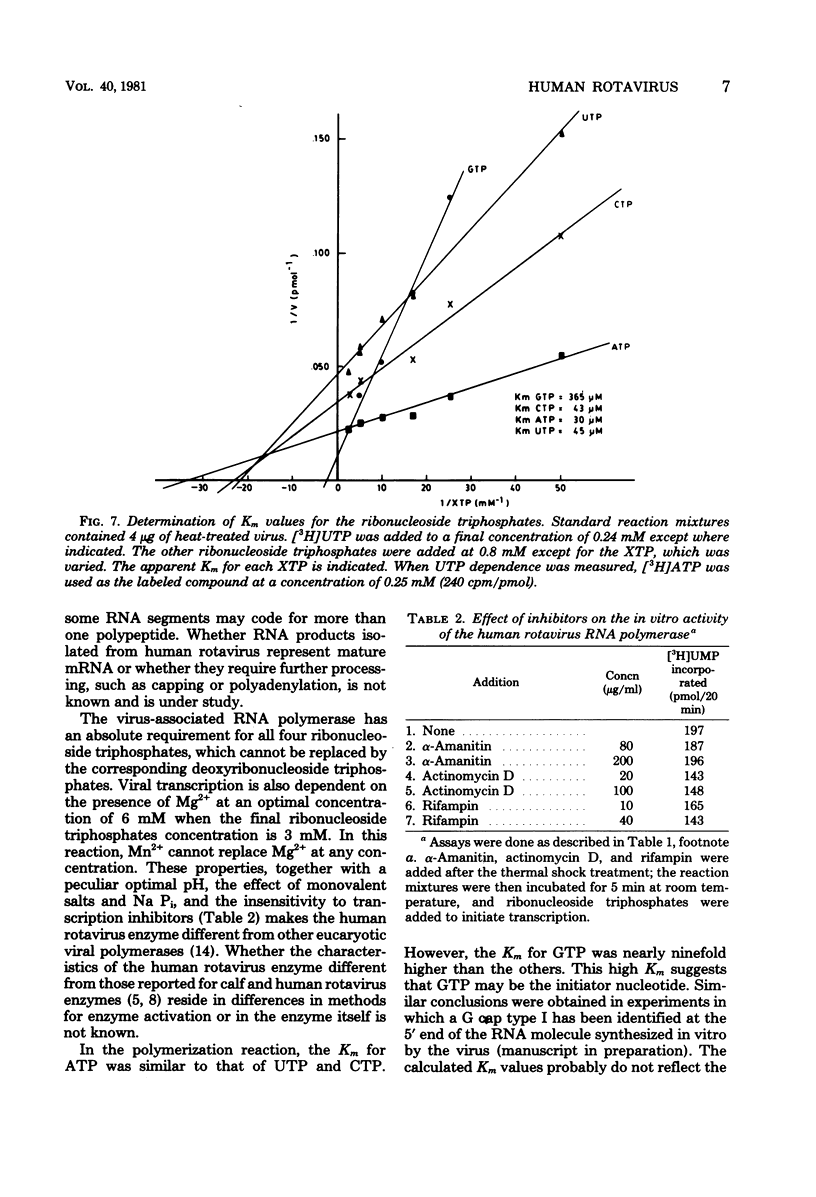
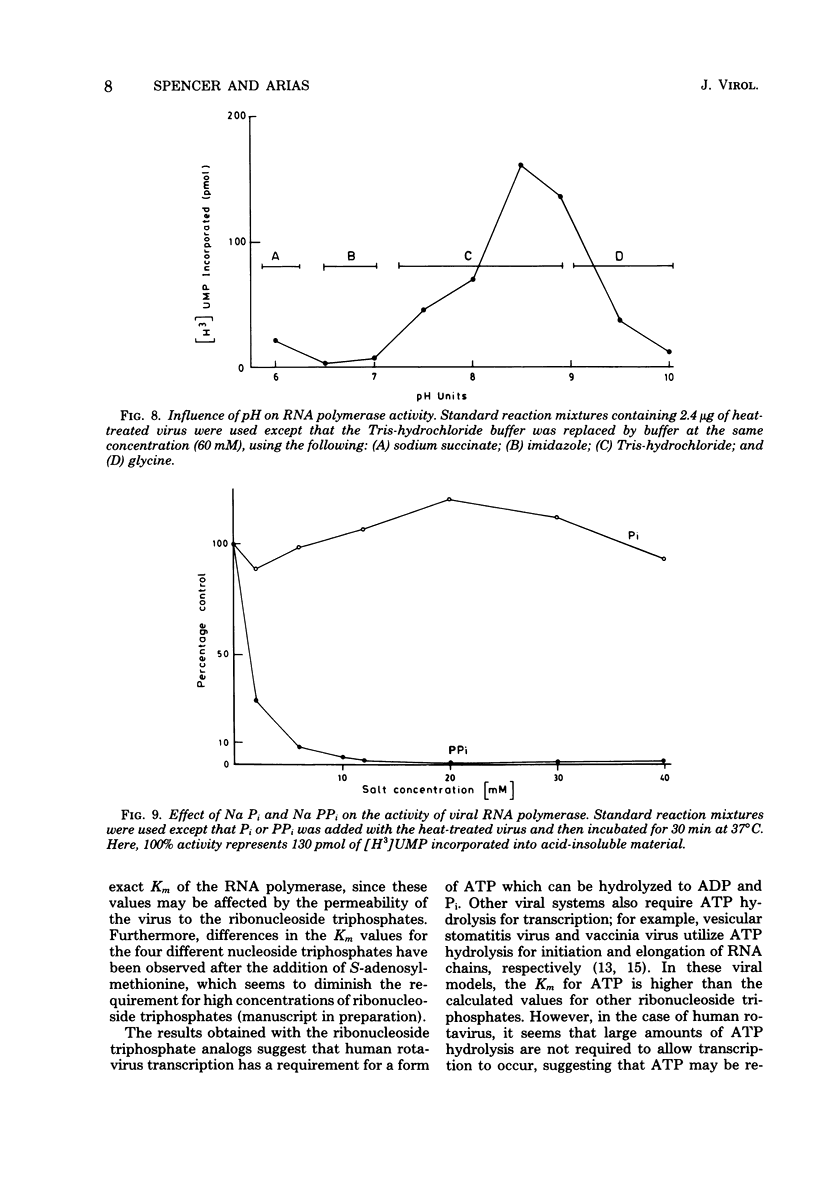
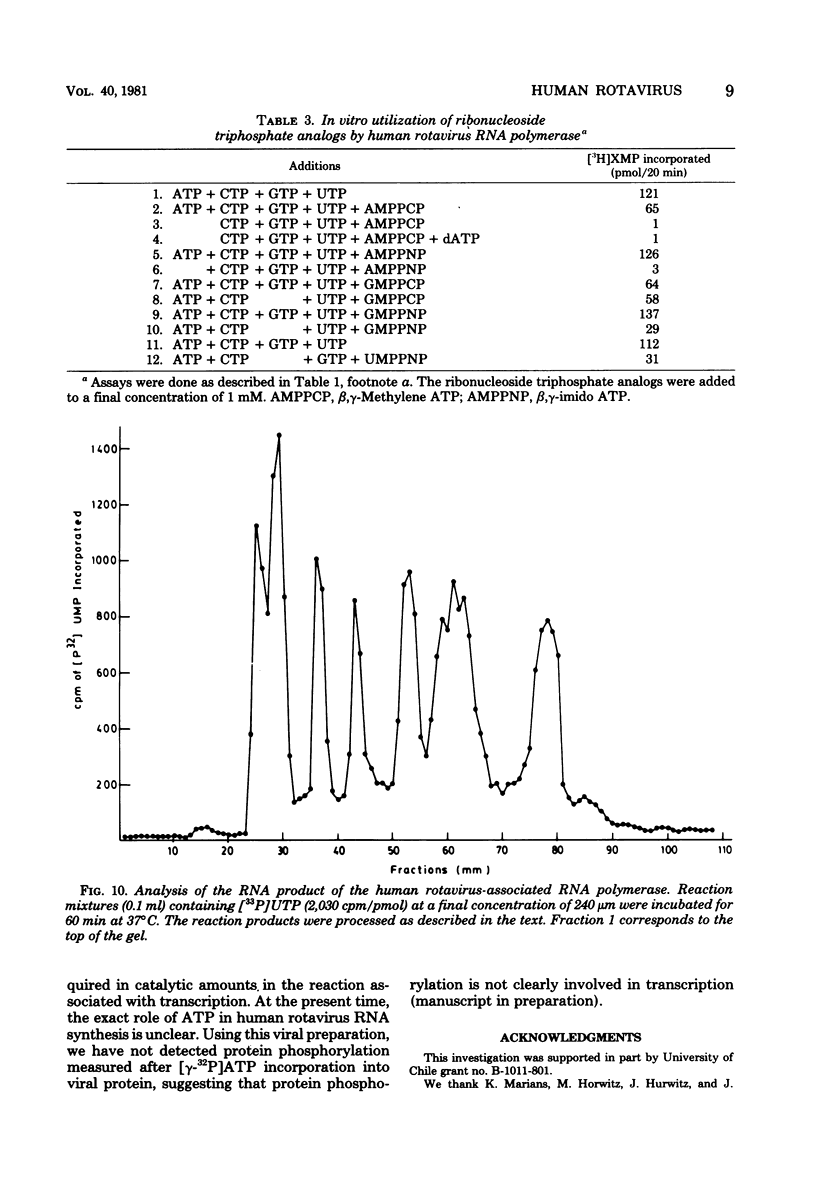
The in vitro characteristics of human rotavirus transcription have been examined. The virus has an associated RNA polymerase activity which was activated after a heat shock treatment. The enzyme required the presence of the four ribonucleoside triphosphates and a divalent cation (Mg2+), and it required an optimum pH of 8.5. The polymerase was activated by monovalent salts and inhibited by Na PPi. The addition of actinomycin D, alpha-amanitin, or rifampin did not inhibit the polymerase activity. After thermal shock of the virus, at least eight different RNA species were synthesized which may correspond to independent transcripts. Transcription also requires a hydrolyzable form of ATP. Analogs such as beta,gamma-imido ATP or beta,gamma-methylene ATP were inhibitory, whereas others, such as the beta-gamma-imido or methylene analogs of CTP, UTP, or GTP, were not inhibitory. This suggests that ATP is related to reactions other than polymerization, probably to initiation or elongation of RNA molecules, as has been described for vesicular stomatitis virus or vaccinia virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Sarkar P., Valenzuela D., Maitra U. Termination of transcription by Escherichia coli RNA polymerase: influence of secondary structure of RNA transcripts on rho-independent and rho-dependent termination. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1613–1617. doi: 10.1073/pnas.76.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chandler P. M., Rimkus D., Davidson N. Gel electrophoretic fractionation of RNAs by partial denaturation with methylmercuric hydroxide. Anal Biochem. 1979 Oct 15;99(1):200–206. doi: 10.1016/0003-2697(79)90063-0. [DOI] [PubMed] [Google Scholar]
- Cohen J., Dobos P. Cell free transcription and translation of rotavirus RNA. Biochem Biophys Res Commun. 1979 Jun 13;88(3):791–796. doi: 10.1016/0006-291x(79)91477-3. [DOI] [PubMed] [Google Scholar]
- Cohen J. Ribonucleic acid polymerase activity associated with purified calf rotavirus. J Gen Virol. 1977 Sep;36(3):395–402. doi: 10.1099/0022-1317-36-3-395. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Muñz O., Serafin F., Romero P. Shift in the prevalent human rotavirus detected by ribonucleic acid segment differences. Infect Immun. 1980 Feb;27(2):351–354. doi: 10.1128/iai.27.2.351-354.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska J. F., Notter M. F., Menegus M. A., Steinhoff M. C. RNA polymerase associated with human rotaviruses in diarrhea stools. J Virol. 1978 May;26(2):544–546. doi: 10.1128/jvi.26.2.544-546.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Wyatt R. G., Kapikian A. Z. Detection of differences among human and animal rotaviruses, using analysis of viral RNA. J Am Vet Med Assoc. 1978 Sep 1;173(5 Pt 2):531–537. [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty M. S. Rotaviruses. J Gen Virol. 1978 Jul;40(1):1–18. doi: 10.1099/0022-1317-40-1-1. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F., Bridger J. C., Woode G. N. Characterisation of a rotavirus.20b. Nature. 1975 Dec 18;258(5536):631–633. doi: 10.1038/258631a0. [DOI] [PubMed] [Google Scholar]
- Shuman S., Spencer E., Furneaux H., Hurwitz J. The role of ATP in in vitro vaccinia virus RNA synthesis effects of AMP-PNP and ATP gamma S. J Biol Chem. 1980 Jun 10;255(11):5396–5403. [PubMed] [Google Scholar]
- Spencer E., Shuman S., Hurwitz J. Purification and properties of vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1980 Jun 10;255(11):5388–5395. [PubMed] [Google Scholar]
- Testa D., Banerjee A. K. Initiation of RNA synthesis in vitro by vesicular stomatitis virus. Role of ATP. J Biol Chem. 1979 Mar 25;254(6):2053–2058. [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]