Abstract
Two clinical isolates of Aspergillus fumigatus, designated AT and DK, were recently obtained from patients failing caspofungin and itraconazole therapy, respectively. The isolates were tested by microdilution for susceptibility to itraconazole, voriconazole, posaconazole, ravuconazole, and caspofungin and by Etest for susceptibility to amphotericin B and caspofungin. Susceptibility testing documented that the DK isolate was azole resistant (itraconazole and posaconazole MICs, >4 μg/ml; voriconazole MIC, 2 μg/ml; ravuconazole MIC, 4 μg/ml), and the resistance was confirmed in a hematogenous mouse model, with mortality and the galactomannan index as the primary and secondary end points. Sequencing of the cyp51A gene revealed the M220K mutation, conferring multiazole resistance. The Etest, but not microdilution, suggested that the AT isolate was resistant to caspofungin (MIC, >32 μg/ml). In the animal model, this isolate showed reduced susceptibility to caspofungin. Sequencing of the FKS1 gene revealed no mutations; the enzyme retained full sensitivity in vitro; and investigation of the polysaccharide composition showed that the β-(1,3)-glucan proportion was unchanged. However, gene expression profiling by Northern blotting and real-time PCR demonstrated that the FKS gene was expressed at a higher level in the AT isolate than in the susceptible control isolate. To our knowledge, this is the first report to document the presence of multiazole-resistant clinical isolates in Denmark and to demonstrate reduced susceptibility to caspofungin in a clinical A. fumigatus isolate with increased expression of the FKS gene. Further research to determine the prevalence of resistance in A. fumigatus worldwide, and to develop easier and reliable tools for the identification of such isolates in routine laboratories, is warranted.
The susceptibility testing of conidium-forming molds has recently been standardized (27, 36), but no breakpoints have yet been established, and susceptibility testing of molds is generally not performed in routine clinical microbiology laboratories. While the end point readings are straightforward for azoles and amphotericin B due to the growth-versus-no-growth pattern of inhibition, the end points for the echinocandins are more difficult to determine due to significant trailing growth. Recently, a number of reports have indicated that azole resistance is emerging in clinical Aspergillus isolates (7, 42, 44, 47). Azoles act by blocking the ergosterol (an essential cell membrane component) biosynthetic pathway through binding to and inhibition of the lanosterol 14-α demethylase enzyme, encoded by the erg11 (cyp51A) gene. Azole resistance may be restricted to itraconazole or may involve cross-resistance to other triazoles as well and has been associated with a number of hot spots in the cyp51A gene with or without a simultaneous tandem repeat in the cyp51A promoter region (15, 25, 42). Resistance to caspofungin has been described for clinical Candida isolates, and recently, breakthrough infections with Aspergillus fumigatus isolates with elevated minimum effective concentrations (MECs) have been reported (18, 19). Finally, manipulated or laboratory-selected strains with various degrees of caspofungin resistance have been described (12, 31, 38). Some of these laboratory-manipulated strains have been found to have mutations in the ECM33 gene (afuEcm33), encoding cell wall proteins important for fungal cell wall organization, and in addition to possess caspofungin resistance to be hypervirulent in animal models (38). Others have mutations in the FKS1 gene, encoding a subunit of the β-1,3-d-glucan synthase enzyme involved in cell wall synthesis; this mechanism has been detected in clinical Candida isolates with reduced susceptibility to caspofungin (12). In still other resistant mutants, the glucan synthase enzyme itself exhibited a wild-type gene sequence, function, level, and caspofungin susceptibility (12).
Here we report the detection and confirmation in an animal model of the first Danish multiazole-resistant A. fumigatus isolate obtained from a patient on itraconazole treatment and the detection of an A. fumigatus isolate displaying decreased caspofungin susceptibility by the Etest, obtained from an Austrian patient failing caspofungin treatment. Our findings indicate a growing need for routine susceptibility testing of clinical A. fumigatus isolates in order to guide treatment and the need for surveillance of the susceptibility epidemiology of Aspergillus isolates in order to monitor changes in the susceptibility patterns of Aspergillus in general.
MATERIALS AND METHODS
Isolates.
The “A. fumigatus DK” isolate was recovered from a 21-year-old cystic fibrosis patient on long-term itraconazole treatment (200 mg twice daily for 14 months). This isolate was multiazole resistant (itraconazole MIC, >4 μg/ml; posaconazole MIC, >4 μg/ml; voriconazole MIC, 2 μg/ml; ravuconazole MIC, 4 μg/ml) but susceptible to amphotericin B (MIC, 0.5 μg/ml), and caspofungin (MIC, 0.125 μg/ml). The other isolate, the “A. fumigatus AT” isolate, was recovered from a patient with invasive pulmonary aspergillosis who failed caspofungin monotherapy. This isolate was susceptible to azoles and amphotericin B (MICs, 0.25 μg/ml for itraconazole, 0.06 μg/ml for posaconazole, 0.125 μg/ml for voriconazole, and 0.5 μg/ml for amphotericin B) but resistant by the Etest to caspofungin (MIC, >32 μg/ml).
Susceptibility testing.
Susceptibility was determined independently (i) at two laboratories by the EUCAST antifungal MIC method for spore-forming molds using Candida krusei ATCC 6258 as a control and (ii) at one laboratory using a modified EUCAST method with a slightly lower inoculum of 5 × 104 cells/ml (36) and (iii) susceptibility tested to caspofungin by the CLSI (formerly NCCLS) method, using Aspergillus flavus ATCC 204304 and Aspergillus fumigatus ATCC 204305 as susceptibility testing procedure controls and A. fumigatus EMFR-S678P as an echinocandin-resistant control (27). Microtiter plates were inoculated with a spore suspension, incubated at 35°C for 24 to 48 h, and read visually and with a spectrophotometer. The azole MIC was defined as the lowest drug dilution yielding no growth (single colonies on the surface are ignored), and the MEC of echinocandin was defined as the lowest drug concentration resulting in aberrant growth. Finally, susceptibilities to amphotericin B and caspofungin were determined by the Etest (AB Biodisk, Solna, Sweden) using RPMI 1640-2% glucose agar plates (SSI Diagnostika, Hillerød, Denmark) according to the manufacturer's recommendations.
DNA sequencing.
The entire coding region of the cyp51A gene from the A. fumigatus DK isolate was amplified and both strands sequenced as previously described (15). Consensus sequences were aligned and mismatches identified using AlignX (Invitrogen, Paisley, United Kingdom). Mutations were confirmed by repeating PCR and sequencing of both strands using the closest forward and reverse primers (15). The sequences were compared to those of a published azole-susceptible strain (GenBank accession no. AF338659) and azole-susceptible clinical isolates (unpublished data from other studies). The A. fumigatus FKS1 gene (GenBank accession no. AFU79728) from the AT isolate was sequenced in hot spot regions between nucleotides 1875 and 4318 by Sanger methodology using a CEQ 8000 Beckman Coulter genetic analysis system. The putative FKS1 gene of Aspergillus lentulus was sequenced between nucleotides 1880 and 2300 and nucleotides 3900 and 4300 (equivalent to A. fumigatus FKS1 hot spots 1 and 2, respectively). A. fumigatus ATCC 13073, A. fumigatus 293, and A. fumigatus CNM-CM-237 (23), as well as A. lentulus CNM-CM-3583, A. lentulus CNM-CM-3599, and A. lentulus CNM-CM-4420 (1), were used as control strains.
Glucan synthase expression by Northern blotting.
For RNA isolation, conidial suspensions (1 × 105 CFU/ml) of a caspofungin-susceptible control clinical isolate (A. fumigatus A29; MEC, 0.04 μg/ml; Etest MIC, 0.094 μg/ml) and the A. fumigatus AT isolate were incubated in the absence and presence of subinhibitory concentrations of caspofungin (0.04 μg/ml) for 24 h at 37°C with shaking. RNA was isolated by use of TRI reagent (Sigma-Aldrich). For Northern blot analysis, 10 μg of total RNA was electrophoresed on 1.2% (wt/vol) agarose-2.2 M formaldehyde gels and blotted onto Hybond N membranes (Amersham Biosciences) (11, 17). The hybridization probes used in this study were generated by PCR using oligonucleotides 5′-GGAAAGCACGGAAAGCAG-3′ and 5′-AAACACACCAGGAGCCAG-3′ for the FKS gene.
Glucan synthase expression profiling by real-time PCR.
A. fumigatus strains were grown in RPMI 1640 and incubated at 37°C with shaking (150 rpm) for 24 h with and without 0.25 μg/ml caspofungin. Total RNA was extracted using the RNeasy minikit (Qiagen). Real-time PCR experiments were performed on a Stratagene Mx3005P multiplex quantitative PCR system using the “quantitative PCR (multiple standards)” setting. A one-step quantitative reverse transcription-PCR kit (Stratagene, La Jolla, CA) combined with molecular beacons was used for all reactions. Each real-time PCR was carried out in a 50-μl reaction volume containing 25 μl of 2× quantitative PCR master mix, 20 pmol of each molecular beacon, 25 pmol of each corresponding primer, and 100 ng of A. fumigatus total RNA. The A. fumigatus molecular beacons and primers were designed using the FKS1 (GenBank accession no. AFU79728), PMA1 (GenBank accession no. AY040608), and beta-tubulin (BTU) (GenBank accession no. AY048754) genes. Relative expression was evaluated using the Pfaffl method (33). The A. fumigatus PMA1 gene and beta-tubulin gene (BTU) were used to normalize the results (4, 25). Two housekeeping genes were used because differences in expression were observed at different time points in A. fumigatus (unpublished data). No significant differences were observed when these two normalizing genes were used under the conditions of these experiments. Molecular beacons were labeled with 5-carboxyfluorescein at the 5′ ends and with benzoic acid succinimidyl ester (dabcyl) at the 3′ ends. All of the molecular beacons were purchased from Biosearch Technologies, Inc. (Novato, CA). PCR primers were designed by using the oligonucleotide design tool of IDT SciTools (Integrated DNA Technologies, Coralville, IA) and were purchased from Integrated DNA Technologies.
Glucan synthase inhibition profiling.
Each A. fumigatus isolate was grown for 24 h with vigorous shaking at 37°C in YPD (2% yeast extract, 4% Bacto peptone, 4% dextrose) broth, and mycelia were collected by centrifugation. Cell disruption, membrane protein extraction, and partial glucan synthase purification by-product entrapment were carried out as described previously (35). Fractions of each step were conserved at −86°C as a procedure control. Susceptibility to the echinocandin drugs was measured by a polymerization assay using a 96-well multiscreen HTS filtration system (Millipore Corporation, Bedford, MA) in a final volume of 100 μl as previously described (30). Serial dilutions of anidulafungin, caspofungin, and micafungin were added (1 μl/well). Control reactions were performed in the presence of 1% dimethyl sulfoxide when anidulafungin was used. The reactions were initiated by addition of the enzyme. Inhibition curves and 50% inhibitory concentrations were determined using a sigmoidal response (variable-slope) curve and a two-site competition fitting algorithm with GraphPad Prism software (version 4.0; Prism Software, Irvine, CA).
Polysaccharide composition.
Cell wall alkali-insoluble extracts were prepared from the caspofungin-susceptible control clinical isolate (A. fumigatus A29; MEC, 0.04 μg/ml; Etest MIC, 0.094 μg/ml) and the resistant A. fumigatus (AT) isolate by following the method developed by Fontaine et al. (10) with slight modifications. The dried samples were boiled twice with 5 ml Tris (50 mM)-EDTA (50 mM)-sodium dodecyl sulfate (2%)-mercaptoethanol (40 mM) reagent (pH 7.5). After centrifugation (at 3,600 rpm for 10 min), the sediment (cell wall ghost) was washed five times with water, followed by freeze-drying. Freeze-dried Tris-EDTA-sodium dodecyl sulfate-mercaptoethanol extracts were then subjected to alkali fractionation (1 M NaOH plus 0.05 M NaBH4, 20 ml, at 70°C, twice). After centrifugation (at 3,600 rpm for 10 min), the sediment (alkali-insoluble fraction) obtained was washed with water until neutrality (four to five times) and freeze-dried (10). Stock solutions were prepared (10-mg/ml contents were dispersed by sonication). One hundred micrograms of these alkali-insoluble fractions was then treated with 10 μl (corresponding to 3.7 μg protein) of LamA, a recombinant endo-β(1,3)-glucanase from Thermotoga neapolitana (49), in acetate buffer (pH 6.0; 50 mM) in a total volume of 200 μl overnight at 37°C. Contents were centrifuged at 3,600 rpm for 10 min; 20 μl of the supernatant (LamA digest) was injected into a Dionex high-performance anion-exchange chromatography system; and 100-μl supernatants were taken for reducing sugar assays by the p-amino-hydroxybenzoic acid hydrazide reagent method, including corresponding blanks with heat-inactivated LamA (9). The hexose composition of the samples was estimated by gas chromatography using meso-inositol as the internal standard (10).
Radial growth velocity evaluation.
A. fumigatus conidia (103 per petri dish) were inoculated into the centers of 90-mm-diameter minimal medium agar and YPD (1% yeast extract, 2% Bacto peptone, 2% dextrose) agar petri dishes. Colony diameters were measured in triplicate every 24 h for 96 h. The average diameter was used to determine the radial growth rate (Kr). Kr was calculated using the linear regression of the radius versus time by a method described previously (34).
Animal model.
A total of 138 NMRI mice (weight, 26 to 30 g; Harlan Scandinavia, Allerød, Denmark) were injected intraperitoneally (i.p.) with 200 mg of cyclophosphamide/kg of body weight on day −3 and 100 mg/kg on day zero to produce prolonged immunosuppression. Mice were kept five to seven to a cage and were allowed free access to food and water. Mice in groups of 10 to 13 were challenged on day zero by intravenous injection of an inoculum of 1.5 × 105 CFU of A. fumigatus in 200 μl with a 25-gauge syringe. Forty-nine mice received the A. fumigatus DK isolate, and 49 and 40 mice received the A. fumigatus AT isolate in two separate experiments. Mice were treated on days 1 to 4 (Monday to Friday) and 7 to 10 (Monday to Thursday) with 0.25 ml of either caspofungin administered i.p. (2.25 mg/kg), low-dose posaconazole given orally (5 mg/kg), high-dose posaconazole given orally (20 mg/kg), or glucose given i.p. (control mice) (29, 40, 46, 48). Mice were observed daily and evaluated by assigning one of the following scores, from 0 to 4: 0, healthy; 1, minor clinical signs of infection and inflammation (e.g., observations of minor signs of distress and pain, changed activity, and social withdrawal); 2, severe signs of infection, such as stiff movements, lack of curiosity, forced ventilation, changed body position, piloerection on the skin, or changes in the pattern of movement (animals scored 2 were reevaluated later the same day); 3, severe suffering and pain (the mouse was sacrificed to minimize its suffering); 4, dead. Surviving mice were sacrificed on day 11 after a total bleed. The experiments were approved by the Danish Animal Experimentation Committee under the Ministry of Justice (number 2004/561-835).
Galactomannan (GM) antigen detection was performed on serum samples obtained on day 11 from surviving mice by use of the commercially available Platelia Aspergillus kit for immunoenzymatic detection of GM antigen (Bio-Rad, France).
Statistics.
Survival was compared by a Mantel-Cox log rank test with a P value of <0.05 as the level of significance. GM levels were compared among the various treatment groups by using the Mann-Whitney test.
RESULTS
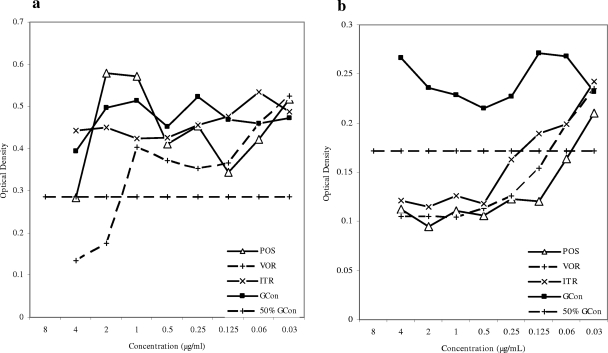
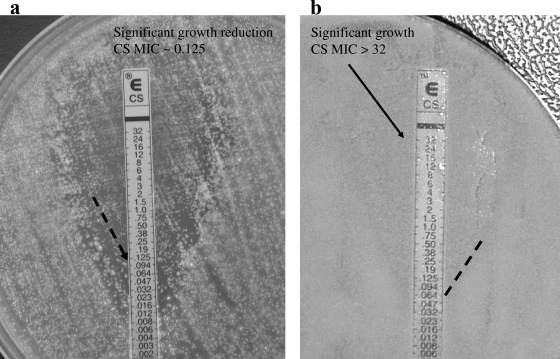
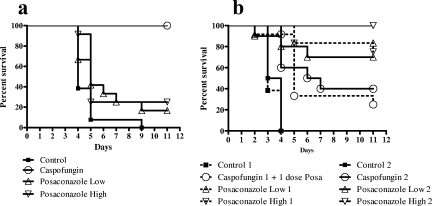
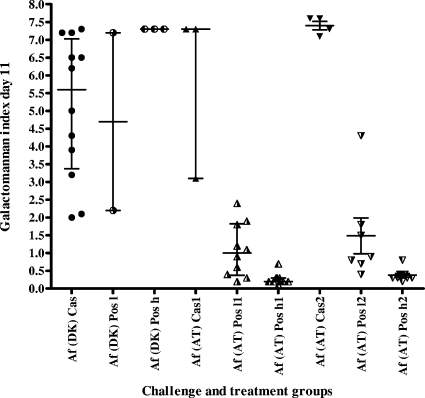
Testing of the susceptibility of the A. fumigatus DK isolate showed it to be azole resistant (Fig. 1a) but caspofungin susceptible, with a microdilution MEC of 0.25 μg/ml and an Etest end point of 0.125 μg/ml (Fig. 2a). The isolate was lethal in the mouse model at inoculum sizes described previously, with a mortality rate of 93% on day 5 and 100% mortality on day 9 (Fig. 3). The survival of mice was 100% in the caspofungin group, in contrast to 16.7 and 25% survival in the two posaconazole-treated groups, respectively (P < 0.0001 and P = 0.0002 for comparisons of caspofungin-treated animals with animals receiving low- and high-dose posaconazole, respectively). The survival of treated animals receiving caspofungin or high-dose posaconazole was significantly better than that of control animals (P < 0.0001 and P = 0.005, respectively). Aspergillus GM index values in the sera of mice surviving on day 11 were determined (Fig. 4). All surviving animals challenged with the A. fumigatus DK isolate were positive for GM antigen, and no statistical difference was found between the index levels for caspofungin-treated and posaconazole-treated animals (P = 0.0887). In order to characterize the mechanism of resistance, the entire coding region of the cyp51A gene was amplified, and both strands were sequenced; revealing an M220K mutation (an alteration from methionine to lysine at codon 220).
FIG. 1.
Growth curves for the A. fumigatus DK (a) and AT (b) isolates determined by the EUCAST microdilution method after 2 days of incubation. POS, posaconazole (4 to 0.03 μg/ml); VOR, voriconazole (4 to 0.03 μg/ml); ITR, itraconazole (4 to 0.03 μg/ml); GCon, growth control (no antifungal).
FIG. 2.
Susceptibilities of the A. fumigatus DK (a) and AT (b) isolates to caspofungin (CS), determined by Etest after 2 days of incubation.
FIG. 3.
Survival curves for mice challenged with A. fumigatus DK (a) or A. fumigatus AT (b) on day zero and subsequently treated with caspofungin, low-dose posaconazole, high-dose posaconazole, or glucose (control group). For the AT isolate, the experiment was performed twice (dashed lines and solid lines indicate results for experiments 1 and 2, respectively), because the caspofungin-treated animals in the first experiment accidentally received one posaconazole dose on day 3.
FIG. 4.
Aspergillus GM antigen index values for surviving mice challenged with the A. fumigatus DK [Af (DK)] or A. fumigatus AT [Af (AT)] isolate. Individual values, medians, and interquartile ranges are displayed. Cas, caspofungin; Pos l, low-dose posaconazole; Pos h, high-dose posaconazole; 2, second (repeated) experiment.
The Etest suggested that the A. fumigatus AT isolate was caspofungin resistant (Fig. 2b). The microdilution assay, however, showed partial growth inhibition of both the AT and DK isolates by caspofungin, and attempts to determine MEC end points by evaluation of micromorphology did not demonstrate differences in susceptibility to caspofungin; both isolates showed aberrant growth at concentrations of 0.25 μg/ml or higher (Fig. 5). In vivo susceptibility tests in the mouse model were performed twice for the A. fumigatus AT isolate, because initially the animals in the caspofungin group accidentally received one dose of posaconazole on day 3 instead of caspofungin, which might have improved their outcome. In both experiments, the survival of animals receiving caspofungin was low (25 to 40%) compared to that of animals receiving posaconazole (70 to 83% survival in the low-dose groups and 75 to 100% survival in the high-dose groups [Fig. 3]). This lower survival for caspofungin-treated animals was statistically significant compared to the survival rates for both posaconazole groups in the first experiment (P values, 0.097 and 0.0115, respectively) and compared to that of the high-dose posaconazole group in the second experiment (P = 0.0039). However, for all treatment groups, survival was better than that for the control groups (P = 0.001 to <0.0001). Animals treated with posaconazole had a significant dose-dependent decrease in the GM indices relative to those of control and caspofungin-treated animals (Fig. 4).
FIG. 5.
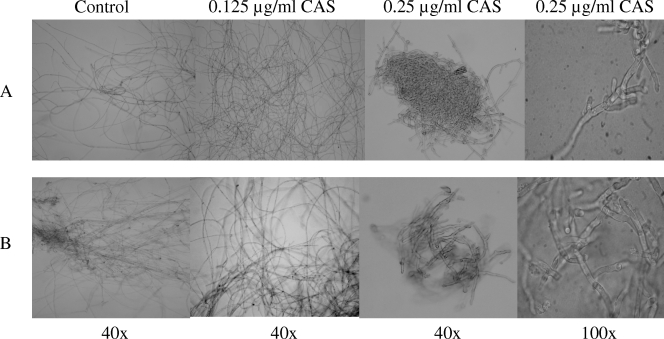
Light microscopic MEC evaluation according to EUCAST methodology after 24 h at 35°C. Shown are changes in the A. fumigatus AT (A) and A. fumigatus DK (B) isolates under caspofungin (CAS) treatment. The positive control shows long, unbranched hyphal elements. CAS treatment at ≥0.25 μg/ml resulted in abnormal, short, and branched hyphal clusters in both the AT isolate (CAS resistant) and the DK isolate (CS sensitive).
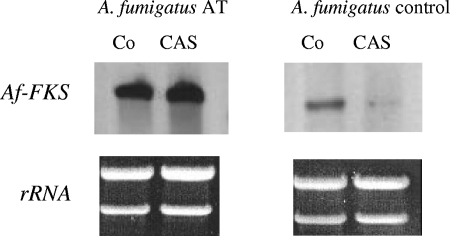
Sequencing of the FKS1 gene of the A. fumigatus AT isolate did not reveal any mutations. Susceptibility profiling of the glucan synthase showed full susceptibility (data not shown), and when the carbohydrate composition was investigated, the total hexose content was decreased in both the AT isolate and the control isolate upon caspofungin pretreatment, but the proportion of β-1,3-d-glucan was unchanged (Table 1). However, Northern blot analysis demonstrated expression of the FKS gene in susceptible and resistant A. fumigatus isolates, yet with marked differences in the expression level, as shown in Fig. 6. Caspofungin treatment did not reduce FKS gene expression in the A. fumigatus AT isolate, in contrast to the reduction in the susceptible control isolate (Fig. 6). This overexpression of the FKS gene was confirmed by real-time PCR. Table 2 compares the FKS1 expression ratios of the A. fumigatus AT isolate and two wild-type strains. The AT isolate showed constitutively higher FKS1 expression (3.09-fold on average) than the wild-type strains. In the presence of a low concentration of caspofungin, the AT isolate showed higher expression ratios than the wild-type strains (3.15-fold on average). These experiments were also performed at higher caspofungin concentrations. When ≥0.5 μg/ml of caspofungin was used, no A. fumigatus total RNA was obtained for the AT isolate, ATCC 13073, or A. fumigatus 293. However, RNA of strain EMFR-S678P (35) was obtained at high caspofungin concentrations (>4 μg/ml).
TABLE 1.
Polysaccharide composition of the AT isolate with and without caspofungin pretreatment in comparison with the control
| Isolate and pretreatmentc | Caspofungin susceptibility | Dry wt (mg) of mycelium | Wt (mg) of AIa | % of dry wt (mg) of mycelium that is AI | % of the AI wt (mg) that is hexosesb | % of total amt of hexoses in the AI that isb
|
% of total amt of hexoses that is a β-(1,3)-glucanb | ||
|---|---|---|---|---|---|---|---|---|---|
| Mannose | Glucose | Galactose | |||||||
| AT | Reduced | ||||||||
| Cas | 30 | 4.7 | 15.7 | 6.5 | 10 | 74 | 16 | 81 | |
| Cas | 60 | 12.5 | 20.8 | ||||||
| None | 142 | 22.6 | 15.9 | 12 | 7 | 86 | 7 | 81 | |
| Control | Normal | ||||||||
| Cas | 15 | 1.5 | 10.0 | 6 | 10 | 74 | 16 | 79 | |
| Cas | 50 | 8.4 | 16.8 | ||||||
| None | 108 | 20.6 | 19.1 | 12 | 5 | 91 | 4 | 77 | |
AI, alkali-insoluble fraction.
Data are averages for two samples for the caspofungin-pretreated isolates.
Cas, caspofungin pretreatment by culturing in the presence of 0.04 μg/ml caspofungin.
FIG. 6.
Expression analysis of the fks gene in caspofungin-resistant (AT) and -susceptible (control) A. fumigatus isolates. Total RNA was extracted from mycelia grown for 24 h at 37°C in the absence (Co) and presence (CAS) of caspofungin. RNA (10 μg) was separated by agarose gel electrophoresis, blotted, and hybridized with an fks probe coding for the β-1,3-glucan synthase complex. Caspofungin treatment did not influence fks gene expression by the resistant A. fumigatus (AT) isolate relative to that by untreated fungi, whereas the susceptible A. fumigatus control isolate showed lower gene expression when cultured in the presence of CAS. Ethidium bromide staining of 26S and 18S rRNA was used as a loading control.
TABLE 2.
FKS1 expression ratios
| Housekeeping gene | Ratios of the FKS1 expressiona in the AT isolate to that in the following reference strains in the indicated mediumb:
|
|||
|---|---|---|---|---|
| ATCC 13073
|
A. fumigatus 293
|
|||
| RPMI 1640 | RPMI 1640 + CSF | RPMI 1640 | RPMI 1640 + CSF | |
| PMA1 | 3.82 | 3.50 | 2.36 | 2.90 |
| BTU | 3.70 | 3.41 | 2.28 | 2.78 |
Calculated as the fold difference between the expression of FKS1 and the expression of the housekeeping gene PMA1 or BTU.
CSF, caspofungin added at a concentration of 0.25 μg/ml.
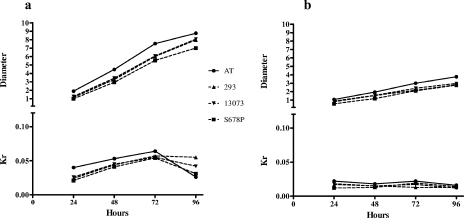
Finally, we observed a higher growth rate for the AT isolate. In order to evaluate these preliminary observations objectively, the radial growth rate was determined. Two different variables were measured: diameter and Kr. The latter variable is independent of time and is related to the specific growth rate when the culture conditions are constant (34). Thus, Kr variations represent metabolic differences between different strains more accurately than diameter. Figure 7 shows the results of measurement of these two variables for 4 days. All the strains reached the exponential, stationary, and death phases at the same times (24 to 48 h, 48 to 72 h, and 72 to 96 h, respectively) independently of the solid medium used. The AT isolate showed a larger colony diameter and a higher Kr but the same slope as the wild type and the caspofungin-resistant FKS1 mutant (Fig. 7). These results may suggest a higher metabolic rate for the AT isolate with no alteration in the cell cycle (higher Kr and no differences in growth curves).
FIG. 7.
Evaluation of the radial growth rate of the A. fumigatus AT isolate in comparison with those of the caspofungin-susceptible control strains ATCC 13073 and A. fumigatus 293 and the caspofungin-resistant A. fumigatus strain EMFR-S678P on YPD agar (a) and minimal medium agar (b). The growth rate is expressed as the colony diameter (in centimeters) and the Kr (in centimeters per hour) after 24 to 96 h.
DISCUSSION
A decade ago, amphotericin B formulations and itraconazole were the only options for antifungal treatment of invasive aspergillosis, but since then, voriconazole has become the first choice for primary therapy due to its superior efficacy in comparison with conventional treatment, and caspofungin has been licensed for salvage therapy (13, 21, 45). With the increasing rates of invasive aspergillosis and candidiasis, the use of expanded-spectrum azoles and echinocandins has increased, and accordingly the potential selection pressure has also increased. Azole resistance, including voriconazole and/or posaconazole resistance, has been reported for clinical Aspergillus isolates in the United Kingdom, Spain, the United States, and, with increasing frequency, The Netherlands (3, 15, 24, 25, 44). Recently, breakthrough infections with A. fumigatus isolates, with MECs in the range of 0.125 to 8 μg/ml, in patients receiving caspofungin prophylaxis/empirical treatment have been reported (19). Finally, slow-sporulating A. fumigatus isolates with multi-drug class resistance have been isolated from patients who received prior fluconazole treatment (3).
The DK isolate described here was resistant to posaconazole in vivo, in an animal model, with mortality and the GM index at the end of therapy as end points. Posaconazole was chosen as the compound because it is metabolized less rapidly in mice than voriconazole (28, 37, 41). The clinical context was prior itraconazole treatment for 14 months. However, infections in azole-naïve patients with resistant strains have been described elsewhere, suggesting the possible presence of azole-resistant isolates in the environment (44). Azoles act by inhibiting lanosterol 14α-demethylase (Cyp51), an enzyme in the ergosterol biosynthetic pathway, resulting in fungal cell instability. The most commonly reported mechanism of azole resistance in Aspergillus is single nucleotide repeats in the cyp51A gene (5, 6, 8, 22, 24, 26). These substituted amino acids may alter drug binding, thus conferring resistance. In the present case, an M220K mutation was detected in the cyp51A gene. Codon 220 is a well-characterized hot spot, for which a replacement of methionine with isoleucine, associated with itraconazole resistance and reduced susceptibility to other azoles, is most commonly reported (5, 6, 24). The effect on cross-resistance is dependent on the specific amino acid substitution. In the present case, the mutation involved the replacement of methionine with lysine, and the isolate also showed cross-resistance to voriconazole, posaconazole, and ravuconazole, in agreement with the findings for a similar clinical isolate from the United Kingdom (14, 24). Despite successful outcomes for the caspofungin-treated mice, the GM levels were not significantly decreased. Several previous studies have reported correlations between GM levels and outcome during caspofungin treatment (20, 43). However, a paradoxical increase in the GM index or in quantitative PCR results despite a histopathological effect and/or clinical improvement has been observed in a number of other studies of animal models and of humans (16, 32, 39, 48). In vitro, higher doses of caspofungin but not voriconazole have been shown to be associated with an increased release of GM into the culture medium (16), and one may speculate that the primarily static inhibition exhibited by caspofungin in comparison to the more fungicidal anti-Aspergillus activity of azoles may contribute to the prolonged circulation of GM despite clinical success.
The results regarding the caspofungin susceptibility of the A. fumigatus AT isolate are less clear. The Etest and animal model results suggested reduced susceptibility, but in our hands the microdilution MEC determination did not identify this isolate as unusual. The studies of the possible mechanism showed that changes at the enzyme level were not relevant, because there were no FKS1 mutations and the semipurified enzyme retained full susceptibility in vitro. The isolate was characterized by rapid growth, and gene expression profiling upon exposure to subinhibitory concentrations of caspofungin demonstrated overexpression of the FKS gene, suggesting that this played a role. Thus, even though the exact mechanism has not yet been determined, it is likely that a conditional resistance mechanism is operational. The clinical context was failure despite caspofungin treatment. However, other factors may have contributed to the poor outcome, and our animal experiments cannot predict if human infection may be treated with or without elevated doses of caspofungin. The EUCAST and CLSI microdilution MEC tests did not categorize this isolate as unusual, and recently, Candida albicans isolates with mutations in the hot spots and markedly elevated Etest MICs were shown to have only slightly elevated caspofungin MECs by microdilution (reference 2 and personal observation). These findings indicate that further studies are needed to define the best methodological parameters for in vitro testing of susceptibility to caspofungin and to determine the true rate of reduced susceptibility in Candida and Aspergillus.
In summary, this is, to our knowledge, the first report to describe and confirm in an animal model a clinical A. fumigatus isolate from Denmark with multiazole resistance and a clinical isolate for which the Etest end point and in vivo susceptibility testing in an animal model suggest decreased susceptibility to caspofungin, possibly due to upregulation of the target enzyme level. Our detection of these isolates illustrates the necessity to be aware of reduced susceptibility among clinical A. fumigatus isolates and the need for monitoring susceptibility not only for epidemiological purposes but also for the guidance of clinical treatment.
Acknowledgments
We thank Jytte Mark Andersen, Frederikke Rosenborg Petersen, and Birgit Brandt for excellent technical assistance. We thank Marianne Skov for providing the information on the azole treatment of the Danish patient, Pfizer for providing voriconazole pure substance, Merck for providing caspofungin pure substance, Schering Plough for providing posaconazole pure substance for the microdilution assays, and Eisai for providing ravuconazole pure substance.
A portion of this work was supported by NIH-NIAID grant AI069397 to D. Perlin.
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Alcazar-Fuoli, L., E. Mellado, A. Alastruey-Izquierdo, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2008. Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob. Agents Chemother. 52:1244-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baixench, M. T., N. Aoun, M. Desnos-Ollivier, D. Garcia-Hermoso, S. Bretagne, S. Ramires, C. Piketty, and E. Dannaoui. 2007. Acquired resistance to echinocandins in Candida albicans: case report and review. J. Antimicrob. Chemother. 59:1076-1083. [DOI] [PubMed] [Google Scholar]
- 3.Balajee, S. A., M. Weaver, A. Imhof, J. Gribskov, and K. A. Marr. 2004. Aspergillus fumigatus variant with decreased susceptibility to multiple antifungals. Antimicrob. Agents Chemother. 48:1197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burghoorn, H. P., P. Soteropoulos, P. Paderu, R. Kashiwazaki, and D. S. Perlin. 2002. Molecular evaluation of the plasma membrane proton pump from Aspergillus fumigatus. Antimicrob. Agents Chemother. 46:615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, J., H. Li, R. Li, D. Bu, and Z. Wan. 2005. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J. Antimicrob. Chemother. 55:31-37. [DOI] [PubMed] [Google Scholar]
- 6.da Silva Ferreira, M. E., J. L. Capellaro, M. E. dos Reis, I. Malavazi, D. Perlin, S. Park, J. B. Anderson, A. L. Colombo, B. A. Arthington-Skaggs, M. H. Goldman, and G. H. Goldman. 2004. In vitro evolution of itraconazole resistance in Aspergillus fumigatus involves multiple mechanisms of resistance. Antimicrob. Agents Chemother. 48:4405-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denning, D. W., K. Venkateswarlu, K. L. Oakley, M. J. Anderson, N. J. Manning, D. A. Stevens, D. W. Warnock, and S. L. Kelly. 1997. Itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1364-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Guerra, T. M., E. Mellado, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2003. A point mutation in the 14α-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 47:1120-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontaine, T., R. P. Hartland, A. Beauvais, M. Diaquin, and J. P. Latge. 1997. Purification and characterization of an endo-1,3-β-glucanase from Aspergillus fumigatus. Eur. J. Biochem. 243:315-321. [DOI] [PubMed] [Google Scholar]
- 10.Fontaine, T., C. Simenel, G. Dubreucq, O. Adam, M. Delepierre, J. Lemoine, C. E. Vorgias, M. Diaquin, and J. P. Latge. 2000. Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J. Biol. Chem. 275:27594-27607. [DOI] [PubMed] [Google Scholar]
- 11.Fourney, R. M., J. Miyakoshi, R. S. Day III, and M. C. Paterson. 1988. Northern blotting: efficient RNA staining and transfer. Focus 10:16-22. [Google Scholar]
- 12.Gardiner, R. E., P. Souteropoulos, S. Park, and D. S. Perlin. 2005. Characterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofungin. Med. Mycol. 43(Suppl. 1):S299-S305. [DOI] [PubMed] [Google Scholar]
- 13.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, and B. de Pauw, for the Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 14.Howard, S. J., A. Albarrag, M. J. Anderson, R. E. Gardiner, S. Park, and D. Perlin. 2006. Azole resistance in clinical Aspergillus fumigatus strains, abstr. M-1753. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA. American Society for Microbiology, Washington, DC.
- 15.Howard, S. J., I. Webster, C. B. Moore, R. E. Gardiner, S. Park, D. S. Perlin, and D. W. Denning. 2006. Multi-azole resistance in Aspergillus fumigatus. Int. J. Antimicrob. Agents 28:450-453. [DOI] [PubMed] [Google Scholar]
- 16.Klont, R. R., M. A. Mennink-Kersten, D. Ruegebrink, A. J. Rijs, N. M. Blijlevens, J. P. Donnelly, and P. E. Verweij. 2006. Paradoxical increase in circulating Aspergillus antigen during treatment with caspofungin in a patient with pulmonary aspergillosis. Clin. Infect. Dis. 43:e23-e25. [DOI] [PubMed] [Google Scholar]
- 17.Kroczek, R. A., and E. Siebert. 1990. Optimization of Northern analysis by vacuum-blotting, RNA-transfer visualization, and ultraviolet fixation. Anal. Biochem. 184:90-95. [DOI] [PubMed] [Google Scholar]
- 18.Krogh-Madsen, M., M. C. Arendrup, L. Heslet, and J. D. Knudsen. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938-944. [DOI] [PubMed] [Google Scholar]
- 19.Madureira, A., A. Bergeron, C. Lacroix, M. Robin, V. Rocha, R. P. de Latour, C. Ferry, A. Devergie, J. Lapalu, E. Gluckman, G. Socié, M. Ghannoum, and P. Ribaud. 2007. Breakthrough invasive aspergillosis in allogeneic haematopoietic stem cell transplant recipients treated with caspofungin. Int. J. Antimicrob. Agents 30:551-554. [DOI] [PubMed] [Google Scholar]
- 20.Maertens, J., A. Glasmacher, D. Selleslag, A. Ngai, D. Ryan, M. Layton, A. Taylor, C. Sable, and N. Kartsonis. 2005. Evaluation of serum sandwich enzyme-linked immunosorbent assay for circulating galactomannan during caspofungin therapy: results from the caspofungin invasive aspergillosis study. Clin. Infect. Dis. 41:e9-e14. [DOI] [PubMed] [Google Scholar]
- 21.Maertens, J., I. Raad, G. Petrikkos, M. Boogaerts, D. Selleslag, F. B. Petersen, C. A. Sable, N. A. Kartsonis, A. Ngai, A. Taylor, T. F. Patterson, D. W. Denning, and T. J. Walsh. 2004. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 39:1563-1571. [DOI] [PubMed] [Google Scholar]
- 22.Mann, P. A., R. M. Parmegiani, S. Q. Wei, C. A. Mendrick, X. Li, D. Loebenberg, B. DiDomenico, R. S. Hare, S. S. Walker, and P. M. McNicholas. 2003. Mutations in Aspergillus fumigatus resulting in reduced susceptibility to posaconazole appear to be restricted to a single amino acid in the cytochrome P450 14α-demethylase. Antimicrob. Agents Chemother. 47:577-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellado, E., T. M. Diaz-Guerra, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2001. Identification of two different 14-α sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 39:2431-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellado, E., G. Garcia-Effron, L. Alcazar-Fuoli, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2004. Substitutions at methionine 220 in the 14α-sterol demethylase (Cyp51A) of Aspergillus fumigatus are responsible for resistance in vitro to azole antifungal drugs. Antimicrob. Agents Chemother. 48:2747-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mellado, E., G. Garcia-Effron, L. Alcazar-Fuoli, W. J. Melchers, P. E. Verweij, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela. 2007. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob. Agents Chemother. 51:1897-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nascimento, A. M., G. H. Goldman, S. Park, S. A. Marras, G. Delmas, U. Oza, K. Lolans, M. N. Dudley, P. A. Mann, and D. S. Perlin. 2003. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob. Agents Chemother. 47:1719-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. NCCLS document M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 28.Nomeir, A. A., P. Kumari, M. J. Hilbert, S. Gupta, D. Loebenberg, A. Cacciapuoti, R. Hare, G. H. Miller, C. C. Lin, and M. N. Cayen. 2000. Pharmacokinetics of SCH 56592, a new azole broad-spectrum antifungal agent, in mice, rats, rabbits, dogs, and cynomolgus monkeys. Antimicrob. Agents Chemother. 44:727-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oakley, K. L., G. Morrissey, and D. W. Denning. 1997. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob. Agents Chemother. 41:1504-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park, S., R. Kelly, J. N. Kahn, J. Robles, M. J. Hsu, E. Register, W. Li, V. Vyas, H. Fan, G. Abruzzo, A. Flattery, C. Gill, G. Chrebet, S. A. Parent, M. Kurtz, H. Teppler, C. M. Douglas, and D. S. Perlin. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perlin, D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, R. L. Schaufele, A. Francesconi, J. Bacher, N. A. Avila, and T. J. Walsh. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob. Agents Chemother. 46:12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeslev, M., and A. Kjoller. 1995. Comparison of biomass dry weights and radial growth rates of fungal colonies on media solidified with different gelling compounds. Appl. Environ. Microbiol. 61:4236-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocha, E. M., G. Garcia-Effron, S. Park, and D. S. Perlin. 2007. A Ser678Pro substitution in Fks1p confers resistance to echinocandin drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 51:4174-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Tudela, J. L., M. C. Arendrup, S. Arikan, F. Barchiesi, J. Bille, E. Chryssanthou, M. Cuenca-Estrella, E. Dannaoui, D. W. Denning, J. P. Donnelly, W. Fegeler, C. Lass-Flörl, C. Moore, M. Richardson, P. Gaustad, A. Schmalreck, A. Velegraki, and P. E. Verweij. July 2008. EUCAST definitive document E.DEF 9.1. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. http://www.escmid.org/Files/EUCAST_moulds_DEFINITIVE_document_V_ISO_April_08%20final.pdf.
- 37.Roffey, S. J., S. Cole, P. Comby, D. Gibson, S. G. Jezequel, A. N. Nedderman, D. A. Smith, D. K. Walker, and N. Wood. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab. Dispos. 31:731-741. [DOI] [PubMed] [Google Scholar]
- 38.Romano, J., G. Nimrod, N. Ben-Tal, Y. Shadkchan, K. Baruch, H. Sharon, and N. Osherov. 2006. Disruption of the Aspergillus fumigatus ECM33 homologue results in rapid conidial germination, antifungal resistance and hypervirulence. Microbiology 152:1919-1928. [DOI] [PubMed] [Google Scholar]
- 39.Scotter, J. M., and S. T. Chambers. 2005. Comparison of galactomannan detection, PCR-enzyme-linked immunosorbent assay, and real-time PCR for diagnosis of invasive aspergillosis in a neutropenic rat model and effect of caspofungin acetate. Clin. Diagn. Lab. Immunol. 12:1322-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sionov, E., S. Mendlovic, and E. Segal. 2005. Experimental systemic murine aspergillosis: treatment with polyene and caspofungin combination and G-CSF. J. Antimicrob. Chemother. 56:594-597. [DOI] [PubMed] [Google Scholar]
- 41.Sugar, A. M., and X. P. Liu. 2000. Effect of grapefruit juice on serum voriconazole concentrations in the mouse. Med. Mycol. 38:209-212. [DOI] [PubMed] [Google Scholar]
- 42.van Leer-Buter, C., R. P. Takes, K. M. Hebeda, W. J. Melchers, and P. E. Verweij. 2007. Aspergillosis—and a misleading sensitivity result. Lancet 370:102. [DOI] [PubMed] [Google Scholar]
- 43.van Vianen, W., S. de Marie, M. T. Ten Kate, R. A. Mathot, and I. A. Bakker-Woudenberg. 2006. Caspofungin: antifungal activity in vitro, pharmacokinetics, and effects on fungal load and animal survival in neutropenic rats with invasive pulmonary aspergillosis. J. Antimicrob. Chemother. 57:732-740. [DOI] [PubMed] [Google Scholar]
- 44.Verweij, P. E., E. Mellado, and W. J. Melchers. 2007. Multiple-triazole-resistant aspergillosis. N. Engl. J. Med. 356:1481-1483. [DOI] [PubMed] [Google Scholar]
- 45.Walsh, T. J., E. J. Anaissie, D. W. Denning, R. Herbrecht, D. P. Kontoyiannis, K. A. Marr, V. A. Morrison, B. H. Segal, W. J. Steinbach, D. A. Stevens, J. A. van Burik, J. R. Wingard, and T. F. Patterson. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327-360. [DOI] [PubMed] [Google Scholar]
- 46.Warn, P. A., A. Sharp, J. Mosquera, J. Spickermann, A. Schmitt-Hoffmann, M. Heep, and D. W. Denning. 2006. Comparative in vivo activity of BAL4815, the active component of the prodrug BAL8557, in a neutropenic murine model of disseminated Aspergillus flavus. J. Antimicrob. Chemother. 58:1198-1207. [DOI] [PubMed] [Google Scholar]
- 47.Warris, A., C. M. Weemaes, and P. E. Verweij. 2002. Multidrug resistance in Aspergillus fumigatus. N. Engl. J. Med. 347:2173-2174. [DOI] [PubMed] [Google Scholar]
- 48.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464-1471. [DOI] [PubMed] [Google Scholar]
- 49.Zverlov, V. V., I. Y. Volkov, T. V. Velikodvorskaya, and W. H. Schwarz. 1997. Highly thermostable endo-1,3-β-glucanase (laminarinase) LamA from Thermotoga neapolitana: nucleotide sequence of the gene and characterization of the recombinant gene product. Microbiology 143:1701-1708. [DOI] [PubMed] [Google Scholar]