Abstract
One hundred twenty-one extended-spectrum β-lactamse-producing enterobacterial clinical isolates were screened for the qepA gene. A single CTX-M-15-positive Escherichia coli isolate (0.8%) that produced the putative pump QepA2 was identified. This qepA2 gene was located onto a 90-kb mobilizable plasmid that conferred reduced susceptibility to hydrophilic fluoroquinolones.
Although resistance to quinolones in Enterobacteriaceae is related mainly to chromosome-encoded mutations (16), emergence of plasmid-mediated quinolone resistance has been reported with three known mechanisms to date: the Qnr determinants (12, 15), aminoglycoside acetyltransferase AAC(6′)-Ib-cr (14), and QepA (13, 19). Whereas QnrA (10), QnrB (8), and QnrS (7) proteins protect target enzymes (DNA gyrase and type IV topoisomerases) from quinolone inhibition (12, 15), the AAC(6′)-Ib-cr determinant acetylates norfloxacin and ciprofloxacin (14).
The QepA determinant has been identified in two Escherichia coli clinical isolates from Japan and Belgium (13, 19). The qepA gene encodes a 14-transmembrane-segment efflux pump belonging to the major facilitator superfamily (13, 19). QepA confers decreased susceptibility to hydrophilic fluoroquinolones (e.g., norfloxacin, ciprofloxacin, and enrofloxacin) with a 32- to 64-fold increase of MICs (13, 19). To date, a single epidemiological survey has evaluated the prevalence of the QepA determinant among a collection of E. coli clinical isolates collected from 2002 to 2006 (cutoff of MICs of norfloxacin, ≥0.025 μg/ml), reporting its low prevalence (0.3%) in Japan (18).
In order to determine whether that determinant may have disseminated further worldwide, we have conducted an epidemiological survey to evaluate the prevalence of the qepA gene among extended-spectrum β-lactamase (ESBL)-producing enterobacterial isolates collected from a French university hospital, since ESBLs are often identified among quinolone-resistant isolates (12, 15).
One hundred twenty-one nonduplicate, ESBL-producing enterobacterial clinical isolates, collected at the Bicêtre Hospital, France, between January and October of 2007 have been studied. Screening was carried out by PCR using standard conditions and specific primers designed in this work: qepA-F (5′-CGTGTTGCTGGAGTTCTTC-3′) and qepA-R (5′-CTGCAGGTACTGCGTCATG-3′), giving a 403-bp product. The qepA gene was detected in a single E. coli isolate, indicating its low prevalence (0.8%) in that collection. Similarly, a low prevalence has also been reported (0.3%) in E. coli (18) from Japan. This work is the first epidemiological survey of the QepA determinant performed with European isolates. To date, only four qepA-positive clinical isolates have been reported from Japan and Belgium, all being E. coli isolates (13, 18, 19).
The qepA gene was identified in E. coli isolate BicA recovered in January 2007 from urine and blood samples of a 79-year-old female patient suffering from pyelonephritis. Antibiotic susceptibility testing by disk diffusion revealed that E. coli BicA was resistant to all β-lactams except carbapenems and cephamycins and exhibited an ESBL phenotype. PCR and sequencing (6) revealed that E. coli BicA harbored the blaTEM-1 and blaCTX-M-15 genes and was also resistant to chloramphenicol, tetracycline, sulfamethoxazole, trimethoprim, nalidixic acid, and fluoroquinolones but remained fully susceptible to aminoglycosides (Table 1), according to the CLSI standard (5). Sequence analysis of the chromosome-encoded quinolone resistance-determining regions (2) revealed that E. coli BicA had a gyrA gene with two mutations, leading to Ser83Leu and Asp87Asn substitutions, and one mutation in parC, leading to a Ser80Ile substitution, compared to the wild-type enzymes of E. coli. No mutation was found in gyrB and parE genes, and no qnr-like or aac(6′)-Ib-cr genes were detected by PCR (4, 11) in E. coli BicA.
TABLE 1.
MICs of antibiotics for the E. coli clinical isolate BicA, its J53 transconjugants harboring natural plasmids pCTX-M (blaCTX-M-15+) or both pCTX-M and pQep (qepA2+), its TOP10 transformant harboring natural plasmid pQep, and reference strains J53 (azide resistant) and TOP10
| Antibiotic | MIC (μg/ml) fora:
|
|||||
|---|---|---|---|---|---|---|
| BicA | TC-1 (pCTX-M) | TC-2 (pCTX-M + pQep) | J53 | TF (pQep) | TOP10 | |
| Nalidixic acid | >256 | 4 | 4 | 4 | 1.5 | 1 |
| Norfloxacin | >256 | 0.06 | 1 | 0.06 | 0.5 | 0.03 |
| Ofloxacin | >32 | 0.12 | 0.12 | 0.12 | 0.03 | 0.01 |
| Pefloxacin | >32 | 0.06 | 0.06 | 0.06 | 0.03 | 0.03 |
| Levofloxacin | >32 | 0.03 | 0.06 | 0.06 | 0.01 | 0.008 |
| Ciprofloxacin | >32 | 0.01 | 0.12 | 0.01 | 0.03 | 0.003 |
| Enrofloxacin | >32 | 0.06 | 0.06 | 0.03 | 0.008 | 0.003 |
| Moxifloxacin | >32 | 0.06 | 0.12 | 0.06 | 0.01 | 0.004 |
| Gatifloxacin | >32 | 0.06 | 0.12 | 0.01 | 0.01 | 0.003 |
| Amoxicillin | >256 | >256 | >256 | 4 | >256 | 4 |
| Amoxicillin-CLAb | 16 | 16 | 16 | 4 | 16 | 4 |
| Ticarcillin | >256 | >256 | >256 | 4 | >256 | 4 |
| Piperacillin | >256 | >256 | >256 | 2 | >256 | 2 |
| Piperacillin-TZBc | 4 | 2 | 2 | 1 | 2 | 1 |
| Cephalothin | >256 | >256 | >256 | 8 | 8 | 4 |
| Cefotaxime | >32 | >32 | >32 | 0.06 | 0.06 | 0.06 |
| Ceftazidime | >32 | 16 | 16 | 0.12 | 0.12 | 0.06 |
| Aztreonam | >32 | 16 | 16 | 0.06 | 0.12 | 0.06 |
| Cefepime | >32 | 16 | 16 | 0.01 | 0.12 | 0.06 |
| Imipenem | 0.5 | 0.25 | 0.25 | 0.06 | 0.25 | 0.12 |
| Gentamicin | 0.5 | 0.12 | 0.12 | 0.12 | 0.25 | 0.25 |
| Tobramycin | 0.5 | 0.25 | 0.25 | 0.12 | 0.5 | 0.5 |
| Amikacin | 2 | 1 | 1 | 1 | 1 | 1 |
| Netilmicin | 0.5 | 0.12 | 0.12 | 0.12 | 0.25 | 0.25 |
| Chloramphenicol | >256 | 8 | >256 | 8 | >256 | 4 |
| Tetracycline | >256 | 2 | 2 | 2 | >256 | 2 |
| Sulfamethoxazole | >256 | 16 | >256 | 16 | >256 | 16 |
| Trimethoprim | >256 | 0.25 | >256 | 0.25 | >256 | 0.12 |
| Rifampin | 16 | 8 | 8 | 8 | 16 | 16 |
TC-1 and TC-2, E. coli J53 transconjugants; TF, E. coli TOP10 transformant.
CLA, clavulanate.
TZB, tazobactam.
The QepA determinant was transferred as previously described (3) from E. coli BicA to the E. coli TOP10 recipient strain by electrotransformation after selection on Trypticase soy agar plates containing ciprofloxacin (0.01 μg/ml). Plasmid analysis performed by using the Kieser technique (9) identified a single ca. 90-kb plasmid (pQep) from the E. coli transformant. PCR-based replicon typing (1) indicated that pQep was a member of the IncFI incompatibility group. Once transferred in E. coli TOP10, pQep conferred increased MICs for hydrophilic fluoroquinolones (such as norfloxacin and ciprofloxacin) as previously described (13, 19) and also increased resistance to amoxicillin, ticarcillin, piperacillin, chloramphenicol, tetracycline, sulfamethoxazole, and trimethoprim (Table 1). In addition, plasmid pQep was confirmed by PCR (6) to harbor the blaTEM-1 gene but not the blaCTX-M-15 gene. A second plasmid pCTX-M of ca. 90 kb and identified as an IncI1-type plasmid carried the blaTEM-1 and blaCTX-M-15 genes that were transferred by conjugation into E. coli J53 after selection with amoxicillin. Transconjugants containing only pCTX-M (TC-1) exhibited an ESBL phenotype without any coresistance (Table 1). Noteworthy, pQep was cotransferred with pCTX-M but was not identified alone after selection with chloramphenicol, suggesting that pQep might be mobilizable and not conjugative, as opposed to pIP1206, which is also classified as a broad-host-range IncFI-type plasmid (13).
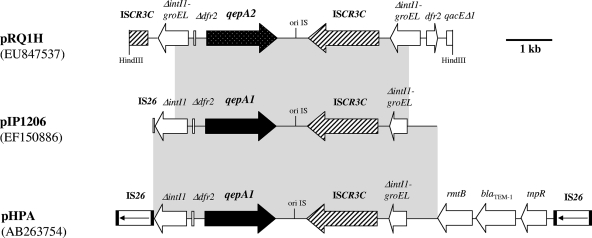
Cloning of HindIII-restricted DNA from whole-cell DNA (3) of E. coli BicA gave recombinant plasmid pRBicAH, containing a 7,020-bp insert including the qepA gene. Sequence analysis revealed that the qepA gene was located inside a genetic structure in large part identical to that of previously described plasmids pHPA (19) and pIP1206 (13), identified from qepA-positive E. coli strains from Japan and Belgium, respectively (Fig. 1). However, detailed analysis of pRBicAH showed that the qepA gene possessed two nucleotide substitutions, leading to Ala99Gly and Val134Ile changes in the deduced 511-amino-acid protein (13, 19). This variant (named QepA2) conferred a phenotype similar to that of the QepA determinant (renamed QepA1) (data not shown). Of note is the fact that the qepA2 gene was not associated with the rmtB gene encoding an aminoglycoside ribosomal methylase, in contrast to what has been reported for plasmids pHPA and pIP1206 (13, 19). Interestingly, the qepA2 gene was identified between two copies of an ISCR-like element and was not associated with IS26 elements, as opposed to what was described in pHPA (Fig. 1). The transposase gene of that ISCR element encoded a protein 100% identical to the putative transposase identified on plasmids pHPA and pIP1206 (13, 19). That transposase showed a single amino acid substitution, Leu331Arg or Leu477Pro, compared to transposases of the ISCR3-like elements ISCR3A (GenBank accession no. AAK02053, AF261825, BAE54320, and ABZ01838) and ISCR3B (GenBank accession no. ACA62823), respectively. This novel IS element was termed ISCR3C. Upstream of the qepA2 gene, a truncated dfr2 gene known to encode resistance to trimethoprim was identified and was located upstream of a truncated integrase gene (ΔintI1) of a class 1 integron (Fig. 1). This truncated integrase gene was identified close to a truncated chaperonin gene (ΔgroEL), thus encoding a fusion protein IntI1/GroEL previously described in association with ISCR-like elements (17).
FIG. 1.
Genetic environment of the qepA2 gene in plasmid pQep from E. coli BicA and comparison with related QepA-positive plasmid structures. Plasmids pHPA and pIP1206 are from E. coli C316 from Japan (19) and E. coli 1540 from Belgium (13), respectively. Recombinant plasmid pRBicAH was obtained in the present study. Open reading frames are indicated by horizontal arrows. The HindIII restriction sites used for cloning experiments are indicated. Shaded areas indicate identities between sequences.
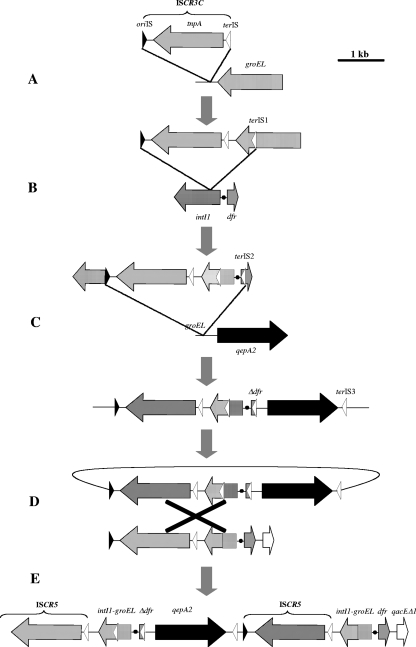
In a study of E. coli C316 from Japan, the region comprising the qepA and rmtB genes was found to be flanked by two copies of IS26, and the authors suggested that those genes were likely part of a composite transposon (Fig. 1) (19). In pQep, the genetic environment of the qepA2 gene was nearly identical to that of pHPA, but no IS26 element was found. The ISCR3-type element might be at the origin of the mobilization of the qepA2 gene, and a possible hypothesis reconstituting the overall process of acquisition of that gene (involving transposition/recombination mechanisms) is proposed in Fig. 2, according to the model described by Toleman et al. (17).
FIG. 2.
Hypothesis of the ISCR3C-mediated mobilization of the qepA2 gene according to the model proposed by Toleman et al. (17). The process may result from several steps. (A) Insertion of ISCR3C downstream of a groEL gene. Aberrant rolling circle (RC) replication of the ISCR3C element (fused to the groEL gene) generates a transposition intermediate starting at oriTS and ending at terIS1, located inside the groEL gene. (B) This intermediate thereafter transposes into a class 1 integrase gene. A second aberrant RC replication generates a transposition intermediate starting at oriTS and ending at terIS2, located inside a dfr2 gene itself downstream of the intI1 gene. (C) This second transposition intermediate transposes upstream of the qepA2 gene. (D) A third aberrant RC replication produces circular intermediates which can then be rescued by recombination events between tnpA or intI1-groEL genes, generating the qepA2-containing complex integron (E). Boxes represent the open reading frames of the various genes, with arrows indicating the direction of their transcription. The integrase-specific recombination sites are indicated as black dots, and oriIS and terIS are represented as black and white triangles, respectively.
By using a 5′ rapid amplification of cDNA ends PCR system (Invitrogen, Cergy-Pontoise, France), the site of initiation of transcription of the qepA2 gene was mapped in E. coli BicA and identified 24 bp upstream of the start codon of that gene. Upstream of this transcriptional start site, a putative −35 promoter sequence (ATGTCG) was found, separated by 17 bp from a putative −10 promoter sequence (TGTCGT). A putative ribosome binding site (GGAAG) was also identified 9 bp upstream of the qepA2 start codon.
This is the first epidemiological survey evaluating the prevalence of the plasmid-mediated quinolone resistance determinant QepA in a European hospital; the prevalence was found to be low among ESBL-producing enterobacterial isolates. Identification of QepA with the widespread ESBL CTX-M-15 on a broad-host-range plasmid and in association with a peculiar ISCR-mediated genetic structure may indicate its further spread.
Nucleotide sequence accession number.
The nucleotide sequence of the qepA2-positive strain E. coli BicA has been submitted to the GenBank nucleotide sequence database (accession no. EU847537).
Acknowledgments
We thank Patrice Courvalin for the gift of the qepA-positive control, Thierry Naas for helpful discussion, and Mark Toleman and Timothy Walsh for their contribution to the ISCR nomenclature.
This work was funded by a grant from the Ministère de l'Education Nationale et de la Recherche (grant UPRES-EA3539), Université Paris XI, Paris, France, and mostly by a grant from the European Community (6th PCRD, LSHM-CT-2005-018705).
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Carattoli, A., A. Bertini, L. Villa, V. Falbo, K. L. Hopkins, and E. J. Threlfall. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219-228. [DOI] [PubMed] [Google Scholar]
- 2.Cattoir, V., P. Lesprit, C. Lascols, E. Denamur, P. Legrand, C. J. Soussy, and E. Cambau. 2006. In vivo selection during ofloxacin therapy of Escherichia coli with combined topoisomerase mutations that confer high resistance to ofloxacin but susceptibility to nalidixic acid. J. Antimicrob. Chemother. 58:1054-1057. [DOI] [PubMed] [Google Scholar]
- 3.Cattoir, V., L. Poirel, C. Aubert, C.-J. Soussy, and P. Nordmann. 2008. Unexpected occurrence of plasmid-mediated quinolone resistance determinants in environmental Aeromonas spp. Emerg. Infect. Dis. 14:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cattoir, V., L. Poirel, V. Rotimi, C.-J. Soussy, and P. Nordmann. 2007. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J. Antimicrob. Chemother. 60:394-397. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Girlich, D., L. Poirel, A. Leelaporn, A. Karim, C. Tribuddharat, M. Fennewald, and P. Nordmann. 2001. Molecular epidemiology of the integron-located VEB-1 extended-spectrum β-lactamase in nosocomial enterobacterial isolates in Bangkok, Thailand. J. Clin. Microbiol. 39:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hata, M., M. Suzuki, M. Matsumoto, M. Takahashi, K. Sato, S. Ibe, and K. Sakae. 2005. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob. Agents Chemother. 49:801-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacoby, G. A., K. E. Walsh, D. M. Mills, V. J. Walker, H. Oh, A. Robicsek, and D. C. Hooper. 2006. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob. Agents Chemother. 50:1178-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kieser, T. 1984. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19-36. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Martinez, L., A. Pascual, and G. A. Jacoby. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797-799. [DOI] [PubMed] [Google Scholar]
- 11.Minarini, L. A., L. Poirel, V. Cattoir, A. L. Darini, and P. Nordmann. Plasmid-mediated quinolone resistance determinants among enterobacterial isolates from outpatients in Brazil. J. Antimicrob. Chemother., in press. [DOI] [PubMed]
- 12.Nordmann, P., and L. Poirel. 2005. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J. Antimicrob. Chemother. 56:463-469. [DOI] [PubMed] [Google Scholar]
- 13.Périchon, B., P. Courvalin, and M. Galimand. 2007. Transferable resistance to aminoglycosides by methylation of G1405 in 16S rRNA and to hydrophilic fluoroquinolones by QepA-mediated efflux in Escherichia coli. Antimicrob. Agents Chemother. 51:2464-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robicsek, A., J. Strahilevitz, G. A. Jacoby, M. Macielag, D. Abbanat, C. H. Park, K. Bush, and D. C. Hooper. 2006. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat. Med. 12:83-88. [DOI] [PubMed] [Google Scholar]
- 15.Robicsek, A., G. A. Jacoby, and D. C. Hooper. 2006. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect. Dis. 6:629-640. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz, J. 2003. Mechanisms of resistance to quinolones: target alterations, decreased accumulation and DNA gyrase protection. J. Antimicrob. Chemother. 51:1109-1117. [DOI] [PubMed] [Google Scholar]
- 17.Toleman, M. A., P. M. Bennett, and T. R. Walsh. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 70:296-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamane, K., J. I. Wachino, S. Suzuki, and Y. Arakawa. 2008. Plasmid-mediated qepA gene among Escherichia coli clinical isolates from Japan. Antimicrob. Agents Chemother. 52:1564-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamane, K., J. Wachino, S. Suzuki, K. Kimura, N. Shibata, H. Kato, K. Shibayama, T. Konda, and Y. Arakawa. 2007. New plasmid-mediated fluoroquinolone efflux pump, QepA, found in an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 51:3354-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]