Abstract
Extended-spectrum β-lactamases (ESBLs), mainly of the CTX-M family, have been associated with Escherichia coli strains of animal origin in Europe. An in vivo experiment was performed to study the effects of veterinary β-lactam drugs on the selection and persistence of ESBL-producing E. coli in the intestinal flora of pigs. Twenty pigs were randomly allocated into three treatment groups and one control group. All pigs were inoculated intragastrically with 1010 CFU of a nalidixic acid (NAL)-resistant mutant derived from a CTX-M-1-producing E. coli strain of pig origin. Treatment with amoxicillin, ceftiofur, or cefquinome according to the instructions on the product label was initiated immediately after bacterial inoculation. Feces were collected from the rectum before inoculation and on days 4, 8, 15, 22, and 25 after the start of treatment. The total and resistant coliforms were counted on MacConkey agar with and without cefotaxime (CTX). Furthermore, MacConkey agar with CTX and NAL was used to count the number of CFU of the inoculated strain. Significantly higher counts of CTX-resistant coliforms were observed in the three treatment groups than in the control group for up to 22 days after the discontinuation of treatment. Ceftiofur and cefquinome exerted larger selective effects than amoxicillin, and the effects persisted beyond the withdrawal times recommended for these cephalosporins. The inoculated strain was detected in only nine animals on day 25. The increase in the number of CTX-resistant coliforms was mainly due to the proliferation of indigenous CTX-M-producing strains and the possible emergence of strains that acquired CTX-M genes by horizontal transfer. The study provides evidence that the cephalosporins used in pig production select for CTX-M-producing E. coli strains. Their use in animals should be carefully considered in view of the critical importance of cephalosporins and the zoonotic potential of ESBL-producing bacteria.
Extended-spectrum β-lactamases (ESBLs) are class A β-lactamases which include three main families: TEM, SHV, and CTX-M (20). The blaCTX-M genes emerged later but were able to spread rapidly, and human infections associated with this ESBL type have recently assumed pandemic proportions (4). Infections with ESBL producers were originally confined to hospitals and nursing homes; but the scenario has rapidly changed, and today community-acquired infections may also occur (18). Furthermore, different animal species and food products have been shown to carry bacteria that harbor these resistance determinants and to be possible reservoirs for human infection (1, 2, 17, 19).
Various studies have shown that cephalosporin use is a risk factor for the acquisition of ESBL-producing strains in humans (20). Cephalosporins licensed for exclusive veterinary use, ceftiofur and cefquinome, are oxyiminocephalosporins (14). According to the instructions on their labels, the two cephalosporins are indicated for use for the treatment of respiratory infections; and cefquinome can also be used for the treatment of mastitis metritis agalaxia syndrome in sows, exudative epidermitis, and meningitis. A recent study in pigs from Danish pig farms revealed that ceftiofur is also used prophylactically in 1-day-old piglets to prevent arthritis, meningitis, septicemia, and diarrhea (15). CTX-M-1-producing Escherichia coli strains were isolated from piglets and slaughter pigs from farms that used ceftiofur but could not be detected on control farms without a recent history of ceftiofur usage (15).
In this study, an in vivo experiment was performed to investigate the selection and persistence of CTX-M-1-producing E. coli strains in the intestinal tracts of pigs treated with three veterinary β-lactam products licensed for use for the treatment of respiratory infections: amoxicillin, ceftiofur, and cefquinome. Changes in the counts of total and cefotaxime (CTX)-resistant coliforms were used to assess the effects of the three β-lactams on the selection of a CTX-M-1-producing E. coli strain inoculated at the beginning of the experiment and indigenous CTX-M-producing E. coli strains occurring in the intestinal flora of the pigs.
MATERIALS AND METHODS
Animals, housing conditions, and ethical issues.
Twenty 8-week-old cross-bred female pigs with weights ranging from 15 to 20 kg were purchased from a specific-pathogen-free farm in Denmark. The animals were housed in a level 2 isolation unit at the Faculty of Life Sciences, University of Copenhagen. All procedures concerning the animal experiments were performed in compliance with the Animals Scientific Act and were performed under the license and approval of the Danish National Animal Experiment Inspectorate (LBK no. 1306 23/11/2007; license no. 2006/561-1141). The animals were observed twice every day for any clinical signs (behavior, gastrointestinal function, respiratory distress, and food and water intake), and rectal temperatures were taken whenever any procedure was done on the animals. At the end of the experiment the animals were euthanized by captive bolt pistol insensibilization and bleeding.
Bacterial strain.
The bacterial strain used to inoculate the pigs was a nalidixic acid (NAL)-resistant mutant (MIC = 32 μg/ml) obtained by spontaneous mutation of E. coli KV7, a CTX-resistant strain (MIC > 2 μg/ml) previously isolated from the feces of a healthy piglet treated with ceftiofur (15). The strain harbored blaCTX-M-1 on an IncN conjugative plasmid. It was additionally resistant to chloramphenicol, trimethoprim, streptomycin, tetracycline, and sulfamethoxazole and belonged to serotype O27 and phylogenetic group D. NAL resistance (MIC ≥ 32 μg/ml) was used as a marker to distinguish the inoculated strain from CTX-resistant coliforms that preexisted in the intestinal flora or that emerged during the experiment as a result of horizontal gene transfer. Preliminary in vitro studies showed that the NAL-resistant mutant had a growth rate similar to that of the wild-type strain (data not shown).
Experimental setup.
Bacterial inoculation was performed immediately before antimicrobial treatment. An endogastric tube was used to inoculate 10 ml of a saline suspension containing approximately 109 CFU/ml of the CTX-M-1-producing strain.
Antimicrobial drugs were purchased as veterinary medical products. Treatment started immediately after bacterial inoculation on day 1, and each antimicrobial product was administered once a day for 3 days by intramuscular injection, according to the standard treatment recommended for respiratory disease in pigs, as follows: amoxicillin at 15 mg/kg of body weight (BW) (group 1), and ceftiofur at 3 mg/kg BW (group 2), cefquinome at 2 mg/kg BW (group 3). The control group was inoculated with the CTX- and NAL-resistant strain but did not receive any antimicrobial treatment (group 0).
Collection and microbiological analysis of fecal samples.
Fecal samples of about 10 g were collected from the rectum prior to antimicrobial treatment (day 0) and on days 4, 8, 15, 22, and 25. Serial 10-fold dilutions were used to count the numbers of viable coliforms on MacConkey agar (Merck) plates, the numbers of CTX-resistant coliforms on MacConkey agar supplemented with 2 μg/ml CTX, and the numbers of the inoculated CTX-M-1-producing strain on MacConkey agar supplemented with 2 μg/ml CTX and 16 μg/ml NAL. All counts (CFU/g) were determined by the spot method. Briefly, 20 μl of each dilution was inoculated as a spot on two replicate plates, followed by 24 h of incubation at 37°C. This method allowed the detection of the coliforms and the quantification of the coliforms at greater than or equal to 500 CFU/g (2.7 log CFU/g) of feces.
On days 0 and 25, representative colonies were isolated from the selective plates containing CTX or CTX and NAL. All isolates were identified as E. coli by the indole, methyl red, Voges-Proskauer, and citrate tests. Isolates confirmed to be E. coli were screened for the presence of the blaCTX-M gene by PCR, and selected amplification products were sequenced for gene identification (13). In order to enable a comparison of the isolates obtained at different time points and the inoculated strain, one or two isolates obtained from each animal and at different sampling times were further characterized by pulsed-field gel electrophoresis (PFGE) with XbaI (5), and their phylogenetic groups were determined by PCR (6).
Statistical analysis.
To compare the effects of each treatment on the selection of resistant ESBL-producing coliforms, a linear model was fit to the data by using the mixed procedure in the SAS program (version 9.1). The response variables were the total counts of CTX-resistant coliforms or CTX- and NAL-resistant coliforms per gram of feces (on the log scale), and the explanatory variables were time and treatment group (amoxicillin, ceftiofur, or cefquinome). Confidence intervals were calculated for the estimated differences, and a difference was deemed significant when the 95% confidence intervals excluded 0. The statistical model included the baseline counts as a covariate to adjust for possible effects on the outcome of the experiment.
RESULTS
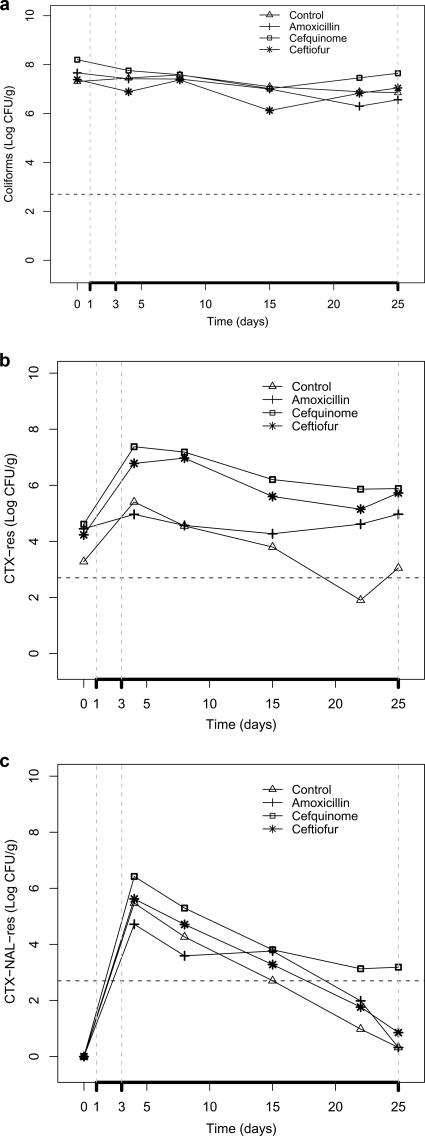
All animals maintained good health status throughout the experiment, and their weights increased from 15 to 20 kg to 22 to 31 kg (average daily weight gain, 302 ± 0.064 g). No significant differences in the average counts of total coliforms were observed between each of the treated groups and the control group (Fig. 1a). Prior to inoculation, the feces of 19 of the 20 pigs contained low counts of CTX-resistant coliforms ranging from 1 × 103 to 1.25 × 105 CFU/g. The counts of CTX-resistant coliforms were significantly higher in the three treatment groups than in the control group, and the effects of treatment were evident until day 25 (Fig. 1b). The inoculated blaCTX-M-1-positive strain resistant to NAL was recovered from all animals until day 8, but its counts gradually dropped (Fig. 1c).
FIG. 1.
Counts (log CFU/g) of total coliforms (a), CXT-resistant (CTX-res) coliforms (b), and CTX- and NAL-resistant (CTX-NAL-res) coliforms (c) in feces of pigs treated with veterinary β-lactam products (amoxicillin, cefquinome, and ceftiofur) and feces of untreated pigs (control). The vertical dashed lines indicate the treatment period (days 1 and 3) and the posttreatment window (days 3 to 25). The horizontal dashed lines indicate the detection limit of the method used (500 CFU/g).
Multiple-comparison tests on CTX-resistant coliform counts showed that the estimates for the effect of treatment (least-squares means) for both ceftiofur and cefquinome differed significantly from the effect of no treatment (control) (2.5 [95% confidence interval {CI} = 1.62, 3.4]) and 2.8 [95% CI = 1.94, 3.73]), respectively), whereas the effect for amoxicillin was only borderline significant (0.9 [95% CI = 0.01, 1.80]). The selection of CTX-resistant E. coli was significantly higher in the groups treated with ceftiofur and cefquinome than in the group treated with amoxicillin (Table 1). The differences in the mean CTX- and NAL-resistant coliform counts were significant only between cefquinome and the control groups (1.5 [95% CI = 0.43, 2.63]) and between the amoxicillin and the cefquinome groups (−1.5 [95% CI = −2.5, −0.35]).
TABLE 1.
Differences in CTX-resistant and CTX- and NAL-resistant coliform counts in feces between the groups treated with amoxicillin, ceftiofur, and cefquinome and the control group
| Coliform resistance and difference evaluated | Estimated difference in coliform count (log CFU/g) | 95% Confidence limita | Adjusted P value |
|---|---|---|---|
| CTX | |||
| Amoxicillin vs control | 0.9 (0.30)b | 0.01, 1.79 | 0.0464c |
| Cefquinome vs control | 2.8 (0.30) | 1.94, 3.73 | <0.0001c |
| Ceftiofur vs control | 2.5 (0.30) | 1.62, 3.40 | <0.0001c |
| Amoxicillin vs cefquinome | −1.9 (0.30) | −2.83, −1.04 | <0.0001c |
| Amoxicillin vs ceftiofur | −1.6 (0.30) | −2.50, −0.71 | 0.0003c |
| Cefquinome vs ceftiofur | 0.3 (0.30) | −0.56, 1.22 | 1.000 |
| NAL and CTX | |||
| Amoxicillin vs control | 0.1 (0.37) | −1.02, 1.18 | 1.000 |
| Cefquinome vs control | 1.5 (0.37) | 0.43, 2.63 | 0.0041c |
| Ceftiofur vs control | 0.6 (0.37) | −0.53, 1.66 | 0.8463 |
| Amoxicillin vs cefquinome | −1.5 (0.37) | −2.5, −0.35 | 0.0066c |
| Amoxicillin vs ceftiofur | −0.5 (0.37) | −1.58, 0.61 | 1.000 |
| Cefquinome vs ceftiofur | −1 (0.37) | −0.13, 2.06 | 0.1062 |
The 95% confidence limits of significant differences exclude 0.
Values in parentheses are standard errors.
Statistically significant difference.
Seventy-two CTX-resistant isolates from days 0 and 25 were identified as E. coli, and the presence of CTX-M in all of them was confirmed by PCR. The blaCTX-M-1 and blaCTX-M-14 genes encoding for ESBL-mediated resistance were found in the indigenous flora of the pigs at the beginning of the experiment (day 0). The E. coli isolates recovered at the end of the experiment (day 25) mostly carried blaCTX-M-1, and two animals additionally harbored isolates with blaCTX-M-14. Sequencing of 14 randomly selected amplicons confirmed the presence of blaCTX-M-1 (group 1) and blaCTX-M-14 (group 9). None of the isolates obtained from the CTX plates on day 25 was genetically related to the inoculated strain. Most of them belonged to phylogenetic group A, and the only two isolates belonging to the phylogenetic group of the inoculated strain (group D) displayed distinct PFGE profiles and harbored a different CTX-M gene (blaCTX-M-14) (Table 2). However, close genetic similarity (≥89%) to the inoculated strain was displayed by 30 isolates obtained from agar plates containing both CTX and NAL, thereby confirming that the counts on these selective agar plates were representative of the inoculated strain.
TABLE 2.
Characterization of CTX-resistant E. coli isolates recovered on days 0 and 25 from pigs treated with the β-lactam drugs amoxicillin, cefquinome, and ceftiofur and the untreated controls
| Treatment group or strain | Animal no. | Day 0
|
Day 25
|
||||
|---|---|---|---|---|---|---|---|
| CTX-M typea | E. coli phylogenetic groupb | PFGE type | CTX-M type | E. coli phylogenetic group | PFGE type | ||
| Control | 1 | 1 | B1 | I | 1 | A | XVI |
| 2 | 1 | B1 | II | 1 | A | XVIIa | |
| 3 | 1 | A | III | 1 | A | XVIII | |
| 4 | 1sc | A | III | 1s | A | XVIII | |
| 5 | 1 | A | III | 1 | A | XVIII | |
| Amoxicillin | 1 | 14 | D | IV | 1 | A | XIX |
| 2 | 14s | D | Va | 1 | A | XIX | |
| 3 | 14s | D | Va | 1 | A | XIX | |
| 4 | —d,e | — | — | 1s | A | XIX | |
| 5 | 14s | D | Va | 1 | A | XX | |
| Ceftiofur | 1 | 1 | A | VI | 1s | A | XXI |
| 2 | 1s | A | VII | 1 | A | XXII | |
| 2 | 14s | D | VIII | 14 | D | NDf | |
| 3 | 1 | B1 | IX | 1 | A | XVIIb | |
| 4 | 1 | A | X | 1 | A | XVIIb | |
| 5 | 1 | A | XI | 14s | D | XXIII | |
| 5 | — | — | — | 1 | A | ND | |
| Cefquinome | 1 | 1s | A | XII | 1s | A | XXIV |
| 2 | 1 | A | XIII | 1 | A | XXVa | |
| 2 | 14s | D | ND | — | — | — | |
| 3 | 14 | D | Va | 1 | A | XXVb | |
| 4 | 1 | A | XIV | 1 | A | XXVI | |
| 4 | 14s | D | Va | — | — | — | |
| 5 | 14 | D | Vb | 1 | A | XXVII | |
| 5 | 1 | A | XV | — | — | — | |
| Inoculated KV7 NALr strain | 1s | D | XXVIII | — | — | — | |
The CTX-M type was determined by general PCR, group PCR, and sequencing of selected amplicons (13).
The E. coli phylogenetic group was determined by multiplex PCR, as described by Clermont et al. (6).
s, sequenced.
—, not tested.
CTX-resistant coliforms were not detected at day 0 in this animal.
ND, not determined.
DISCUSSION
Treatment with amoxicillin, ceftiofur, or cefquinome resulted in the selection of CTX-M-producing E. coli in the intestinal flora of pigs. With the exception of cefquinome, the observed increase in the counts of CTX-M-producing E. coli was not due to the inoculated strain but to E. coli strains occurring in the indigenous flora of the pigs. Surprisingly, most pigs (19/20) were carriers of CTX-M-producing E. coli before inoculation, including CTX-M-1 and CTX-M-14 variants. Although no information was available on possible factors contributing to their selection on the specific-pathogen-free farm of origin, this finding suggests that it may be difficult to control the risk for colonization of animals with ESBL-producing E. coli. The indigenous CTX-M-producing strains had an ecological advantage over the inoculated strain, as indicated by the drop in the counts on plates containing NAL and by the genetic characterization of the isolates obtained at the end of the experiment. Several factors might have contributed to this result, such as host factors or a possible loss of fitness due to the quinolone resistance mutation in the inoculated strain, which could have reduced the ability of the strain to colonize the pigs in vivo (16). It has previously been observed that a predominant E. coli clone is normally associated with the individual intestinal flora and that colonization with externally introduced strains is difficult due to competition with the adapted indigenous flora (9, 10).
The effect of treatment on the counts of the CTX-M-producing coliforms was statistically significant for all β-lactams tested in comparison with the effect of no treatment (control). However, the effect was more marked in the groups treated with ceftiofur and cefquinome (Table 1). On average, the estimated difference between the counts of CTX-M-producing E. coli in the feces of cephalosporin-treated pigs and those in the feces of the control group was about 100-fold higher, and these higher counts persisted for at least 22 days after the end of treatment. Accordingly, the selective effects exerted by ceftiofur and cefquinome persisted beyond their withdrawal times (the time required to ensure that drug residues are below the maximum residue limit in the edible tissues), which were 9 and 5 days, respectively. Previous studies on the selective effects of other antimicrobial classes on antimicrobial-resistant Salmonella enterica serovar Typhimurium DT104 have indicated that recent antimicrobial treatment is likely to increase the numbers of resistant bacteria in slaughtered animals (7, 8, 21). Similarly, our results indicate that pigs treated with cephalosporins and sent to slaughterhouses shortly after the end of the withdrawal time may shed high numbers of ESBL-producing bacteria (3.0 × 104 to 9.5 × 106 CFU/g feces).
Treatment with amoxicillin induced a significant quantitative increase in CTX-resistant coliform counts, although to a lesser extent (less than a 10-fold difference compared with the counts in the control group). However, the withdrawal time of this drug is 30 days, and thus, the risk of meat contamination at the slaughterhouse appears to be lower for this drug. The effect of cephalosporins on the selection of CTX-M-producing members of the family Enterobacteriaceae has been reported by other authors. The longer persistence of CTX-M-9-positive Salmonella enterica serovar Virchow was shown following cefixime treatment in an human fecal flora rat model, but no transfer of the resistance determinant to a recipient strain or to the commensal flora was observed (12). In our experiment, the increase in the numbers of ESBL-producing bacteria following treatment with ceftiofur or cefquinome was mainly due to the proliferation of indigenous CTX-M-producing strains and to the possible emergence of strains that acquired CTX-M genes by horizontal transfer, as indicated by the high degree of diversity of the population of CTX-resistant E. coli isolates observed by PFGE analysis.
This in vivo study clearly indicates that veterinary cephalosporins select for ESBL-producing E. coli in the intestinal flora of pigs and that such a selective effect persists for a period longer than the withdrawal time required for these antimicrobials. The quantitative data generated by this study might be useful for assessment of the risk of acquisition of antimicrobial resistance from cephalosporin use in pig production. According to the WHO and OIE classifications, broad-spectrum cephalosporins are critically important antimicrobials for both human and animal health (11). The use of cephalosporins for the treatment of animals should be limited to cases in which the use of narrower-spectrum antimicrobials is not possible due to resistance problems or a proven lack of clinical efficacy by alternative antimicrobial drugs (3).
Acknowledgments
The study was supported by a grant from the EU Marie Curie Programme TRAINAU (grant MEST-CT-2004-007819).
We thank Peter Damborg, Arshnee Moodley, Tony Bønnelycke, and Frei Bindslev for their assistance in the experiments with the pigs.
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Aarestrup, F. M., H. Hasman, Y. Agerso, L. B. Jensen, S. Harksen, and B. Svensmark. 2006. First description of blaCTX-M-1-carrying Escherichia coli isolates in Danish primary food production. J. Antimicrob. Chemother. 57:1258-1259. [DOI] [PubMed] [Google Scholar]
- 2.Brinas, L., M. A. Moreno, M. Zarazaga, C. Porrero, Y. Saenz, M. Garcia, L. Dominguez, and C. Torres. 2003. Detection of CMY-2, CTX-M-14, and SHV-12 beta-lactamases in Escherichia coli fecal-sample isolates from healthy chickens. Antimicrob. Agents Chemother. 47:2056-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burch, D., C. Duran, and F. M. Aarestrup. 2008. Guidelines for antimicrobial use in swine p. 102-125. In L. Guardabassi, L. Jensen, and H. Kruse (ed.), Guide to antimicrobial use in animals. Blackwell Publishing, Oxford, United Kingdom.
- 4.Canton, R., and T. M. Coque. 2006. The CTX-M beta-lactamase pandemic. Curr. Opin. Microbiol. 9:466-475. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2004. One-day (24-28 h) standardized laboratory protocol for molecular subtyping of Escherichia coli O157:H7, non-typhoidal Salmonella serotypes, and Shigella sonnei by pulsed field gel electrophoresis (PFGE). PulseNet manual. Centers for Disease Control and Prevention, Atlanta, GA.
- 6.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delsol, A. A., M. Anjum, M. J. Woodward, J. Sunderland, and J. M. Roe. 2003. The effect of chlortetracycline treatment and its subsequent withdrawal on multi-resistant Salmonella enterica serovar Typhimurium DT104 and commensal Escherichia coli in the pig. J. Appl. Microbiol. 95:1226-1234. [DOI] [PubMed] [Google Scholar]
- 8.Delsol, A. A., M. J. Woodward, and J. M. Roe. 2004. Effect of a 5 day enrofloxacin treatment on Salmonella enterica serotype Typhimurium DT104 in the pig. J. Antimicrob. Chemother. 53:396-398. [DOI] [PubMed] [Google Scholar]
- 9.Duval-Iflah, Y., J. Chappuis, R. Ducluzeau, and P. Raibaud. 1983. Intraspecific interactions between Escherichia coli strains in human newborns and in gnotobiotic mice and piglets. Prog. Food Nutr. Sci. 7:107-116. [PubMed] [Google Scholar]
- 10.Duval-Iflah, Y., P. Raibaud, and M. Rousseau. 1981. Antagonisms among isogenic strains of Escherichia coli in the digestive tracts of gnotobiotic mice. Infect. Immun. 34:957-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FAO/WHO/OIE. 2007. Report of the Joint FAO/WHO/OIE Expert Meeting on Critically Important Antimicrobials. FAO, Rome, Italy.
- 12.Faure, S., A. Perrin-Guyomard, and M. Laurentie. 2007. Horizontal transfer of blaCTX-M-9 gene from animal strains of Salmonella enterica Virchow to Escherichia coli in the human-faecal-flora-associated (HFF) rat model: impact of therapeutic doses of cefixime. Proc. 2nd Symp. Antimicrob. Resist. Anim. Environ.
- 13.Hasman, H., D. Mevius, K. Veldman, I. Olesen, and F. M. Aarestrup. 2005. Beta-lactamases among extended-spectrum beta-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J. Antimicrob. Chemother. 56:115-121. [DOI] [PubMed] [Google Scholar]
- 14.Hornish, R. E., and S. F. Kotarski. 2002. Cephalosporins in veterinary medicine—ceftiofur use in food animals. Curr. Top. Med. Chem. 2:717-731. [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen, C. J., L. M. Cavaco, H. Hasman, H. D. Emborg, and L. Guardabassi. 2007. Occurrence of CTX-M-1-producing Escherichia coli in pigs treated with ceftiofur. J. Antimicrob. Chemother. 59:1040-1042. [DOI] [PubMed] [Google Scholar]
- 16.Lindgren, P. K., L. L. Marcusson, D. Sandvang, N. Frimodt-Moller, and D. Hughes. 2005. Biological cost of single and multiple norfloxacin resistance mutations in Escherichia coli implicated in urinary tract infections. Antimicrob. Agents Chemother. 49:2343-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, J. H., S. Y. Wei, J. Y. Ma, Z. L. Zeng, D. H. Lu, G. X. Yang, and Z. L. Chen. 2007. Detection and characterisation of CTX-M and CMY-2 beta-lactamases among Escherichia coli isolates from farm animals in Guangdong Province of China. Int. J. Antimicrob. Agents 29:576-581. [DOI] [PubMed] [Google Scholar]
- 18.Livermore, D. M., R. Canton, M. Gniadkowski, P. Nordmann, G. M. Rossolini, G. Arlet, J. Ayala, T. M. Coque, I. Kern-Zdanowicz, F. Luzzaro, L. Poirel, and N. Woodford. 2007. CTX-M: changing the face of ESBLs in Europe. J. Antimicrob. Chemother. 59:165-174. [DOI] [PubMed] [Google Scholar]
- 19.Meunier, D., E. Jouy, C. Lazizzera, M. Kobisch, and J. Y. Madec. 2006. CTX-M-1- and CTX-M-15-type beta-lactamases in clinical Escherichia coli isolates recovered from food-producing animals in France. Int. J. Antimicrob. Agents 28:402-407. [DOI] [PubMed] [Google Scholar]
- 20.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiuff, C., J. Lykkesfeldt, O. Svendsen, and F. M. Aarestrup. 2003. The effects of oral and intramuscular administration and dose escalation of enrofloxacin on the selection of quinolone resistance among Salmonella and coliforms in pigs. Res. Vet. Sci. 75:185-193. [DOI] [PubMed] [Google Scholar]