Abstract
Weak electric currents generated using conductive electrodes have been shown to increase the efficacy of antibiotics against bacterial biofilms, a phenomenon termed “the bioelectric effect.” The purposes of the present study were (i) to find out whether insulated electrodes that generate electric fields without “ohmic” electric currents, and thus are not associated with the formation of metal ions and free radicals, can inhibit the growth of planktonic bacteria and (ii) to define the parameters that are most effective against bacterial growth. The results obtained indicate that electric fields generated using insulated electrodes can inhibit the growth of planktonic Staphylococcus aureus and Pseudomonas aeruginosa and that the effect is amplitude and frequency dependent, with a maximum at 10 MHz. The combined effect of the electric field and chloramphenicol was found to be additive. Several possible mechanisms underlying the observed effect, as well as its potential clinical uses, are discussed.
The use of physical means as an aid for modern medicine in the continuous battle against pathogenic microorganisms holds new prospects that only recently have begun to be widely recognized. Light sources of various types are being used for photodynamic therapy in dentistry and dermatology (10, 13, 33). Ultrasound waves are used for human dental plaque removal (16) and, in combination with antibiotics, for the eradication of bacterial biofilms in vitro and in vivo (3, 7, 19, 21). In addition, thermotherapy, originally developed as a tool against cancerous tumors, has been found to be effective against cutaneous leishmaniasis (22).
The major drawback of the methods mentioned above is their limited selectivity; thus, ultrasonic waves and thermotherapy nonspecifically produce heat that may cause severe collateral damage. Similarly, the illumination of the photosensitizers in photodynamic therapy can harm tissues in the vicinity of the target area. Other downsides of photodynamic therapy include the need to deliver the photosensitizers to the treated area and the low tissue penetration of the radiation, limiting the application of this treatment to topical infections (13, 14).
The use of an additional physical means, weak electric currents, to inhibit bacterial growth was suggested by Rosenberg et al. (24), who observed that electrolysis resulted in the arrest of Escherichia coli cell division. Further investigation of this phenomenon revealed that transition platinum complexes produced at the platinum electrodes during electrolysis were responsible for the bacterial growth inhibition. These derivatives were found not to be specific to bacteria; they were also toxic to human cells. In fact, this work eventually led to the discovery of the known chemotherapeutic agent cisplatin (25). In the years to follow, it was demonstrated that low-intensity electric currents, mostly direct current (DC) (1, 5, 20, 31, 32), as well as alternating electric fields of as much as 10 MHz (4, 18), can enhance the efficacy of antibacterial agents against bacterial biofilms. In all of these studies, the electric currents were generated using conductive electrodes, allowing for the formation of metal ions and free radicals at the electrode surface. Like cisplatin, these products are toxic to human cells, and therefore the use of such electric currents was limited.
Recently it was demonstrated that low-intensity alternating electric fields at frequencies of 100 to 200 kHz can inhibit the growth of proliferating cancerous cell lines, both in vitro and in vivo, without affecting normal quiescent cells (11, 12). These fields, termed “tumor-treating fields” (TTFields), were generated by means of electrically insulated ceramic electrodes, thus ensuring that during the application of the field there is no electrolysis and that no biocides or ions are produced at the electrode surface and released into the medium. Clinical investigations, supported by in vitro studies, have demonstrated the safety of the use of TTFields. Evidence was presented indicating that the mechanism at the basis of this inhibitory effect was related to the unidirectional dielectrophoresis forces produced by the nonhomogeneous electric fields generated in the vicinity of the cleavage plain that gradually develops and separates the newly formed daughter cells from each other. Since a similar process occurs in rapidly replicating prokaryotic organisms, it is reasonable to assume that they can be targeted by appropriately tuned electric fields. The field parameters required for affecting bacteria are expected to be significantly different from those affecting mammalian cells due to the significant differences in size (see Kirson et al. [11]).
In view of these considerations, the objectives of the present study were to test the feasibility of using alternating electric fields generated by insulated electrodes for the inhibition of planktonic bacteria and to define the effective field parameters for the inhibition process.
MATERIALS AND METHODS
Reagents.
Unless stated otherwise, all reagents were purchased from Sigma (Israel). Dehydrated culture media were purchased from Difco Laboratories (Detroit, MI).
Test strains and growth conditions.
Pseudomonas aeruginosa strain PAO1 was a generous gift from Shiri Navon-Venezia (Division of Infectious Diseases, Sourasky Medical Center, Tel Aviv, Israel). Staphylococcus aureus strain SH1000 was a generous gift from Yair Aharonowitz (Department of Molecular Microbiology and Biotechnology, Tel Aviv University). All strains were grown in LB medium (1.0% Bacto tryptone, 0.5% yeast extract, 1.0% NaCl) (Frutarom). Broth cultures of freshly plated bacterial strains were grown in 3 ml of liquid medium at 37°C for 16 h in an orbital shaker (220 rpm; New Brunswick Scientific, NJ) and diluted in fresh LB broth to a predetermined absorbance at 595 nm (Biowave cell density meter; WPA, United Kingdom), which yielded the desired CFU per ml.
AMFields generation system.
AMFields (antimicrobial fields) were generated inside a 50-mm-diameter glass petri dish by pairs of parallel 15-mm-long, 5-mm-high electrodes placed 23 mm apart (Fig. 1A). The metal electrodes were completely insulated from the medium by a ceramic material (lead magnesium niobate-lead titanate [PMN-PT]) with a very high dielectric constant (ɛ, >5,000) such that the capacitance of the electrodes was approximately 10 nF each. The back of the electrodes was insulated by a 5-mm-thick layer of 353ND medical-grade epoxy (Epoxy Technology, Billerica, MA). Each chamber contained two electrode pairs positioned perpendicularly to each other so as to generate sequentially, in the medium between them, electric fields at 90° to each other. The four electrodes were held in position by a polycarbonate structure, as shown in Fig. 1B. The electrodes were connected to a radio frequency amplifier (75A250; AR Worldwide, Souderton, PA) activated by a sine wave generator (model 662; OR-X, Israel). The entire AMFields-generating system was placed inside a Faraday cage in order to meet the guidelines of the International Non-Ionizing Radiation Committee (INIRC) for limiting exposure to time-varying electric, magnetic, and electromagnetic fields. The temperature at the center of the chamber was monitored continuously using an insulated T-type thermocouple (Omega, Stamford, CT). (A diagram of the system is given in Fig. 1C.) At the highest frequencies used (30 to 50 MHz), the fields interfered with the temperature measurements. Therefore, under these conditions, temperatures were measured periodically with the field turned off briefly.
FIG. 1.
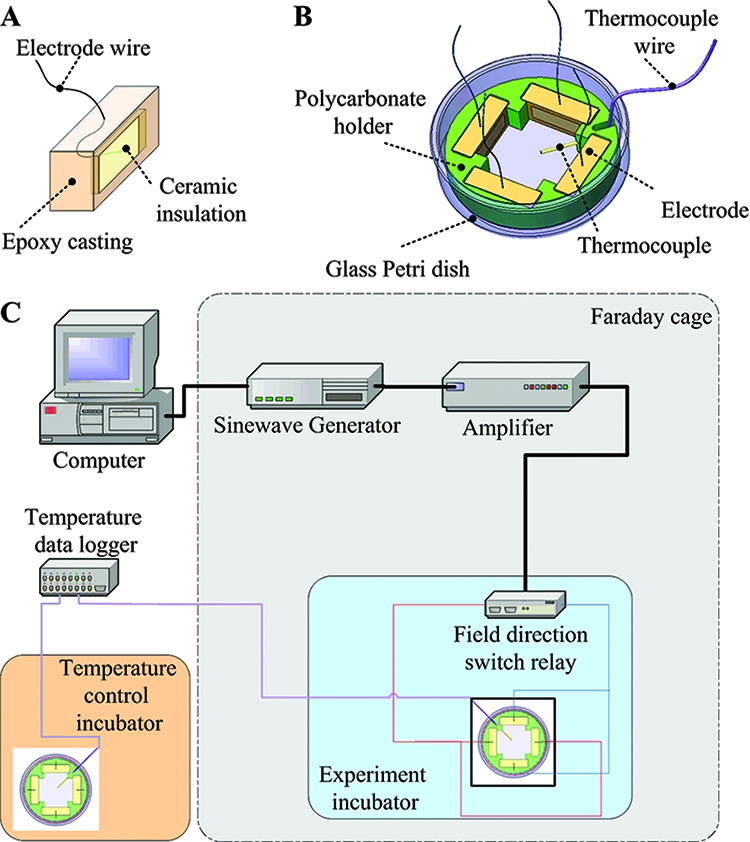
Schematic design of an insulated AMFields electrode (A), an AMFields bacterium-treating chamber (B), and the AMFields generation system (C).
Because AMFields are associated with heat production, the chamber temperature was kept constant at the desired level by computer feedback control of the waveform amplitude. The field generation was switched between the two perpendicular directions every 300 ms by activating two pairs of perpendicular electrodes. These cycle times were found to minimize the creation of thermal gradients within the treated area that could affect the bacterial growth rate. The intensity of the electric field was measured using a shielded coaxial probe with two exposed tips positioned 1 cm apart. The probe was connected, through a coaxial cable, to a floating input oscilloscope (190B; Fluke, The Netherlands). Field intensities were measured at the end of each treatment by dipping the probe in the culture medium, such that the two measuring points were in parallel with the lines of the electric field. Field intensities are expressed as peak-to-peak volts per centimeter of distance.
Application of AMFields to bacteria.
Overnight bacterial cultures were diluted in fresh LB broth to an optical density (OD) corresponding to bacterial counts of 1 × 107 CFU/ml. Petri plates containing the AMFields chamber (Fig. 1B) were filled with 7 ml of the diluted cultures and placed inside an incubator set to maintain the proper culture temperature of 37°C once the fields were applied (FOC 225I; Velp Scientifica). Fields were applied for 2 h for S. aureus and 2.5 h for P. aeruginosa. Preliminary experiments indicated that these treatment durations were sufficient to allow for the growth of the control culture group (not exposed to AMFields) by approximately 1 order of magnitude. Control bacterial groups, placed in inactive AMFields chambers, were positioned in a prewarmed incubator at 37°C. The AMFields chamber temperature reached 37.0 ± 0.2°C within 5 min for both the treated and control groups. At the end of treatment, cultures were suspended by pipetting. Four 250-μl aliquots were dispensed into a 96-microwell plate (NunclonΔ; Nunc, Denmark), and the OD at 750 nm (OD750) was determined spectrophotometrically with a microplate reader (Infinite 200; Tecan, Austria). The percentage of growth for each well was calculated as (OD750 of the treated well/OD750 of the control well) × 100.
Combined effect of AMFields and antibiotics.
Chloramphenicol was obtained as a powder (Sigma, Israel) and dissolved in 95% ethanol (Frutarom, Israel). All the stock solutions were filter sterilized and held at −20°C until use. Serial twofold dilutions of each antibiotic agent were prepared by following the CLSI guidelines.
The MIC of an antibiotic was defined as the lowest concentration that completely inhibited the growth of the organism. MICs were determined using microdilution susceptibility tests. Briefly, a 100-μl bacterial suspension (approximately 5 × 105 CFU/ml) in LB medium was added to 100 μl of culture medium containing either no antibiotic or an antibiotic at one of several concentrations (serial twofold dilutions) in 96-well plates. Inhibition of proliferation was determined by measurement of the OD750 after overnight incubation at 37°C. The MIC of AMFields was defined as the lowest intensity that inhibited growth by 80% or more relative to the growth of the control, as determined using the microplate reader.
Drug interactions with AMFields were assessed according to the checkerboard method, with the following modifications. S. aureus inocula were diluted in LB medium containing the antibiotic to a final concentration of 0.5 × 105 to 1.0 × 105 CFU/ml. The final concentrations of chloramphenicol ranged from 0.25 to 4 μg/ml. AMFields-treated plates and control plates were handled as described for the AMFields application experiments, except that the treatment time was 6 h. At the end of the treatment, cultures were sampled and tested for bacterial growth inhibition as described above. The percentage of growth for each well was calculated as described above. Samples were also subjected to serial 10-fold dilutions from which 20-μl aliquots were plated onto LB agar plates (1.5% agar, 1.0% Bacto tryptone, 1% NaCl, 0.5% yeast extract). CFU counts were performed after overnight incubation at 37°C.
To evaluate the effect of the combination treatment, the fractional inhibitory concentration (FIC) (6) was calculated for the AMFields and for each antibiotic. The following formulas were used to calculate the FIC index: FIC of AMFields = (MIC of AMFields in combination)/(MIC of AMFields alone); FIC of chloramphenicol = (MIC of chloramphenicol in combination)/(MIC of chloramphenicol alone); FIC index = (FIC of AMFields) + (FIC of chloramphenicol). Synergy was defined as a FIC index of ≤0.5. Indifference was defined as a FIC index of >0.5 but ≤4. Antagonism was defined as a FIC index of >4.
Finite-element simulations of electric-field distribution in bacteria.
Numerical calculations, based on a finite-element mesh, were used to reconstruct the electric-field distribution inside dividing P. aeruginosa and S. aureus cells. The following geometries and parameters were used for the calculations. P. aeruginosa was considered an ellipse, with a large radius of 2.0 μm and a small radius of 0.6 μm, having two membranes (external and internal) 8 nm thick. The two membranes were assumed to be separated by a periplasmic space of 50 nm. The dividing bacterium furrow diameter was taken as 0.2 μm, and the applied external field was 20 V/cm. Since no data regarding the electric properties of P. aeruginosa have been published, we used the following data for the electric properties of E. coli in the calculations: inner membrane conductivity, 1 μS/m; outer membrane conductivity, 3 mS/m; medium conductivity, 0.5 S/m; cytoplasm conductivity, 0.5 S/m; conductivity of the periplasmic space, 50 mS/m (9, 26). S. aureus was considered a sphere with a radius of 0.6 μm and a membrane thickness of 8 nm. The bacterial cell wall thickness was 20 nm. The dividing bacterium furrow diameter was taken as 0.2 μm, and the applied external field was 20 V/cm. In the simulation, the membrane conductivity was 1 μS/m and the cell wall conductivity was 10 mS/m. The conductivity of the medium was 0.5 S/m, and the conductivity of the cytoplasm was 0.8 S/m (26).
RESULTS
Inhibition of bacterial growth as a function of AMFields frequency.
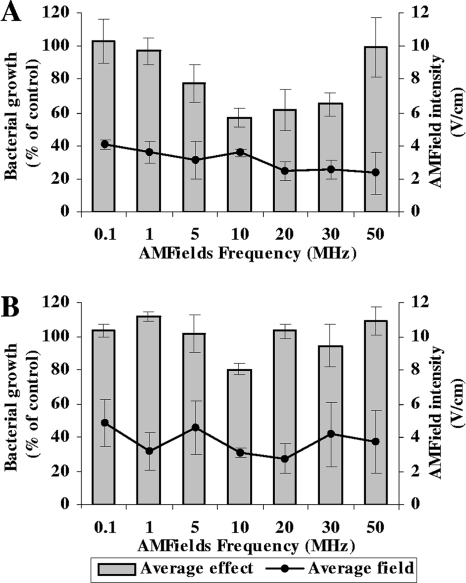
The frequency dependence of the effect of the AMFields was tested between 100 kHz and 50 MHz. The results in Fig. 2 show that AMFields at 2 to 4 V/cm inhibit the growth of the two bacterial species tested. The effect is frequency dependent, with maximum growth inhibition at 10 MHz for both S. aureus and P. aeruginosa. Note that the AMFields-generating system was designed to maintain a constant temperature in the chamber by adjusting the field intensity. Therefore, the intensity of the fields varied between different frequencies within a range of ±15%. The results presented are means ± standard deviations (SD) for at least 16 pooled samples. Analysis of variance using XLSTAT (version 2008.5.01; Addinsoft) demonstrated the high significance of the frequency dependency (P < 0.0001) for both species. Higher field frequencies were not tested due to equipment limitations.
FIG. 2.
Average relative growth of microorganisms exposed to AMFields at various frequencies. (A) S. aureus (strain SH1000) after a 2-h treatment; (B) P. aeruginosa (strain PAO1) after 2.5 h of treatment. The effect, based on OD measurements, is expressed as a percentage of the growth of the heat control. Averages for at least 16 independent experiments ± SD are presented. The solid lines indicate the corresponding average field intensities ± SD.
Inhibition of S. aureus growth as a function of AMFields intensity.
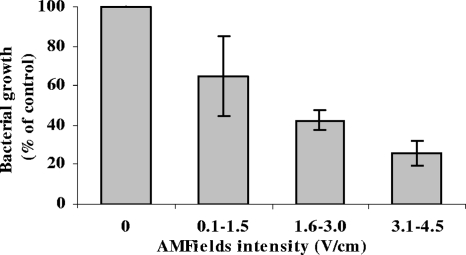
Once we had identified 10 MHz as the most effective frequency against S. aureus, we tested the relationship between the intensity of AMFields and bacterial growth inhibition at 10 MHz. As seen in Fig. 3, the growth inhibition is field intensity dependent, with larger inhibitory effects at higher intensities. The results presented are means ± standard errors for at least three independent experiments (P < 0.001).
FIG. 3.
Relative growth of S. aureus (strain SH1000) after a 6-h exposure to 10-MHz AMFields of various intensities. The relative growth, based on OD measurements, is expressed as a percentage of the growth of the heat control (mean ± standard error for at least three independent experiments). The initial S. aureus concentration in these experiments was 0.5 × 105 to 1 × 105 CFU/ml.
Combined effect of AMFields and antibiotics.
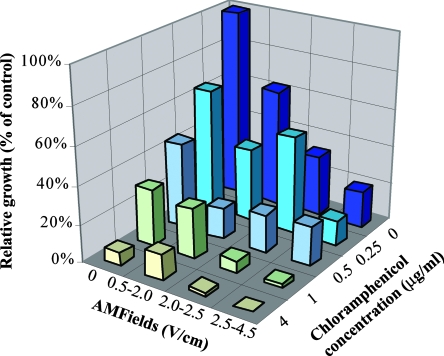
The MIC of chloramphenicol against S. aureus was found to be 4 μg/ml, a value similar to those reported by the CLSI. The separate and combined effects of AMFields and chloramphenicol on the growth of S. aureus, as determined by OD measurements, are given in Fig. 4. Similar results were obtained using CFU counts (data not shown). As seen, in the presence of 4 V/cm, 10-MHz AMFields, much lower concentrations of chloramphenicol (1 μg/ml) are sufficient to produce >95% inhibition of the growth of S. aureus. The FIC index was found to be 0.6, indicating that there is an additive effect for the combined exposure to AMFields and chloramphenicol.
FIG. 4.
Separate and combined effects of 10-MHz AMFields of various intensities and chloramphenicol at different concentrations on the growth of S. aureus (strain SH1000) after 6 h of exposure. The calculations are based on OD measurements.
Finite-element simulations of the electric-field distribution.
The electric-field distribution in and around P. aeruginosa and S. aureus, calculated using the finite-element mesh method, is shown in Fig. 5. In the simulation (Fig. 5A), it is seen that inside the dividing bacterium, close to the furrow, the electric field is strongest and is nonuniform. The nonuniformity generates dielectrophoresis forces that approach a maximal value at AMFields frequencies of ∼2.0 MHz for P. aeruginosa (Fig. 5B) and ∼7.0 MHz for S. aureus (Fig. 5C).
FIG. 5.
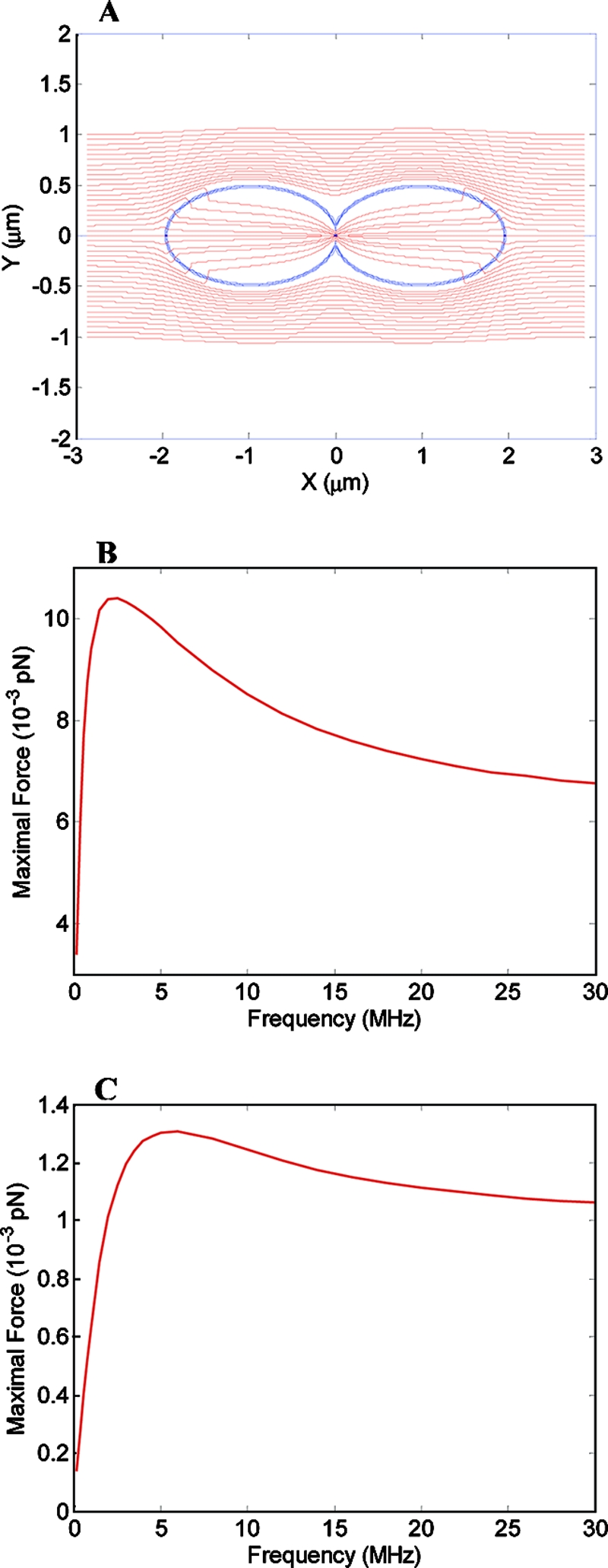
(A) Electric field inside and around a dividing rod-like bacterium. The simulation is based on 10-MHz electric fields with an intensity of 20 V/cm. The dividing bacterium has a furrow diameter of 0.2 μm, located at 0 μm on the x axis. (B and C) Magnitude of the force acting on a dipole of 3,000 debye units inside a dividing P. aeruginosa (B) or S. aureus (C) cell when the field intensity is 20 V/cm as a function of the AMFields frequency.
DISCUSSION
The use of electric current as a bacterial growth inhibitor was reported more than 40 years ago (24), with many subsequent reports over the years (27, 30). Most of the literature on the subject deals with enhancement of the efficacy of antibiotics against microbial biofilms by the application of weak DC currents, a phenomenon termed “the bioelectric effect” by Costerton et al. (5). Several mechanisms were suggested for this inhibition, depending on the nature of the current: relatively low intensity direct current and low-frequency alternating electric fields, when applied using conductive electrodes, can lead to electrolysis, the production of toxic derivatives and free radicals, modification of the pH (28), and alterations in bacterial biofilm structure (29). High-intensity pulsed electric currents (generating electric fields of >1,000 V/cm) cause electroporation (17, 34).
High-frequency alternating currents (10 MHz) were reported by Caubet et al. (4) to enhance the susceptibility of bacterial E. coli biofilms to antibiotics and to decrease the number of bacteria in biofilms by >60%, even in the absence of an antibiotic. These effects were attributed by the authors to changes induced in the biofilm's exopolysaccharide matrix due to the interaction of the electromagnetic field with charged particles present in that matrix. However, since these experiments were carried out using conductive electrodes, the effects may be due in part to the production of toxic elements at the electrode surface.
Unlike all the reports discussed above, in which electric currents were applied using conductive electrodes, in the present study we investigated bacterial growth inhibition by high-frequency, low-intensity electric fields generated by completely insulated electrodes. Thus, the electric fields were not associated with electrolysis or the production of free radicals, toxic metal ions, etc., at the electrode surface. Furthermore, the low intensity of the fields applied here (0.5 to 4 V/cm) rules out the possibility of electroporation, which occurs at field intensities in the range of 1,000 V/cm. The continuous control of the medium temperature eliminates the possibility of thermal effects. In addition, the reported enhancement of the transfer of ions and antibiotics through biofilms by electric currents is not relevant to the present study, because the bacteria treated here were planktonic and the inhibitory effect was observed even in the absence of antibiotics.
Since the commonly accepted mechanisms mentioned above are unlikely to be responsible for the bacterial growth inhibition reported in the present study, we must look for alternative mechanisms to explain the reported effects. In analogy to the mechanism suggested by Kirson et al. (11, 12) for mammalian cells, it seems logical that high-frequency alternating electric fields affect dividing bacteria during cytokinesis due to the nonhomogeneous electric fields generated near the bridge separating the daughter cells. These nonhomogeneous fields exert unidirectional dielectrophoresis forces on charged and polar particles and molecules and thus may result in their movement toward the furrow (12). In order to evaluate whether AMFields can induce such effects in dividing bacteria, the field distribution and the associated electric forces were modeled using finite-element mesh simulations. The results of these simulations (Fig. 5) indicate that the gradient of the electric field inside a dividing bacterium exerts forces of 10−2 to 10−3 pN directed toward the furrow on small dipoles. This force in a bacterial cell, which is of the same order of magnitude as the calculated force in a mammalian cell (11, 12), is sufficient to induce particle and macromolecule distortion and movement at frequencies shown to be effective against bacterial growth. Considering the bacterial cytoplasm viscosity to be 1 centipoise, the terminal velocity of a small dipole is expected to be ∼4 μm/s for P. aeruginosa, and ∼0.6 μm/s for S. aureus, per V/cm of external electric field. Since the electric force is directed toward the furrow, it will lead to the accumulation of molecules around the furrow within a few seconds. Such forces may affect the structural integrity of the cells or interfere with processes in which electrostatic forces play a role. In eukaryotic cells, the cellular structures that are expected to be mostly affected are the highly polar spindle microtubules. Kirson et al. (12) demonstrated that an alternating electric field interfered with the orientation of the spindle microtubules as well as with the polymerization-depolymerization processes involved in the chromosome separation process. While the common paradigm is that bacteria lack the highly organized microtubules present in eukaryotic cells, a tubulin homologue, FtsZ, has been found to be present in all bacteria (2). The structural similarity between FtsZ and tubulin regarding their large dipole moment, as calculated by the protein dipole moment server (8), as well as the analogy between the repeated nature of the Z-ring and the spindle apparatus, raises the possibility that FtsZ is a target for the action of AMFields. Of course, other polar and structurally oriented molecules may be influenced by the AMFields, thus disrupting various cellular processes within the cell.
An additional explanation for the frequency-dependent inhibition of bacterial growth relates to the suggested effect of alternating electric fields on the enzyme-substrate reaction equilibrium (23). According to the suggested model, the electric charge distribution on many enzymes varies with the conformational changes associated with enzyme-substrate interaction. Because the AMFields may affect molecular charge distributions, they could interfere with some enzymatic reactions. The effect is expected to be larger for membrane enzymes, because the membrane prevents the enzyme from rotating and thus escaping the effect of the field, and because the electric field is magnified in the membrane. Assuming that one or more pivotal enzymes are indeed influenced by the electric fields, exposure of these enzymes to the properly tuned frequency can inhibit bacterial growth by depleting the cell of the enzymes' products. Two exemplary proteins that are expected to be influenced by external electric fields and that are present in P. aeruginosa and S. aureus are glycerol-3-phosphate dehydrogenase (36) and FtsK (2, 15). Glycerol-3-phosphate dehydrogenase is involved in respiration, glycolysis, and phospholipid biosynthesis, and FtsK is an essential cell division protein. Both are membrane proteins with large dipole moments (1,793 and 1,579 debye units, respectively, as calculated by the protein dipole moment server [8]). These values are very similar to the dipole moment of tubulin, the structure and function of which during mitosis have been shown to be disrupted by alternating electric fields of a few hundred kHz (11, 12).
The combined effect of AMFields and chloramphenicol against S. aureus was investigated in detail and was found to be additive. The combination of chloramphenicol and electric currents had not been tested before; however, the majority of studies reported that the combined effect of other antibiotics and electric current was synergistic (4, 35). The synergism reported could be the result of the strong effect of the biocides generated during the application of direct electric current using conductive electrodes. The production of biocides constitutes a major obstacle to the clinical use of electric-current treatment due to the nonspecific nature of these toxic derivatives. This stands in contrast to the specific and safe use of AMFields applied by insulated electrodes with frequencies that have no known effect on human cells (11, 12). The results of these preliminary experiments indicate enhanced antibiotic efficacy in combination with AMFields. One of the major drawbacks of treating pathogens with antibacterial agents is the ability of bacteria to develop resistance to antibiotics. So far, there is no evidence that the bacterial strains used in this study acquired resistance to the inhibitory effect of the fields (data not shown). Assuming that the effect is a result of the nonhomogeneous fields created at the bridge separating the daughter cells of the dividing bacterium, in order for a bacterium to escape inhibition by AMFields, its physical properties would have to change radically—an unlikely outcome.
There are three main limitations to the use of DC currents, including weak currents, for the treatment of infection. The first is that such currents may stimulate nerves and muscles, causing pain and muscular contractions in the patient. The second relates to the spread of the currents in the body, which can be regarded as a volume conductor. Thus, unless the lesion is superficial or unless there is a conductor leading from the surface to a deeply situated lesion, a current density of sufficient intensity at the target can be obtained only when the density near the electrodes is of a damaging and stimulating magnitude. The third limitation is that DC currents cannot be generated by insulated electrodes and are therefore always associated with electrolysis, metal ions, free radicals, etc. A recent report demonstrated the applicability of weak DC currents in reducing the level of pin tract infections associated with external fixators in a goat model (31). The authors were able to deliver the DC current to the infected implants because the pins protruded externally, thus allowing for a direct connection with the current source. The currents used were far too low to induce nerve stimulation; however, the risks of electrolysis and the formation of toxic derivatives still remain, requiring long-term, thorough follow-up to ensure the safety of the use of such currents.
The relatively high frequencies at which the AMFields effect was observed allow for the application of high intensities required for deep treatment without nerve or muscle stimulation. Thus, the door is open for future applications of AMFields as a treatment for resistant infections and difficult-to-treat chronic conditions such as infected diabetic ulcers and infections associated with implants. Infected ulcers could be treated by placing the infected body part in a container filled with a high-electric-impedance solution. Insulated electrodes, connected to the AMFields generator, would generate the necessary fields. Alternatively, the AMFields could be delivered to infected orthopedic implants by means of properly positioned surface electrodes. Similar methodology is currently being used in treating recurrent glioblastoma patients with TTFields (11). Note also that because the bacterial division cycle is about 20 min long, effective treatment is achieved within a few hours (in contrast to cancer cell treatment). Thus, AMFields can be used to accelerate the treatment of common infections, such as tonsillitis, pharyngitis, and otitis, in parallel with antibiotics.
In summary, AMFields constitute a promising new antimicrobial modality in the continuous battle against microbial pathogens. Unlike the electric currents whose use has been reported previously, AMFields are not expected to have any toxicity for human cells. The efficacy of AMFields against bacterial pathogens will most likely be enhanced in vivo by the activity of the immune system as well as by cotreatment with various antibiotics, making AMFields a potential new antiinfection treatment modality.
Acknowledgments
Y. Palti has a minority holding in NovoBiotics, Ltd., and is a member of the company's board of directors. M. Giladi, A. Blatt, and Y. Porat are employed in full by NovoBiotics, Ltd. E. Dekel is a consultant to NovoBiotics, Ltd.
Footnotes
Published ahead of print on 28 July 2008.
REFERENCES
- 1.Blenkinsopp, S. A., A. E. Khoury, and J. W. Costerton. 1992. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 58:3770-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carballido-López, R., and J. Errington. 2003. A dynamic bacterial cytoskeleton. Trends Cell Biol. 13:577-583. [DOI] [PubMed] [Google Scholar]
- 3.Carmen, J. C., B. L. Roeder, J. L. Nelson, B. L. Beckstead, C. M. Runyan, G. B. Schaalje, R. A. Robison, and W. G. Pitt. 2004. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biofilms in vivo. J. Biomater. Appl. 18:237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caubet, R., F. Pedarros-Caubet, M. Chu, E. Freye, M. de Belem Rodrigues, J. M. Moreau, and W. J. Ellison. 2004. A radio frequency electric current enhances antibiotic efficacy against bacterial biofilms. Antimicrob. Agents Chemother. 48:4662-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costerton, J. W., B. Ellis, K. Lam, F. Johnson, and A. E. Khoury. 1994. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob. Agents Chemother. 38:2803-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliopoulos, G. M., and R. C. Moellering. 1991. Antimicrobial combinations, p. 432-492. In V. Lorian (ed.), Antibiotics in laboratory medicine, 3rd ed. The Williams & Wilkins Co, Baltimore, MD.
- 7.Ensing, G. T., B. L. Roeder, J. L. Nelson, J. R. van Horn, H. C. van der Mei, H. J. Busscher, and W. G. Pitt. 2005. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J. Appl. Microbiol. 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felder, C. E., J. Prilusky, I. Silman, and J. L. Sussman. 2007. A server and database for dipole moments of proteins. Nucleic Acids Res. 35:W512-W521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hölzel, R. 1999. Non-invasive determination of bacterial single cell properties by electrorotation. Biochim. Biophys. Acta 1450:53-60. [DOI] [PubMed] [Google Scholar]
- 10.Jori, G., C. Fabris, M. Soncin, S. Ferro, O. Coppellotti, D. Dei, L. Fantetti, G. Chiti, and G. Roncucci. 2006. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers Surg. Med. 38:468-481. [DOI] [PubMed] [Google Scholar]
- 11.Kirson, E. D., V. Dbaly, F. Tovarys, J. Vymazal, J. F. Soustiel, A. Itzhaki, D. Mordechovich, S. Steinberg-Shapira, Z. Gurvich, R. Schneiderman, Y. Wasserman, M. Salzberg, B. Ryffel, D. Goldsher, E. Dekel, and Y. Palti. 2007. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 104:10152-10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirson, E. D., Z. Gurvich, R. Schneiderman, E. Dekel, A. Itzhaki, Y. Wasserman, R. Schatzberger, and Y. Palti. 2004. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 64:3288-3295. [DOI] [PubMed] [Google Scholar]
- 13.Maisch, T. 2007. Anti-microbial photodynamic therapy: useful in the future? Lasers Med. Sci. 22:83-91. [DOI] [PubMed] [Google Scholar]
- 14.Maisch, T., C. Bosl, R. M. Szeimies, N. Lehn, and C. Abels. 2005. Photodynamic effects of novel XF porphyrin derivatives on prokaryotic and eukaryotic cells. Antimicrob. Agents Chemother. 49:1542-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massey, T. H., C. P. Mercogliano, J. Yates, D. J. Sherratt, and J. Lowe. 2006. Double-stranded DNA translocation: structure and mechanism of hexameric FtsK. Mol. Cell 23:457-469. [DOI] [PubMed] [Google Scholar]
- 16.Mourad, P. D., F. A. Roberts, and C. McInnes. 2007. Synergistic use of ultrasound and sonic motion for removal of dental plaque bacteria. Compend. Contin. Educ. Dent. 28:354-358. [PubMed] [Google Scholar]
- 17.Oshima, T., and M. Sato. 2004. Bacterial sterilization and intracellular protein release by a pulsed electric field. Adv. Biochem. Eng. Biotechnol. 90:113-133. [PubMed] [Google Scholar]
- 18.Pareilleux, A., and N. Sicard. 1970. Lethal effects of electric current on Escherichia coli. Appl. Microbiol. 19:421-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitt, W. G., M. O. McBride, J. K. Lunceford, R. J. Roper, and R. D. Sagers. 1994. Ultrasonic enhancement of antibiotic action on gram-negative bacteria. Antimicrob. Agents Chemother. 38:2577-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabinovitch, C., and P. S. Stewart. 2006. Removal and inactivation of Staphylococcus epidermidis biofilms by electrolysis. Appl. Environ. Microbiol. 72:6364-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rediske, A. M., N. Rapoport, and W. G. Pitt. 1999. Reducing bacterial resistance to antibiotics with ultrasound. Lett. Appl. Microbiol. 28:81-84. [DOI] [PubMed] [Google Scholar]
- 22.Reithinger, R., M. Mohsen, M. Wahid, M. Bismullah, R. J. Quinnell, C. R. Davies, J. Kolaczinski, and J. R. David. 2005. Efficacy of thermotherapy to treat cutaneous leishmaniasis caused by Leishmania tropica in Kabul, Afghanistan: a randomized, controlled trial. Clin. Infect. Dis. 40:1148-1155. [DOI] [PubMed] [Google Scholar]
- 23.Robertson, B., and R. D. Astumian. 1990. Michaelis-Menten equation for an enzyme in an oscillating electric field. Biophys. J. 58:969-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg, B., L. Vancamp, and T. Krigas. 1965. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 205:698-699. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg, B., L. VanCamp, J. E. Trosko, and V. H. Mansour. 1969. Platinum compounds: a new class of potent antitumour agents. Nature 222:385-386. [DOI] [PubMed] [Google Scholar]
- 26.Sanchis, A., A. P. Brown, M. Sancho, G. Martinez, J. L. Sebastian, S. Munoz, and J. M. Miranda. 2007. Dielectric characterization of bacterial cells using dielectrophoresis. Bioelectromagnetics 28:393-401. [DOI] [PubMed] [Google Scholar]
- 27.Spadaro, J. A., T. J. Berger, S. D. Barranco, S. E. Chapin, and R. O. Becker. 1974. Antibacterial effects of silver electrodes with weak direct current. Antimicrob. Agents Chemother. 6:637-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart, P. S., W. Wattanakaroon, L. Goodrum, S. M. Fortun, and B. R. McLeod. 1999. Electrolytic generation of oxygen partially explains electrical enhancement of tobramycin efficacy against Pseudomonas aeruginosa biofilm. Antimicrob. Agents Chemother. 43:292-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoodley, P., D. deBeer, and H. M. Lappin-Scott. 1997. Influence of electric fields and pH on biofilm structure as related to the bioelectric effect. Antimicrob. Agents Chemother. 41:1876-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valle, A., E. Zanardini, P. Abbruscato, P. Argenzio, G. Lustrato, G. Ranalli, and C. Sorlini. 2007. Effects of low electric current (LEC) treatment on pure bacterial cultures. J. Appl. Microbiol. 103:1376-1385. [DOI] [PubMed] [Google Scholar]
- 31.van der Borden, A. J., P. G. Maathuis, E. Engels, G. Rakhorst, H. C. van der Mei, H. J. Busscher, and P. K. Sharma. 2007. Prevention of pin tract infection in external stainless steel fixator frames using electric current in a goat model. Biomaterials 28:2122-2126. [DOI] [PubMed] [Google Scholar]
- 32.van der Borden, A. J., H. van der Werf, H. C. van der Mei, and H. J. Busscher. 2004. Electric current-induced detachment of Staphylococcus epidermidis biofilms from surgical stainless steel. Appl. Environ. Microbiol. 70:6871-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wainwright, M. 1998. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 42:13-28. [DOI] [PubMed] [Google Scholar]
- 34.Weaver, J. C. 1993. Electroporation: a general phenomenon for manipulating cells and tissues. J. Cell. Biochem. 51:426-435. [DOI] [PubMed] [Google Scholar]
- 35.Wellman, N., S. M. Fortun, and B. R. McLeod. 1996. Bacterial biofilms and the bioelectric effect. Antimicrob. Agents Chemother. 40:2012-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh, J. I., U. Chinte, and S. Du. 2008. Structure of glycerol-3-phosphate dehydrogenase, an essential monotopic membrane enzyme involved in respiration and metabolism. Proc. Natl. Acad. Sci. USA 105:3280-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]