Abstract
New drugs are needed to shorten the duration of tuberculosis treatment. R207910, a diarylquinoline, is very active against Mycobacterium tuberculosis both in vitro and in mice. In healthy volunteers, the coadministration of R207910 and rifampin induced the increased metabolism of R207910, resulting in a 50% reduction in the level of R207910 exposure. We assessed the impact of reducing the dose of R207910 on its efficacy when R207910 was combined with a background regimen of isoniazid, rifampin, and pyrazinamide. Addition of 25 mg/kg of body weight or 12.5 mg/kg R207910 to the background regimen resulted in faster bacterial clearance and culture negativity. The difference in efficacy between the two doses was not statistically significant. The minimal bactericidal dose of R207910 when it was tested as part of the combination was identical to that when it was tested as monotherapy. Because of the drug-drug interaction in humans, the activity of R207910 in humans could be less than that expected from studies with mice. Our data from the mouse model demonstrate that R207910 has significant activity, even when its exposure is reduced by 50% and when it is added to a strong background regimen of isoniazid, rifampin, and pyrazinamide. In killing kinetic studies, the bactericidal effect of R207910 in mice was modest during the first week of treatment, but it increased in the following 3 weeks, while the bactericidal activity of isoniazid was limited to the first week of treatment.
New antituberculosis (anti-TB) drugs that have the potential to shorten the current duration of therapy for both active and latent tuberculosis and to improve the efficacy of treatment regimens for multidrug-resistant (MDR) TB are urgently needed.
R207910 (TMC207), a diarylquinoline, is a new compound that exhibits a new mode of action (inhibition of ATP synthase) against mycobacteria (1, 6). The compound is being assessed in a phase IIb trial for the treatment of active tuberculosis in patients with MDR-TB. In vitro, R207910 is active against sensitive and resistant strains of Mycobacterium tuberculosis (1, 2). In mice, it accelerates bacterial clearance when it is combined with regimens active against both sensitive and MDR Mycobacterium tuberculosis (1, 10), Mycobacterium leprae (4), and Mycobacterium ulcerans (5). In the established murine model of tuberculosis, monotherapy with R207910 is as active as the standard WHO regimen, which combines rifampin (RIF), isoniazid (INH), and pyrazinamide (PZA); and when it was combined with RIF-INH-PZA (RHZ), INH-PZA, or RIF-PZA, the lungs of mice became culture negative after just 2 months of treatment (1).
When R207910 is combined with PZA, R207910 acts synergistically in the murine model (3). In fact, after only 2 months of treatment, the combination of R207910 and PZA led to the complete eradication of the 7.2 log10 CFU that was present in the lungs of mice at the onset of treatment. Such a level of efficacy needed 4 months of treatment with the triple-drug combination RIF-moxifloxacin-PZA (11).
An extended early bactericidal assay was conducted with human patients who were treated for 7 days with R207910 given at 25 mg, 100 mg, and 400 mg per day. Patients treated with either 600 mg per day of RIF or 300 mg per day of INH were used as controls. This study showed that R207910 given at 400 mg per day showed a significant decrease in CFU counts in the sputum of treated patients in comparison with the pretreatment levels (13).
A drug-drug interaction study was conducted with 16 healthy volunteers who received a single dose of 300 mg of R207910 alone and seven daily doses of 10 mg/kg of body weight of RIF. The area under the concentration-time curve (AUC) from time zero to 336 h for R207910 after its coadministration with RIF was about half that when it was dosed alone, indicating that the metabolism of R207910 is induced by coadministration with RIF (unpublished data).
In the current study, we aimed to determine the efficacies of different doses of R207910 in combination with RHZ in order to mimic the decrease in the level of exposure to R207910 when it is combined with RIF. In addition, we wanted to measure the efficacy of R207910 and INH after 1 week of treatment in order to compare the efficacy with the early bactericidal effect observed in patients.
MATERIALS AND METHODS
Antimicrobial agents.
The following compounds were provided by Johnson & Johnson (Beerse, Belgium): RIF, INH, PZA, and R207910.
Mycobacterium tuberculosis strain.
The drug-sensitive H37Rv strain of M. tuberculosis was obtained from V. Jarlier (Paris, France) and was grown on Löwenstein-Jensen medium. Colonies were subcultured in 7H9 broth with 10% oleic acid-albumin-dextrose catalase (Difco, le Pont de Claix, France) for 7 days at 37°C. The turbidity of the resulting suspension was adjusted with phosphate-buffered saline to match that of a McFarland 2 suspension that was estimated to have 108 CFU/ml of microorganisms and that was used for mouse inoculation. The MICs of RIF, INH, and R207910 for strain H37Rv were determined on 7H11 agar medium and were 0.25, 0.06, and 0.03 μg/ml, respectively. The MIC of PZA was determined on Löwenstein-Jensen medium at pH 5.5 and was 10 μg/ml.
Infection of mice.
For experiments I and II (a drug-drug interaction study and a study of treatment with PZA and R207910 [ZJ] with the established infection model, respectively), 150 and 62 4-week-old female outbred Swiss mice, respectively, were purchased from the Janvier Breeding Center (Le Genest Saint-Isle, France) and were inoculated in the tail vein with 0.2 ml of a bacterial suspension containing 3.5 × 107 and 3 × 106 CFU of M. tuberculosis H37Rv, respectively. The animal experimentation guidelines of Johnson & Johnson were followed. This study was approved by the Johnson & Johnson ethical committee for the use of animals.
Chemotherapy.
In experiment I (drug-drug interaction study), following infection, the mice were randomly allocated to two control groups and four test groups (Table 1), each of which consisted of 24 to 30 mice. The first group was a negative control group that consisted of mice that were infected but left untreated. The second group was a positive control group that consisted of mice that were treated with the standard regimen for drug-susceptible TB, i.e., 2 months of the RHZ combination (15). Mice in the third, fourth, fifth, and sixth groups were treated with different dosages of R207910 (3, 6.25, 12.5, and 25 mg/kg, respectively) in combination with RHZ.
TABLE 1.
Experimental design for drug-drug interactions study (experiment I)
| Treatment groupa | Total no. of mice | No. of mice killed on:
|
|||||
|---|---|---|---|---|---|---|---|
| D−13 | D0 | W2 | W4 | W6 | W8 | ||
| Control | 30 | 6 | 12 | 6 | 6 | ||
| RHZ | 24 | 6 | 6 | 6 | 6 | ||
| RHZJ3 | 24 | 6 | 6 | 6 | 6 | ||
| RHZJ6.25 | 24 | 6 | 6 | 6 | 6 | ||
| RHZJ12.5 | 24 | 6 | 6 | 6 | 6 | ||
| RHZJ25 | 24 | 6 | 6 | 6 | 6 | ||
The dosages were as follows: INH, 25 mg/kg; RIF, 10 mg/kg; PZA, 150 mg/kg; R207910, 3, 6.25, 12.5, or 25 mg/kg.
In experiment II (the study of ZJ treatment with the established infection model), mice were randomly allocated following infection to two control groups and three test groups (Table 2), each of which consisted of 5 to 18 mice. The first group was a negative control group that consisted of mice that were infected but not treated. The second was a positive control group that consisted of mice that were treated with the first-line drug INH. The mice in the third, fourth, and fifth groups were treated either with R207910 at 25 mg/kg alone or with the combination of compound R207910 at two different dosages (2.5 and 25 mg/kg) along with PZA, respectively.
TABLE 2.
Early bactericidal activities of R207910 and the other anti-TB drugs against Mycobacterium tuberculosis infection in the established infection mouse model (experiment II)
| Treatment groupa | Total no. of mice | No. of mice killed onb:
|
|||
|---|---|---|---|---|---|
| D0 | W1 | W2 | W4 | ||
| Control | 18 | 8 | 10 | ||
| INH | 5 | 3 | 2 | ||
| R207910c | 15 | 5 | 5 | 5 | |
| ZJ25 | 15 | 5 | 5 | 5 | |
| ZJ2.5 | 9 | 3 | 4 | 2 | |
The mice were treated 5 days per week.
The mice were infected on D−14.
R207910 was used at 25 mg/kg.
In experiments I and II, treatment was initiated 2 weeks after infection in order to mimic the large bacterial populations observed in human cavities and was administered 5 days a week.
In experiment I, to provide baseline values, 6 and 12 infected and untreated mice were killed on day 1 (D1) and D14 after infection (D−13 and D0, respectively, in relation to the time of initiation of treatment). For all groups treated with RHZ and RHZ plus R207910 (RHZJ), the mice were killed after 2, 4, 6, and 8 weeks of treatment. In experiment II, to provide baseline values, eight infected and untreated mice were killed on D14 (D0 in relation to the time of initiation of treatment) after infection, and the treated mice were killed at week 1 (W1), W2, and W4 postinfection.
In both experiments, solutions of R207910 and RIF were prepared in 20% hydroxypropyl-β-cyclodextrin, and those of PZA and INH were prepared in 10% hydroxypropyl-β-cyclodextrin and water, respectively. The solutions were stored at 4°C. All the drugs were given orally by gavage.
The drugs were administered at the following dosages: 10, 25, and 150 mg/kg/day for RIF, INH, and PZA, respectively. On the basis of the AUCs, these dosages, which are similar to those used in previous experiments (1, 8, 9, 10, 14), were chosen because they are as potent as the usual dosages administered to humans.
Assessment of infection and treatment.
The severity of infection and the effectiveness of the treatments were assessed by survival rate, spleen weight, gross lung lesion (scored from 0 to ++, with the last score referring to a lung that was extensively occupied by tubercles), and the numbers of CFU in the spleens.
In experiment I, at D−13, D0, W2, and W4 of treatment, the numbers of CFU in the spleens were determined by plating six serial 10-fold dilutions of homogenized suspensions onto Löwenstein-Jensen plates. At W6 and W8 of treatment, the entire suspension prepared from each individual spleen, which was expected to contain only a few bacilli, was plated without dilution on 12 Löwenstein-Jensen plates. In experiment II, at D0, W1, and W2 of treatment, the numbers of CFU in the spleens were determined by plating six serial 10-fold dilutions of homogenized suspensions onto Löwenstein-Jensen plates. At W4 postinfection, the entire suspension prepared from each spleen, which was expected to contain only a few bacilli, was plated without dilution on 12 Löwenstein-Jensen plates.
In both experiments, the culture results were recorded after incubation at 37°C for 4 weeks. The treatment was defined to have a bactericidal effect if there was a significant decrease in the mean number of CFU in the treated group compared to the pretreatment value.
Statistical analysis.
In experiments I and II, the Student t test was used to analyze the spleen weights and the CFU count data. Since multiple comparisons were made, the Bonferroni correction of the P value was used. Since six and five groups were compared, the P values were adjusted to 0.0033 and 0.005, respectively.
RESULTS
Survival rate.
In experiment I, all 12 infected and untreated mice died between D14 and D28 postinfection. The mortality rates in the treated groups were 4, 10, 3, 1, and 1 mice in the groups treated with RHZ, RHZJ3, RHZJ in which R207910 was used at 6.25 mg/kg (RHZJ6.25), RHZJ in which R207910 was used at 12.5 mg/kg (RHZJ12.5), and RHZJ in which R207910 was used at 25 mg/kg (RHZJ25), respectively. The high mortality rates observed were due to the high CFU counts achieved in mice at the time that treatment was started (7.92 log10 CFU in the spleens). In experiment II, all untreated mice were dead by W2. The mortality of the treated mice was limited and was related to gavage accidents: two mice in the group treated with R207910 at 25 mg/kg died and one mouse in the group treated with PZA and R207910 at 2.5 mg (ZJ2.5) died.
Spleen weight.
In experiment I, the mean spleen weight was 172 ± 20 mg at D1 postinfection. It increased significantly 2 weeks later to reach 437 ± 94 mg (P = 0.001). Two weeks of treatment with RHZ or RHZJ (in which R207910 was given at 3, 6.25, 12.5, or 25 mg/kg) was not able to reduce the increase in the mean spleen weight. Treatment with RHZ or RHZJ (at all concentrations) for 4, 6, and 8 weeks was able to prevent further increases in the spleen weights (P > 0.0033). In experiment II, the mean spleen weight of the infected and untreated mice increased significantly from 125 ± 16 mg on D1 postinfection to 441 ± 145 mg on D14 (P = 0.0001), which was the time that treatment was started. Treatment with either INH, R207910, or the ZJ combination for 1, 2, and 4 weeks was able to prevent further spleen weight increases but did not reduce them (P > 0.005).
Gross lung lesions.
In experiment I, at W2 postinfection, all 12 infected and untreated mice harbored severe (++ score) gross lung lesions. After 2 weeks of treatment, there was no change in the gross lung lesions. The number of mice displaying severe lung lesions decreased after 4 weeks of treatment. After 6 and 8 weeks of treatment, almost all mice treated with different doses of R207910 in combination with RHZ displayed moderate (+ score) gross lung lesions and a few mice receiving at least 6.25 mg/kg of R207910 were free of lesions. In experiment II, gross lung lesions developed in the infected and untreated mice at W2 postinfection (++ score). Treatment with INH, R207910 at 25 mg/kg, ZJ2.5, and PZA and R207910 at 25 mg (ZJ25) for 1, 2, or 4 weeks did not improve the lung lesion scores.
Killing kinetics for RHZ and RHZJ regimens.
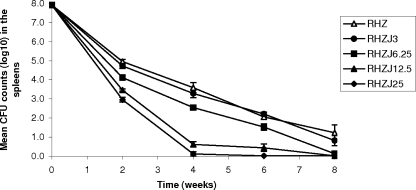
Between D1 and D14 postinfection, when the treatment was started, the mean CFU counts in the spleens increased from 6.56 ± 0.22 to 7.92 ± 0.23 log10 CFU (P = 0.0001). Treatment with RHZ resulted in potent bactericidal activity in the first 2 weeks (−3.0 log10 CFU), followed by a weaker bactericidal effect in the following three 2-week intervals (−1.4, −1.5, and −0.8 log10 CFU, respectively) (Fig. 1). The addition of 3 or 6 mg/kg of R207910 to the RHZ regimen slightly improved the efficacy of R207910 after 2 weeks of treatment (0.2 log10 CFU [P = 0.36] and 0.8 log10 CFU [P = 0.003], respectively), but no additional bactericidal activity was observed between W2 and W8 (P > 0.0033). However, only one of five mice became culture negative after 8 weeks of treatment with RHZ, whereas four of six mice treated with RHZJ6.25 became culture negative. The use of R207910 at 12.5 and 25 mg/kg resulted in very significant additional bactericidal activity, removing an additional 1.5 to 2.0 log10 CFU (compared to that achieved with RHZ) after just 2 weeks and an additional 3.0 to 3.4 log10 CFU after 4 weeks of treatment (P < 0.0033). The killing kinetics in subsequent weeks could not be assessed, as less than 0.5 log10 CFU survived after the first 4 weeks of treatment with these combinations (P > 0.0033). In the group treated with the combination of 25 mg/kg of R207910 and RHZ, all mice became culture negative after just 6 weeks of treatment.
FIG. 1.
Bactericidal activity of daily (5 days per week) RHZJ combinations in comparison with that of standard daily therapy with RHZ in M. tuberculosis-infected mice. Error bars represent the standard errors of the means.
Killing kinetics of INH, PZA, R207910, and ZJ combinations.
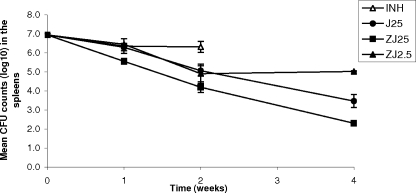
INH killed 0.6 log10 CFU during the first week (P = 0.27 compared with the results for the untreated controls) and displayed no further killing during the second week of treatment (Fig. 2). R207910 at 25 mg/kg had activity similar to that of INH during the first week (0.6 log10 CFU; P = 0.028 compared with the results for the untreated controls); but in contrast to INH, it displayed accelerated killing in the second week (1.3 log10 CFU), and killing continued during W3 and W4 (average, 0.95 log10 CFU per week; P = 0.001 compared with the results for the untreated controls). Compared to R207910 25 mg/kg monotherapy, ZJ25 killed an extra 0.8 log10 CFU after 1 week and an extra 1.2 log10 CFU after 4 weeks (P = 0.0001). The combination of PZA with a 10-fold lower dose of R207190 (ZJ2.5) matched the activity of monotherapy with R207910 at 25 mg/kg during the first 2 weeks but did not kill any additional bacilli in W3 and W4.
FIG. 2.
Bactericidal activity of daily (5 days per week) ZJ combinations in comparison with that of INH in M. tuberculosis-infected mice. Error bars represent the standard errors of the means.
DISCUSSION
A pharmacokinetic interaction with RIF reduces the AUC of R207910 by 50% in humans (unpublished data). Because of this drug-drug interaction, the expected efficacy of R207910 in humans may be less than that observed in mice. To estimate the effect of this interaction on the efficacy of R207910 in humans, we studied lower doses of R207910 in combination with RHZ in the mouse model. When it was dosed at 6.25, 12.5, and 25 mg/kg, R207910 was able to improve the activity of the RHZ regimen by decreasing the CFU counts in the spleens after 2, 4, 6, and 8 weeks of treatment and by increasing the proportion of mice with negative cultures after 8 weeks of treatment. The minimal bactericidal dose (MBD; the dose that leads to the killing of at least 2 log10 CFU) of R207910 when it was used in combination with RHZ was 12.5 mg/kg. This is in full agreement with the MBD of R207910 when it was used as monotherapy (1). The fact that identical MBDs were obtained when R207910 was used as monotherapy and in combination with RHZ suggests that a strong background regimen (RHZ) is not able to conceal the activity of even the lowest bactericidal dose of R207910. This is a surprising finding, as this has so far not been observed with other new anti-TB drugs. When PA-824 was combined with RHZ for 8 weeks and used at its MBD of 100 mg/kg, it was not able to increase the bactericidal activity of the RHZ regimen (12). When moxifloxacin was given at 100 mg/kg in combination with RHZ 5 days a week for 8 weeks, the increase in bactericidal activity was modest (11). The addition of sparfloxacin to RHZ for 8 weeks did not improve the activity of RHZ (7).
We studied the speed and extent of killing of some individual drugs and combinations in a subsequent experiment (Fig. 2). ZJ25 killed about 1 log10 CFU more than R207910 alone did. ZJ2.5, which included a dose of R207910 well below the MBD, was as active as R207910 at 25 mg/kg during the first 2 weeks of treatment. The synergy between R207910 and PZA was able to compensate for a 10-fold decrease in the R207910 dose in that time frame (Fig. 2). It is not clear why the ZJ2.5 combination did not succeed in achieving further killing in W3 and W4 of treatment. The addition of RIF-INH to the ZJ25 group (RHZJ25 in Fig. 1 compared to ZJ25 in Fig. 2) resulted in accelerated killing and achieved culture negativity for all animals after just 6 weeks of treatment. The contribution of ZJ to the efficacy of this RHZJ combination appeared to be at least as important as the contribution of RH.
Some interesting observations regarding the killing kinetics can be made. First, the efficacy of R207910 monotherapy during the first week was not impressive (Fig. 2; 0.65 log10 CFU [P = 0.0028 compared with the results for the untreated controls]), although it matched that of INH (Fig. 2; 0.59 log10 CFU [P = 0.9 compared with the results for R207910]). In the first early bactericidal activity trial performed with TB patients, the 400-mg dose of R207910 killed 0.77 log10 CFU in the first week of treatment, whereas INH killed 1.88 log10 CFU (13). The fact that INH had greater efficacy in the 1-week human study than it did in the mouse study may be explained by the presence of more rapidly replicating bacilli in the human cavities. Interestingly, INH did not kill any additional bacilli during the second week in the mouse study, while the level of killing by R207910 accelerated in the second week (1.2 log10 CFU) and continued in the last 2 weeks (average per week, 0.8 log10 CFU). The ZJ25 combination killed 1.4 log10 CFU in both the first and the second weeks, but this decreased to 0.95 log10 CFU per week in the subsequent 2 weeks. The RHZ combination killed 3.0 log10 CFU in the first 2 weeks and 1.4, 1.5, and 0.8 log10 CFU in the subsequent 2-week intervals. The addition of 25 mg/kg R207910 to that regimen killed an extra 2.0 log10 CFU in the first 2 weeks and an extra 3.4 log10 CFU in the next 2 weeks, or 7.8 log10 CFU in total, with four of six animals reaching culture negativity after just 4 weeks of treatment. The combination of potent drugs apparently compensated for the decreased killing speeds of the individual drugs over time.
In conclusion, our study suggests that the addition of R207910 to the standard RHZ regimen remains an interesting option for study in clinical trials, despite the observed drug-drug interactions between RIF and R207910. As a rapid rate of killing may or may not translate into a durable effect in patients, the relapse rates obtained with reduced doses of R207910 should be studied in the mouse model.
Footnotes
Published ahead of print on 21 July 2008.
REFERENCES
- 1.Andries, K., P. Verhasselt, J. Guillemont, H. W. H. Göhlmann, J. M. Neefs, H. Winkler, J. V. Gestel, P. Timmerman, M. Zhu, E. Lee, P. Williams, D. De Chaffoy, E. Huitric, S. Hoffner, E. Cambau, C. Truffot-Pernot, N. Lounis, and V. Jarlier. 2005. R207910, a diarylquinoline active on ATP synthase of Mycobacterium tuberculosis. Science 307:223-227. [DOI] [PubMed] [Google Scholar]
- 2.Huitric, E., P. Verhasselt, K. Andries, and S. Hoffner. 2007. In vitro antimycobacterial spectrum of a diarylquinoline ATP synthase inhibitor. Antimicrob. Agents Chemother. 51:4202-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibrahim, M., K. Andries, N. Lounis, A. Chauffour, C. Truffot-Pernot, V. Jarlier, and N. Veziris. 2007. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob. Agents Chemother. 51:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji, B., A. Chauffour, K. Andries, and V. Jarlier. 2006. Bactericidal activities of R207910 and other newer antimicrobial agents against Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 50:1558-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji, B., S. Lefrançois, J. Robert, A. Chauffour, C. Truffot-Pernot, and V. Jarlier. 2006. The in vitro and in vivo activities of rifampin, streptomycin, amikacin, moxifloxacin, R207910, linezolid, and PA-824 against Mycobacterium ulcerans. Antimicrob. Agents Chemother. 50:1921-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koul, A., N. Dendouga, K. Vergauwen, B. Molenberghs, L. Vranckx, R. Willebrords, Z. Ristic, H. Lill, I. Dorange, J. Guillemont, D. Bald, and K. Andries. 2007. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 3:323-324. [DOI] [PubMed] [Google Scholar]
- 7.Lalande, V., C. Truffot-Pernot, A. Paccaly-Moulin, J. Grosset, and B. Ji. 1993. Powerful bactericidal activity of sparfloxacin (AT-4140) against Mycobacterium tuberculosis in mice. Antimicrob. Agents Chemother. 37:407-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lounis, N., B. Ji, C. Truffot-Pernot, and J. Grosset. 1997. Which aminoglycoside or fluoroquinolone is more active against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 41:607-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lounis, N., A. Bentoucha, C. Truffot-Pernot, B. Ji, R.J. O'Brien, A. Vernon, G. Roscigno, and J. Grosset. 2001. Effectiveness of once-weekly riafpentine and moxifloxacin regimens against Mycobacterium tuberculosis in mice. Antimicrob. Agents Chemother. 45:3482-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lounis, N., N. Veziris, A. Chauffour, C. Truffot-Pernot, K. Andries, and V. Jarlier. 2006. Combinations of R207910 with drugs used to treat multidrug-resistant tuberculosis have the potential to shorten treatment duration. Antimicrob. Agents Chemother. 50:3543-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuermberger, E., T. Yoshimatsu, S. Tyagi, R. J. O'Brien, A. N. Vernon, R. E. Chaisson, W. R. Bishai, and J. Grosset. 2004. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am. J. Respir. Crit. Care Med. 169:421-426. [DOI] [PubMed] [Google Scholar]
- 12.Nuermberger, E., I. Rosenthal, S. Tyagi, K. N. Williams, D. Almeeda, C. A. Peloquin, W. R. Bishai, and J. Grosset. 2006. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 50:2621-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rustomjee, R., A. Diacon, J. Allen, A. Venter, C. Reddy, R. F. Patienta, T. C. Mthiyane, T. De Marez, R. Van Heeswijk, R. Kerstens, A. Koul, K. De Beule, P. R. Donald, and D. F. McNeeley. 2008. Early bactericidal activity and pharmacokinetics of the investigational diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob. Agents Chemother. 52:2831-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veziris, N., C. Truffot-Pernot, A. Aubry, V. Jarlier, and N. Lounis. 2003. Fluoroquinolone-containing third-line regimen against Mycobacterium tuberculosis in vivo. Antimicrob. Agents Chemother. 47:3117-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. 1997. Treatment of tuberculosis: guidelines for national programmes, 2nd ed. Report WHO/TB/97.220. World Health Organization, Geneva, Switzerland.