Abstract
We report a case of Candida glabrata invasive candidiasis that developed reduced susceptibility to caspofungin during prolonged therapy. Pre- and posttreatment isolates were confirmed to be isogenic, and sequencing of hot spots known to confer echinocandin resistance revealed an F659V substitution within the FKS2 region of the glucan synthase complex.
The echinocandins have become first-line therapy in many centers for the treatment of invasive candidiasis due to their proven efficacy, the infrequency of side effects, and the favorable drug interaction profile (12, 16, 20, 23). However, reduced susceptibility to these agents has been reported in patients receiving therapy for invasive candidiasis and is primarily due to mutations within highly conserved regions of FKS1 and FKS2, genes encoding subunits of the glucan synthase enzyme complex (8, 9, 21). We report a case of invasive candidiasis caused by Candida glabrata that developed reduced susceptibility to caspofungin during a prolonged course of therapy with this agent.
A 41-year-old previous orthotopic liver recipient, who had no previous antifungal exposure, developed C. glabrata candidemia 8 months after transplantation. Intravenous caspofungin (70-mg load, followed by 50 mg daily) was initiated, and the fungemia cleared within 24 h. Yet cultures of multiple sites remained positive: bronchoalveolar lavage cultures, thought to represent colonization, were positive on days 23 and 52 of therapy; peritoneal fluid and an abdominal wall abscess were positive on day 40; and blood cultures returned positive on day 53. Dialysis dependence, hepatic dysfunction, and drug interaction concerns precluded alternative antifungal agents. The patient died on day 61 of caspofungin therapy after the development of multiorgan failure. Broth microdilution testing performed according to CLSI (formerly NCCLS) standard M27-A2 methodology (17) demonstrated reduced caspofungin susceptibility (MICs of 2 and 8 μg/ml at 24 and 48 h, respectively) for C. glabrata isolate 7755 recovered from the peritoneal fluid on day 40 compared to isolate 7754 (MIC of 0.25 μg/ml) recovered from the blood prior to antifungal therapy.
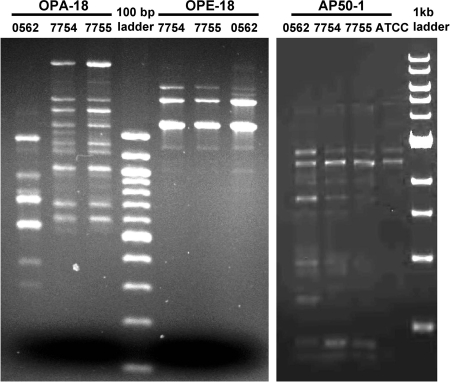
Random amplification of polymorphic DNA using previously described methods and primers (AP50-1, OPA-18, and OPE-18) (1, 2) strongly suggested strain isogenicity for isolates 7754 and 7755 recovered from this patient. Band patterns were identical for these two isolates with each of the three primers used, while differences in band intensity and location were observed compared to the unrelated isolate 0562 with primers OPA-18 and AP50-1 (Fig. 1).
FIG. 1.
Random amplification of polymorphic DNA gel patterns for C. glabrata isolates 7754, 7755, and 0562 obtained with primers OPA-18, OPE-18, and AP50-1.
Conserved regions of the glucan synthase enzyme complex hot spot regions were identified within the C. glabrata genome sequence (http://cbi.labri.fr/Genolevures/index.php) for C. glabrata FKS1 (CgFKS1) (CAGL0G01034g) and CgFKS2 (CAGL0K04037g). Genomic DNA was exracted using a commercially available kit (MasterPure yeast DNA purification kit; Epicentre Biotechnologies, Madison, WI), and regions of interest were sequenced with primers prepared at the UTHSCSA Advanced Nucleic Acid Core facility (Table 1). Sequence analysis of susceptible isolate 7754 revealed wild-type sequences in hot spots 1 and 2 of CgFKS1 and CgFKS2. However, a mutation within hot spot 1 of CgFKS2 that conferred an F659V amino acid substitution in CgFks2p was found in isolate 7755 with reduced caspofungin susceptibility.
TABLE 1.
Primer sequences used for amplification and sequencing of the hot spot regions within CgFKS1 and CgFKS2
| Primer | Sequence |
|---|---|
| FKS1 | |
| HS1 | |
| Forward | 5′-CCATTGGGTGGTCTGTTCACG |
| Reverse | 5′-GATTGGGCAAAGAAAGAAATACGAC |
| Sequencing | 5′-CTCAAACCTTCACTGCCTC |
| HS2 | |
| Forward | 5′-GGTATTTCAAAGGCTCAAAAGGG |
| Reverse | 5′-ATGGAGAGAACAGCAGGGCG |
| Sequencing | 5′-CGGTATGAATGCCCTATTACG |
| FKS2 | |
| HS1 | |
| Forward | 5′-GTGCTCAACATTTATCTCGTAGG |
| Reverse | 5′-CAGAATAGTGTGGAGTCAAGACG |
| Sequencing | 5′-GCTTCTCAGACTTTCACCG |
| HS2 | |
| Forward | 5′-CGTAGACCGTTTCTTGACTTC |
| Reverse | 5′-CTTGCCAATGTGCCACTG |
| Sequencing | 5′-TCTTGACTTTCTACTATGCG |
Although rare, recent reports have illustrated the potential for echinocandin resistance to emerge during therapy (7, 10, 13, 14, 22). Many of these reports have identified mutations within genes encoding subunits of the glucan synthase complex, and all mutations described to date reside within highly conserved regions of FKS1 or its homolog, FKS2 (5, 6, 11, 21). Candida albicans isolates comprise the majority of these cases, with mutations leading to codon changes F641S, S645F, S645Y, S645P, and R1361H (13, 14, 21). Additionally, a mutation resulting in amino acid change R1361G within the FKS1 homolog in Candida krusei has been described (8).
Reduced echinocandin susceptibility and clinical failure have also been reported with C. glabrata. One case report detailed the emergence of caspofungin resistance and clinical failure after prolonged therapy, a finding supported by both in vitro and in vivo studies (10). However, no sequence analysis of either CgFKS1 or CgFKS2 was reported. Conversely, another study described a mutation within CgFKS2 resulting in an F659V codon change. Although no clinical information was provided, this mutation was proven to confer caspofungin resistance (9). The same mutation within CgFKS2 was also found in isolate 7755 in our patient and was associated with a marked increase in caspofungin MICs. Similarly, another recent case report also demonstrated a mutation within hot spot 1 of Fks1p in a C. glabrata isolate during caspofungin therapy leading to reduced susceptibility and clinical failure (3).
Despite the 8- to 32-fold increases in the caspofungin 24- and 48-h MICs for isolate 7755, anidulafungin and micafungin maintained potency against both 7754 and 7755 (Table 2). However, this difference in potencies between caspofungin and the other echinocandins was no longer present when susceptibility testing was repeated in the presence of 50% human serum. In this setting, the 24- and 48-h MICs for anidulafungin and micafungin increased 8- to 32-fold against isolate 7754 and 4- to 16-fold for isolate 7755. The prospect of using a different echinocandin when caspofungin resistance is encountered has been proposed (7, 10, 22) and is based on enhanced potency of anidulafungin and micafungin against Candida isolates observed in vitro (4, 18). Unfortunately, these observations have not translated into improved efficacy in murine models of invasive fungal infections. In these studies, in vivo efficacy correlated better with in vitro potency when tested in the presence of human serum (19, 25). The effect of serum on the activity of echinocandins is not fully understood. One potential explanation proposes the observed reduction in susceptibility is due to significant protein binding associated with these agents. Although this reduction in potency may be secondary to protein binding, significantly higher drug activity has been measured for micafungin than that predicted by the free drug concentration using protein binding data (15). The clinical relevance of reduced in vitro potency for the echinocandins in the presence of serum is unknown.
TABLE 2.
Caspofungin, anidulafungin, and micafungin MICs in the presence and absence of 50% human serum
| C. glabrata isolate | MIC (μg/ml) with no human serum/50% human seruma
|
|||||
|---|---|---|---|---|---|---|
| Caspofungin
|
Anidulafungin
|
Micafungin
|
||||
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| 7754 | 0.25/0.5 | 0.25/0.5 | 0.125/1 | 0.125/2 | 0.125/1 | 0.125/4 |
| 7755 | 2/2 | 8/8 | 0.5/4 | 1/4 | 0.25/4 | 0.5/4 |
MICs were read at 24 and 48 h as the lowest concentration of drug resulting in a significant (≥50%) decrease in turbidity compared to the growth control.
Continued exposure to antimicrobials is often associated with the development of resistance, and as our case and previous reports illustrate, this also may lead to the development of echinocandin resistance in Candida species, including non-C. albicans isolates, during continued drug pressure with members of this antifungal class. A heightened suspicion for reduced echinocandin susceptibility as a possible cause of patient failure is needed as the use of these agents continues to increase.
Acknowledgments
We thank A.W. Fothergill, C. Kelly, J. E. Patterson, and M. G. Rinaldi for their work (24), which led us to further pursue studies with these isolates that are presented in this article.
This research was funded in part by a National Institute of Health research grant to T.F.P. (5RO1DE018096).
G.R.T., A.C.V., and N.C.V. report no conflicts of interest. N.P.W. has received research support from Pfizer and Schering-Plough. J.S.L. has served as a consultant and speaker for Astellas, Merck, Pfizer, and Schering-Plough. T.F.P. has received research support from Merck, Pfizer, Schering-Plough, and Nektar Therapeutics and has served on the speakers' bureau for Merck and Pfizer and as a consultant for Astellas, Basilea, Merck, Nektar, Pfizer, Schering-Plough, and Stiefel Laboratories.
Footnotes
Published ahead of print on 1 August 2008.
REFERENCES
- 1.Bautista-Muñoz, C., X. M. Boldo, L. Villa-Tanaca, and C. Hernández-Rodriguez. 2003. Identification of Candida spp. by randomly amplified polymorphic DNA analysis and differentiation between Candida albicans and Candida dubliniensis by direct PCR methods. J. Clin. Microbiol. 41:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, K., D. Badehorn, S. Deiwick, G. Peters, and W. Fegeler. 2000. Molecular genotyping of Candida species with special respect to Candida (Torulopsis) glabrata strains by arbitrarily primed PCR. J. Med. Microbiol. 49:575-581. [DOI] [PubMed] [Google Scholar]
- 3.Cleary, J. D., G. Garcia-Effron, S. W. Chapman, and D. S. Perlin. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob. Agents Chemother. 52:2263-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cota, J., M. Carden, J. R. Graybill, L. K. Najvar, D. S. Burgess, and N. P. Wiederhold. 2006. In vitro pharmacodynamics of anidulafungin and caspofungin against Candida glabrata isolates, including strains with decreased caspofungin susceptibility. Antimicrob. Agents Chemother. 50:3926-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douglas, C. M., J. A. D'Ippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, W. Li, G. K. Abruzzo, A. Flattery, K. Bartizal, A. Mitchell, and M. B. Kurtz. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas, C. M., J. A. Marrinan, W. Li, and M. B. Kurtz. 1994. A Saccharomyces cerevisiae mutant with echinocandin-resistant 1,3-β-d-glucan synthase. J. Bacteriol. 176:5686-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez, S., J. L. López-Ribot, L. K. Najvar, D. I. McCarthy, R. Bocanegra, and J. R. Graybill. 2004. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob. Agents Chemother. 48:1382-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahn, J. N., G. Garcia-Effron, M.-J. Hsu, S. Park, K. A. Marr, and D. S. Perlin. 2007. Acquired echinocandin resistance in a Candida krusei isolate due to modification of glucan synthase. Antimicrob. Agents Chemother. 51:1876-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katiyar, S., M. Pfaller, and T. Edlind. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krogh-Madsen, M., M. C. Arendrup, L. Heslet, and J. D. Knudsen. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938-944. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz, M. B., G. Abruzzo, A. Flattery, K. Bartizal, J. A. Marrinan, W. Li, J. Milligan, K. Nollstadt, and C. M. Douglas. 1996. Characterization of echinocandin-resistant mutants of Candida albicans: genetic, biochemical, and virulence studies. Infect. Immun. 64:3244-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuse, E. R., P. Chetchotisakd, C. A. da Cunha, M. Ruhnke, C. Barrios, D. Raghunadharao, J. S. Sekhon, A. Freire, V. Ramasubramanian, I. Demeyer, M. Nucci, A. Leelarasamee, F. Jacobs, J. Decruyenaere, D. Pittet, A. J. Ullmann, L. Ostrosky-Zeichner, O. Lortholary, S. Koblinger, H. Diekmann-Berndt, and O. A. Cornely. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 369:1519-1527. [DOI] [PubMed] [Google Scholar]
- 13.Laverdiere, M., R. G. Lalonde, J. G. Baril, D. C. Sheppard, S. Park, and D. S. Perlin. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57:705-708. [DOI] [PubMed] [Google Scholar]
- 14.Miller, C. D., B. W. Lomaestro, S. Park, and D. S. Perlin. 2006. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy 26:877-880. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki, N., M. Aibiki, and Y. Matsumoto. 2006. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1598, p. 423.
- 16.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 17.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, NCCLS document M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 18.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paderu, P., G. Garcia-Effron, S. Balashov, G. Delmas, S. Park, and D. S. Perlin. 2007. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 51:2253-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappas, P. G., C. M. Rotstein, R. F. Betts, M. Nucci, D. Talwar, J. J. De Waele, J. A. Vazquez, B. F. Dupont, D. L. Horn, L. Ostrosky-Zeichner, A. C. Reboli, B. Suh, R. Digumarti, C. Wu, L. L. Kovanda, L. J. Arnold, and D. N. Buell. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883-893. [DOI] [PubMed] [Google Scholar]
- 21.Park, S., R. Kelly, J. N. Kahn, J. Robles, M. J. Hsu, E. Register, W. Li, V. Vyas, H. Fan, G. Abruzzo, A. Flattery, C. Gill, G. Chrebet, S. A. Parent, M. Kurtz, H. Teppler, C. M. Douglas, and D. S. Perlin. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelletier, R., I. Alarie, R. Lagace, and T. J. Walsh. 2005. Emergence of disseminated candidiasis caused by Candida krusei during treatment with caspofungin: case report and review of literature. Med. Mycol. 43:559-564. [DOI] [PubMed] [Google Scholar]
- 23.Reboli, A. C., C. Rotstein, P. G. Pappas, S. W. Chapman, D. H. Kett, D. Kumar, R. Betts, M. Wible, B. P. Goldstein, J. Schranz, D. S. Krause, and T. J. Walsh. 2007. Anidulafungin versus fluconazole for invasive candidiasis. N. Engl. J. Med. 356:2472-2482. [DOI] [PubMed] [Google Scholar]
- 24.Villareal, N. C., A. W. Fothergill, C. Kelly, J. E. Patterson, M. G. Rinaldi, and T. F. Patterson. 2004. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1034, p. 417.
- 25.Wiederhold, N. P., L. K. Najvar, R. Bocanegra, D. Molina, M. Olivo, and J. R. Graybill. 2007. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob. Agents Chemother. 51:1616-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]