Abstract
Pneumococcal surface protein A (PspA) is an important virulence factor of Streptococcus pneumoniae. PspA exists as two major families, which include variable but serologically cross-reactive proteins. Previous studies with a family 1 PspA antigen suggested that children develop low concentrations of anti-PspA after pneumococcal carriage or infection. In this study, antibody to PspA families 1 and 2 was measured by an enzyme immunoassay of the serum and saliva of children with a history of culture-proven pneumococcal colonization and/or acute otitis media and in the serum and saliva of adults. The PspA families of the pneumococcal strains isolated from children were determined. The majority of the children had high serum and salivary anti-PspA concentrations to the PspA family they had encountered and low concentrations to the other, whereas adults had high antibody concentrations to both PspA families, both in serum and in saliva. The results suggest that children have a relatively family-specific antibody response to the PspA family they have been exposed to and that any PspA vaccine for children should contain members of both major PspA families.
Virulence proteins play important roles at different stages of pneumococcal infection, from nasopharyngeal colonization to progression to pneumonia and bacteremia (10). Proteins essential to the virulence of Streptococcus pneumoniae (pneumococcus) could be effective and affordable vaccine antigens. Pneumococcal surface protein A (PspA) serves as an important virulence tool for pneumococcus by, e.g., interfering with complement opsonization (25-27, 39). PspA also binds lactoferrin (8, 9) and thereby protects pneumococci from apolactoferrin-mediated killing (30), which can be important at mucosal surfaces. In animal models, PspA is able to induce a mucosal and systemic antibody response and protective immunity against pneumococcal colonization, otitis media, and invasive disease (3, 4, 41, 42). In adult humans, PspA was one of the only two proteins that induced a statistically significant rise in serum immunoglobulin G (IgG) titer during experimental colonization (18), and preexisting antibodies to PspA appeared to offer protection against carriage (17).
PspA is highly variable at the level of DNA sequence, and few PspA molecules are exactly the same (12). Nevertheless, PspAs share many sequence similarities and serologically cross-reactive epitopes (12). The epitopes shown to elicit protective antibodies are located in the surface-exposed N-terminal part of PspA (19, 43). Based on sequence similarities, PspAs have been divided into three PspA families and further into six clades; family 1 (clades 1 and 2) and family 2 (clades 3, 4, and 5) collectively cover 98% of the PspAs (12), whereas to date, only a few pneumococcal strains have been classified into family 3 (clade 6) (11). Slight differences in the distributions of PspA families 1 and 2 have been reported in different parts of the world (11, 23, 29, 36, 40).
The data from animal models on cross-protection of immunity evoked by different PspAs are somewhat contradictory, speaking both for (19, 22, 38) and against (20, 28) cross-protection. With a DNA vaccine, the cross-reactivity of antibodies was restricted to the same PspA family, and protection was limited to the same clade that mice were immunized with (20). Mice immunized with a hybrid protein containing portions of family 1 and family 2 PspAs were protected against strains with either PspA family, but protection against family 2 appeared to be clade specific (6).
Few studies have addressed the cross-reactivity of human antibodies. Adults immunized with a single family 1 PspA had antibodies cross-reacting with different PspAs from families 1 and 2 (21) that were competent for protecting mice after passive immunization from challenge strains expressing PspAs of either family (2). The extent of the cross-reactivity of PspA clades followed roughly the amount of amino acid sequence homology of the heterologous antigens (21). Depending on the cross-reactive properties of each individual PspA molecule, one or more PspAs may be recognized by antiserum raised to a single PspA (11). In a human colonization model, serum IgG antibodies raised to the PspA of the colonizing strain cross-reacted with some other PspA molecules as well, but recognition was strongest with the most similar PspA (17, 18).
We have previously assessed the development of natural immunity to pneumococci in relation to pneumococcal carriage and disease (14, 24, 31-35) in the children of the Finnish Otitis Media (FinOM) Cohort Study (15, 37). Studies suggested that infants and children develop only low or undetectable concentrations of serum and salivary anti-PspA antibodies after exposure to pneumococci (24, 33). Similar kinetics were observed for Kenyan children: anti-PspA IgG and IgA developed later than antibodies to other surface proteins, with concentrations slowly increasing until early childhood (16). In the sera of young Filipino infants, antibodies to PspA were detected only rarely in spite of frequent pneumococcal colonization (13).
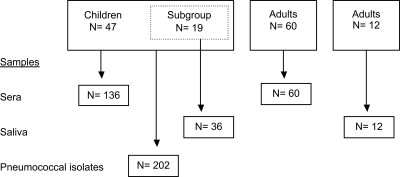
Given that in mice PspA is an immunogenic and protective surface protein (2, 3, 5), the results of the studies of anti-PspA antibody production in young children were unexpected. In the above studies (13, 16, 24, 33), only one PspA antigen, family 1 PspA from strain Rx1, was used as a target antigen in an enzyme immunoassay (EIA), which may be insufficient to detect antibodies to representatives of the other major PspA family. Anti-PspA antibodies might develop in a PspA family-specific manner in early life, depending on the types of PspAs the children have encountered. In this study, we reanalyzed the sera and saliva of children and adults by using both family 1 and family 2 antigens. The sera for this study were selected from the first 47 consecutive children with pneumococcal exposures (acute otitis media [AOM] and/or nasopharynx carriage) and from 60 adults participating in the FinOM Cohort Study (24). The PspA families of the pneumococci isolated from the children have been analyzed in another study (M. M. Melin et al., submitted for publication). In this study, we describe the PspA family-specific antibody development in children in relation to the PspA family of the pneumococcus they have encountered. We also compare the antibody profiles of children to those of adults.
MATERIALS AND METHODS
Study population.
In the FinOM Cohort Study, a cohort of 329 Finnish children was followed from 2 to 24 months of age in order to assess natural immunity to pneumococcal carriage and disease. Pneumococcal carriage in the nasopharynx was examined by 10 consecutive nasopharyngeal-swab cultures taken from 2 to 24 months of age (37). Additional samples were collected and cultured at sick visits; aspirate was collected during respiratory infection and a middle-ear-fluid (MEF) sample when AOM was diagnosed (15). Serum samples were taken at scheduled “healthy” visits at ages 6, 12, 18, and 24 months. Additional paired serum samples were obtained when the child had AOM. Sera were also collected from 325 mothers of the children (24). For salivary antibody measurements, salivary samples were obtained from the study children at the ages of 6, 12, 18, and 24 months and from healthy adults not enrolled in the FinOM study (33).
Clinical samples selected for this study. (i) Serum samples.
For this study, we selected a subset of the FinOM cohort, comprising the children with pneumococcal exposures, who were thus expected to have antibodies to PspA. At least two sera (n = 136) from the first 47 consecutive children with pneumococcal exposures were selected (Fig. 1). Most children had multiple pneumococcal cultures. Serum samples collected prior to and closely after the pneumococcal exposures were selected for analysis. Sera taken at less than 3 months of age were excluded because of the possible existence of maternal antibodies in infant sera. Serum antibodies to PspA and other pneumococcal antigens in Filipino infants decreased consistently with the disappearance of maternal antibodies up to 18 weeks of age (13). Most of the 47 children in this study had had pneumococcal exposures before the first selected serum sample, and thus, the majority of the sera in this study were collected after pneumococcal exposure (122/136). Approximately half of the selected serum samples were negative for anti-PspA (family 1) in the previous study (24). To compare the antibodies of children and adults, randomly picked sera of 60 mothers of the FinOM Cohort Study (24) were analyzed (Fig. 1).
FIG. 1.
Numbers of subjects and samples selected for the study from among the whole cohort of 329 children and adults participating in the FinOM Cohort Study. The adult saliva samples were from donors not enrolled in the FinOM Cohort Study.
(ii) Saliva samples.
Salivary antibodies to PspA were measured from the same children whose serum samples were analyzed in this study (Fig. 1). The saliva samples were stored at −70°C, and they had been thawed once (33) before use in this study. Due to the small volume left, the saliva of only 19 of the 47 children (n = 36; 1 to 3 samples/child) was available for these reanalyses. Eleven of the saliva samples were obtained before any cultured pneumococcal exposures, and 25 were postexposure samples. In addition, 12 out of the 17 adult saliva samples (33) were still available for analysis (Fig. 1).
(iii) Pneumococcal strains.
Nasopharyngeal carriage isolates (n = 172) and isolates from MEF samples (n = 30) collected prior to or at the same time as the serum samples of the 47 children were included in this study (Fig. 1). The isolation and capsular serotyping of pneumococcal strains have been described elsewhere (15, 37). In case multiple pneumococcal isolates of the same capsular serotype were cultured from different anatomical sites at the same time, only one was selected for analysis, since isolates of the same serotype found in aspirate/nasopharyngeal-swab and MEF samples are believed to be the same strain (7). PspA families of the isolates were determined as described elsewhere (Melin et al., submitted).
Antibody determinations by EIA. (i) PspA antigens.
Two PspA antigens (representatives of families 1 and 2) were used for separate EIA analyses of anti-PspA family 1 and anti-PspA family 2 antibodies in serum and salivary samples. The recombinant PspA/Rx1aa1…303 is a family 1, clade 2 antigen (PspA1) comprised of the N-terminal 303 amino acids of Streptococcus pneumoniae strain Rx1. The antigen PspA/V-24aa1…350 belongs to family 2 and clade 3 (PspA2), and it consists of amino acids 1 to 350 of the wild-type mature PspA from S. pneumoniae strain V-24. A third PspA antigen, PspA/JCP56aa1…330 belongs to family 2 and clade 4. It was used for analyses of a small subset of serum samples that remained negative for anti-PspA2 with the family 2, clade 3 antigen. PspA antigens were purified as previously described (1). The PspA antigen used in the previous studies (24, 33) consisted of the first 315 amino acids of Rx1 PspA and was thus nearly identical to the family 1 antigen used in this study, except that it was manufactured by Sanofi Pasteur (formerly Pasteur Mérieux Connaught).
(ii) EIA for serum IgG.
IgG-specific antibodies to PspA1 and PspA2 were measured by EIA as described previously (24), with minor modifications. The wells of microtiter plates (Maxi sorp; Nunc Roskilde, Denmark) were coated with 2 μg/ml recombinant antigen in sterile phosphate-buffered saline. Polyclonal alkaline phosphatase-conjugated goat anti-human IgG (Sigma Immuno Chemicals, St. Louis, MO) was used for detection. IgG concentrations were calculated in μg/ml by using a reference serum (human serum pool A) for which the anti-PspA1 and anti-PspA2 IgG concentrations had been determined previously (21). Samples with antibody concentrations below the lower limit of quantitation (0.22 μg/ml for PspA1 and 0.26 μg/ml for PspA2) were assigned values equivalent to half of the quantitation limit.
(iii) EIA for salivary IgA.
The EIA method described previously (33), with minor modifications, was used. The coating concentration was 2 μg/ml in phosphate-buffered saline for each PspA antigen, and the coating volume was 100 μl/well. Monoclonal anti-human IgA antibodies produced in mice (M207; Bionostics) were used for detection. An optical density (OD) of ≥0.04 for anti-PspA was considered positive. Samples with ODs of <0.04 were given a value of 0.02. Since no reference for IgA anti-PspA was available, the actual concentrations could not be calculated, and the average ODs of triplicate wells are reported.
Statistical methods.
Statistical analyses on anti-PspA concentrations and ODs were performed with log-transformed data. The levels of serum IgG anti-PspA are given as geometric mean concentrations with 95% confidence intervals. Anti-PspA levels of salivary IgA are given as the geometric means of ODs. The Pearson coefficient of correlation was calculated for anti-PspA1 and anti-PspA2 in the sera of children and adults. Student's paired t test (two-tailed) was applied in the comparison of IgG anti-PspA1 and anti-PspA2 geometric mean concentrations in the sera of children and adults. Due to the high proportion of anti-PspA negative salivary samples, the Wilcoxon signed-rank test was applied in the comparison of anti-PspA IgA to families 1 and 2. In both tests, P values of less than 0.05 were considered statistically significant.
RESULTS
Development of IgG antibodies to PspA1 and PspA2 in serum.
Antibody to PspA1 and/or PspA2 was detected in 90% of the sera collected from children after pneumococcal exposure (n = 122); 79% of the sera had antibody to PspA1 and 80% to PspA2. Antibodies to both antigens were found in 75% of the sera. In 15% of the sera, there was detectable antibody to one PspA family only. Ten percent of the sera remained below the limits of quantitation for both PspA families, in spite of pneumococcal exposures. The samples of the six children with exposure to family 2 PspA but no detectable (n = 9) or very low (n = 5) antibody concentrations were reanalyzed by using another family 2 PspA antigen, but antibody concentrations remained equally low with both clade 3 and clade 4 antigens (data not shown).
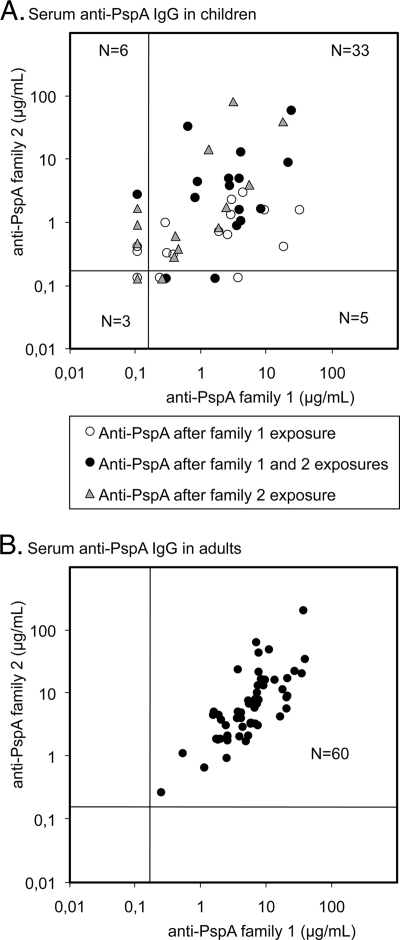
All pneumococcal isolates from 16 children had PspA family 1, and all isolates from 15 children had PspA family 2. Pneumococcal strains representing both PspA families were isolated from 16 children. The sera of children were divided into three groups according to the PspA families of the pneumococci isolated before the serum sample was obtained: (i) family 1, (ii) family 2, or (iii) both family 1 and 2 PspAs. For statistical analyses, only one postcontact serum sample (the last one) from each child (n = 47) was included (Table 1). The concentrations of serum antibodies to the two PspA families were often unequal (Fig. 2). The correlation of antibody concentrations between PspA family 1 and family 2 in children's sera was better after exposure to both PspA families (r = 0.60) than after exposure only to family 1 (r = 0.27) or family 2 (r = 0.47). Anti-PspA concentrations to the PspA family the children had been exposed to were twice as high compared to those to the other PspA family (Table 1). Anti-PspA antibody concentrations were generally higher in adults than in children (Table 1). All adults had similar antibody concentrations to both PspA families, which correlated well with each other (r = 0.76; Fig. 2).
TABLE 1.
Antibodies to PspA in serum
| Subject and PspA exposure | Mean age in days (SD)a | No. of subjects | GMC (95% CI)b
|
|
|---|---|---|---|---|
| PspA family 1 | PspA family 2 | |||
| Children | 47 | |||
| PspA family 1 exposure | 435 (148) | 16 | 1.26 (0.46, 3.44) | 0.56 (0.33, 0.97) |
| PspA family 2 exposure | 490 (153) | 15 | 0.60 (0.24, 1.52) | 1.23 (0.40, 3.77) |
| PspA family 1 and 2 exposures | 557 (173) | 16 | 2.30 (1.07, 4.95) | 2.88 (1.18, 7.02) |
| Adults | 60 | 5.47 (4.24, 7.06) | 5.71 (4.21, 7.75) | |
SD, standard deviation.
The geometric mean concentrations (GMCs) and 95% confidence intervals (CI) of serum PspA antibodies in the sera of children and adults. Only one serum per child was included in the comparison; the average ages of the children at the time the sera were collected are given. Children developed more antibodies to the same PspA family they had been exposed to, but the differences in mean anti-PspA1 and anti-PspA2 concentrations were not statistically significant (family 1, P = 0.059; family 2, P = 0.058; Student's t test, paired, two-tailed).
FIG. 2.
Anti-PspA antibodies in the sera of children (47 sera) with previous culture-proven pneumococcal exposures (nasopharyngeal or middle-ear isolates) and in the sera of adults (60 sera). The samples are distributed below and above the limits of detection in the four fields (0.22 μg/ml for PspA1 and 0.26 μg/ml for PspA2), and the number of samples in each of the four sections is given.
Salivary IgA antibodies to PspA1 and PspA2.
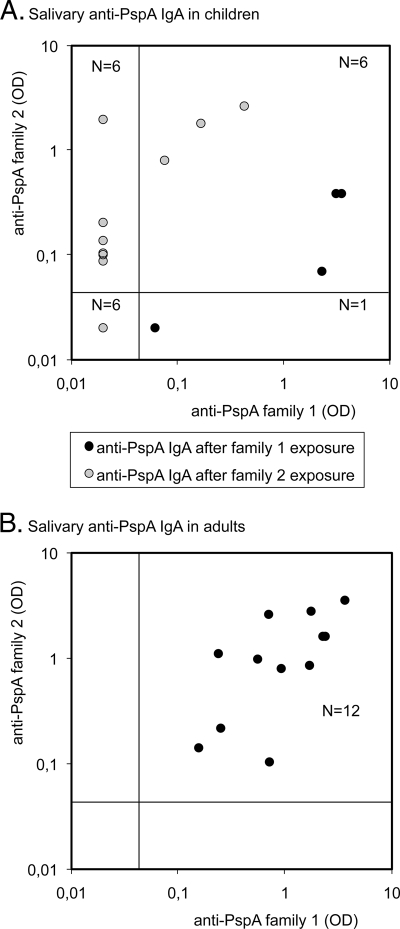
All preexposure saliva samples (n = 11) of the children were negative for anti-PspA, but after pneumococcal exposure, 13/19 children had salivary antibodies to either PspA family 1 or 2, and 6/19 had detectable antibodies to both PspA families (Fig. 3). However, when the children were exposed to only one PspA family, the ODs for that PspA family were higher than those for the other family (Table 2). Each of the 12 adult saliva samples contained antibodies to both PspA families, and the levels were very similar to those of antibodies to PspA families 1 and 2 (Fig. 3 and Table 2).
FIG. 3.
Salivary antibodies to PspA in children with recent pneumococcal exposures (n = 19) and in 12 adults. Only one sample per child was included in the comparison; the last sample was preceded by exposures to strains with either family 1 (n = 6) or family 2 PspAs (n = 13). Detection limits are indicated in the figures; samples with an OD of <0.04 remain beyond the antibody detection range; samples below that OD are assigned a common OD value equal to half of the detection limit (OD = 0.02).
TABLE 2.
Antibodies to PspA in saliva
| Subject and PspA exposure | No. of subjects | GM (95% CI)a
|
|
|---|---|---|---|
| PspA family 1 | PspA family 2 | ||
| Children | 19 | ||
| PspA family 1 exposure | 6 | 0.29 (0.02, 4.35) | 0.07 (0.01, 0.30) |
| PspA family 2 exposure | 13 | 0.03 (0.02, 0.06) | 0.15 (0.05, 0.47) |
| Adults | 12 | 0.85 (0.45, 1.63) | 0.85 (0.40, 1.80) |
The geometric means (GM) and 95% confidence intervals (CI) of PspA antibodies (ODs) in the saliva of children and adults. Only one sample per child was included in the comparison. Children developed more antibodies to the same PspA family they had been exposed to. The difference in geometric mean anti-PspA1 and anti-PspA2 was statistically significant in children after family 2 exposure (family 1, P = 0.068; family 2, P = 0.008; Wilcoxon signed-rank test).
Kinetics of serum anti-PspA1 and -PspA2 in relation to the PspA family of the prior pneumococcal exposures.
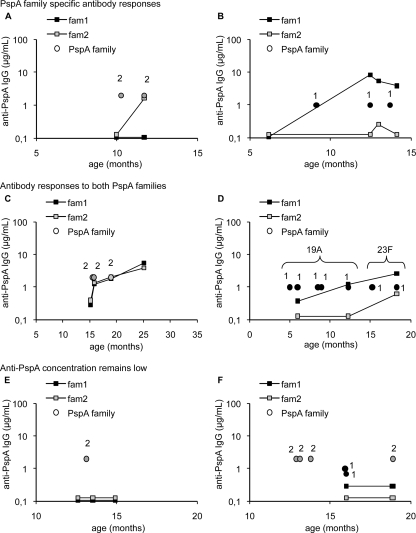
Graphs depicting both the kinetics of serum antibody development and the PspA families of pneumococcal strains isolated were made individually for each child. Based on the nature of the response, the graphs were divided into three groups: (i) specific responses to the same PspA family as in the prior culture(s) (n = 28), (ii) responses to both PspA families in spite of an exposure to one PspA family only (n = 10), or (iii) no response in spite of pneumococcal exposure (n = 9). Representatives of each group are presented in Fig. 4.
FIG. 4.
Kinetics of serum anti-PspA1 and -PspA2 antibody development in individual children. The circles indicate positive pneumococcal cultures, and the squares indicate the kinetics of antibodies to either PspA1 or PspA2. The children were divided into three groups based on the pattern of their serum anti-PspA antibody kinetics. Two representatives of each group are shown: PspA family-specific response (A and B), antibody responses to both PspA families in spite of exposure to only one PspA family (C and D), and no anti-PspA response in spite of exposure (E and F). Half of the children had an antibody response specific to the PspA family they had been exposed to (A and B). A simultaneous increase in both anti-PspA1 and -PspA2 concentrations was found in one-fifth of the children who had been exposed to only one of the PspA families (C and D). Acquisition of a strain with a different capsular serotype is indicated in panel D. One-fifth of the children had antibody concentrations below the detection limit (E), or they did not have any response to the PspA that was found in the isolated pneumococci (F).
Half of the children (28/47) had a marked antibody response only to the same PspA family/families to which they had been exposed. Fourteen of these 28 children had a strictly PspA family-specific antibody response (Fig. 4A), and the rest produced antibodies to both families but clearly more to the one they had been exposed to (as in Fig. 4B). A simultaneous increase in both anti-family 1 and -family 2 PspA concentrations was found in 10 children who had been exposed to pneumococci of one PspA family only. Five of these 10 children had similar antibody concentrations as well as identical antibody kinetics for the two PspAs (Fig. 4C), and the other five produced antibodies to one PspA family only at first, but additional pneumococcal exposures with the same PspA family elevated the antibody concentration to the other PspA family as well (Fig. 4D). Nine of the 47 children produced neither anti-PspA1 nor anti-PspA2 antibodies in spite of culture-proven pneumococcal exposure (Fig. 4E and F). For these children either the anti-PspA concentrations were below the detection limits (n = 4) or the antibody concentrations did not rise (n = 5).
DISCUSSION
In the present study, we have shown that young children are more likely to produce antibodies recognizing only the PspA family of their infecting strain than a strong response to both PspA families. The natural exposure of humans to S. pneumoniae is likely to contribute to the breadth of cross-reactivity of antibody to PspA—the more divergent the encounters with different PspAs, the more cross-reacting the antibodies are likely to be. Antibody to PspA is thought to have a role in pneumococcal acquisition (18, 42) and disease (34). In the first years of life, children's antibody to PspA may not recognize all pneumococci, which may have significance in protection against pneumococcal disease or carriage. The situation may be different later in life; adult sera were observed to have more cross-reacting antibodies to PspA.
The results of our previous studies measuring antibodies to pneumolysin, pneumococcal surface adhesin A, and a PspA family 1 antigen suggested that young children develop only low concentrations of antibodies to PspA after pneumococcal carriage or AOM (24, 33). By the age of 24 months, only 58% of the sera from children with pneumococcal exposure contained serum antibodies to PspA, whereas antibodies to the two other pneumococcal proteins were found in well over 90% of the sera (24). However, almost every adult serum sample (320/325) was positive for anti-PspA family 1 antibodies (24). Similarly, salivary IgA to PspA family 1 was found in 15/17 of the samples of adults, but only 25% of the salivary samples from children with pneumococcal exposure had PspA antibodies at 24 months of age (33). The higher coating concentration and the use of an additional PspA antigen increased the number of positive serum and saliva samples in our previous study (34). In this study, too, the number of anti-PspA-positive samples was higher; antibody to family 1 PspA was detected in 79% of the sera and 48% of the saliva from children with pneumococcal exposure. The use of an additional PspA antigen for PspA family 2 further increased the proportion of anti-PspA-positive samples; as much as 90% of the sera and 80% of the saliva collected after pneumococcal exposure were positive for either anti-PspA family 1 or 2 or both.
Most of the children with pneumococcal carriage or AOM included in this study had antibodies detectable with either of the two PspA antigens, which indicates that antibodies targeted against clades within a PspA family are generally cross-reactive within the PspA family. Sera that were negative to family 2 anti-PspA, despite family 2 exposure, were reanalyzed with another family 2 antigen to exclude the possibility of clade specificity of anti-PspA; however, the result remained the same.
Even though as many as 75% of the children's serum samples were positive for both anti-PspA family 1 and 2, the anti-PspA concentrations to the two PspA antigens could be very different, being generally higher for the PspA family the child had been exposed to. When looking at the individual antibody development (in Fig. 4), half of the children had “specific” responses, meaning a response was found only to the PspA family the children had been exposed or that the concentration of anti-PspA was higher to that PspA family. Some children produced antibodies to both PspA families, although only one of the PspA families was detected. One possible explanation is that some pneumococcal infections or carriages may not have been cultured. Nasopharyngeal cultures were taken monthly during the first 6 months of age but only once in 3 months after that (37).
The different anti-PspA antibody responses of the children might partly be explained by variability in the cross-reactivity of the PspAs of the pneumococci the children have been exposed to. The results of a previous study suggested that some PspA molecules may share a more cross-reacting pattern; pneumococci with such a cross-reacting PspA were detected with both antisera raised against family 1 (clade 1) and family 2 (clades 3 and 4) (11). Thus, children who have carried a pneumococcus with a cross-reacting character could have elicited antibodies that recognize both family 1 and 2 antigens. On the other hand, a child with a strictly specific antibody response to one PspA family only may have been infected with a strain with a more “pure” antigenic profile.
Adult sera contained substantial and rather similar concentrations of antibodies to both family 1 and 2 PspAs. Even though the children selected for this study all had frequent pneumococcal exposures, their overall mean anti-PspA IgG concentrations were significantly lower than those of adults (Table 1). It can be speculated that this could be associated with the low protective capacity of natural anti-PspA in young children. Moreover, only one-third of the children had encountered both family 1 and family 2 PspAs. The anti-PspA concentration in the sera of the children who had been exposed to both PspA families was higher than that in the sera of the children with exposure to only one PspA family, suggesting that these children had had more pneumococcal encounters. We have previously shown that in young children, serum anti-PspA IgG rises as a result of an increasing number of pneumococcal exposures (34). It is likely that the adults are well immunized as a result of several pneumococcal exposures. Because of this, the adult sera presumably contain more cross-reactive antibodies, or a wider repertoire of antibodies recognizing different kinds of PspAs, than the sera of children. The sera from children with exposures to both PspA families appeared to be more cross-reacting. The sera from adults immunized with recombinant PspA (21), like the sera from healthy adults shown here, showed virtually no family specificity. These findings strongly argue that when immunizing infants, a PspA vaccine will need to contain PspAs from both families.
Acknowledgments
Eeva Koskenniemi is acknowledged for data management. We thank all the children and parents who participated in the FinOM Cohort Study. Written, informed consent was obtained at the time of study enrollment from the parents of all children participating in the FinOM Cohort Study. The study protocol was evaluated before the start of the trial by the National Public Health Institute ethics committee and by local health authorities (ethics committee and health board of Tampere).
The FinOM studies were supported by Merck & Co. Inc., Sanofi Pasteur, Wyeth Vaccines, and the World Health Organization (GPV/VRD contract V23/181/116). This work was supported in part by the Meningitis Research Foundation (project 13/00). D.E.B. and S.K.H. were supported by grant AI21548 from the National Institutes of Health.
T.M.K. has consulted on advisory boards for GSK Bio and Wyeth Vaccines, has had travel paid for by Sanofi Pasteur and Wyeth as an expert at symposia, and has received honoraria from Wyeth. H.M.K. has consulted on advisory boards for GSK Bio, has had travel paid for by GSK Bio and Wyeth Vaccines as an invited speaker or expert at symposia, and has received honoraria from GSK Bio. D.E.B. and S.K.H. are consultants with Sanofi Pasteur and have done research in collaboration with Sanofi Pasteur that has been supported in part or in total by Sanofi Pasteur.
Footnotes
Published ahead of print on 27 August 2008.
REFERENCES
- 1.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briles, D. E., S. K. Hollingshead, J. King, A. Swift, P. A. Braun, M. K. Park, L. M. Ferguson, M. H. Nahm, and G. S. Nabors. 2000. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694-1701. [DOI] [PubMed] [Google Scholar]
- 3.Briles, D. E., S. K. Hollingshead, J. C. Paton, E. W. Ades, L. Novak, F. W. van Ginkel, and W. H. Benjamin, Jr. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339-348. [DOI] [PubMed] [Google Scholar]
- 4.Briles, D. E., S. K. Hollingshead, E. Swiatlo, A. Brooks-Walter, A. Szalai, A. Virolainen, L. S. McDaniel, K. A. Benton, P. White, K. Prellner, A. Hermansson, P. C. Aerts, H. Van Dijk, and M. J. Crain. 1997. PspA and PspC: their potential for use as pneumococcal vaccines. Microb. Drug Resist. 3:401-408. [DOI] [PubMed] [Google Scholar]
- 5.Briles, D. E., R. C. Tart, H. Y. Wu, B. A. Ralph, M. W. Russell, and L. S. McDaniel. 1996. Systemic and mucosal protective immunity to pneumococcal surface protein A. Ann. N. Y. Acad. Sci. 797:118-126. [DOI] [PubMed] [Google Scholar]
- 6.Darrieux, M., E. N. Miyaji, D. M. Ferreira, L. M. Lopes, A. P. Lopes, B. Ren, D. E. Briles, S. K. Hollingshead, and L. C. Leite. 2007. Fusion proteins containing family 1 and family 2 PspA fragments elicit protection against Streptococcus pneumoniae that correlates with antibody-mediated enhancement of complement deposition. Infect. Immun. 75:5930-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray, B. M., G. M. Converse III, and H. C. Dillon, Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 8.Hakansson, A., H. Roche, S. Mirza, L. S. McDaniel, A. Brooks-Walter, and D. E. Briles. 2001. Characterization of binding of human lactoferrin to pneumococcal surface protein A. Infect. Immun. 69:3372-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammerschmidt, S., G. Bethe, P. H. Remane, and G. S. Chhatwal. 1999. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 67:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hava, D. L., J. LeMieux, and A. Camilli. 2003. From nose to lung: the regulation behind Streptococcus pneumoniae virulence factors. Mol. Microbiol. 50:1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollingshead, S. K., L. Baril, S. Ferro, J. King, P. Coan, and D. E. Briles. 2006. Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J. Med. Microbiol. 55:215-221. [DOI] [PubMed] [Google Scholar]
- 12.Hollingshead, S. K., R. Becker, and D. E. Briles. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmlund, E., B. Quiambao, J. Ollgren, H. Nohynek, and H. Kayhty. 2006. Development of natural antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A and pneumolysin in Filipino pregnant women and their infants in relation to pneumococcal carriage. Vaccine 24:57-65. [DOI] [PubMed] [Google Scholar]
- 14.Holmlund, E., B. Simell, T. Jaakkola, M. Lahdenkari, J. Hamel, B. Brodeur, T. Kilpi, and H. Kayhty. 2007. Serum antibodies to the pneumococcal surface proteins PhtB and PhtE in Finnish infants and adults. Pediatr. Infect. Dis. J. 26:447-449. [DOI] [PubMed] [Google Scholar]
- 15.Kilpi, T., E. Herva, T. Kaijalainen, R. Syrjanen, and A. K. Takala. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654-662. [DOI] [PubMed] [Google Scholar]
- 16.Laine, C., T. Mwangi, C. M. Thompson, J. Obiero, M. Lipsitch, and J. A. Scott. 2004. Age-specific immunoglobulin G (IgG) and IgA to pneumococcal protein antigens in a population in coastal Kenya. Infect. Immun. 72:3331-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCool, T. L., T. R. Cate, G. Moy, and J. N. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCool, T. L., T. R. Cate, E. I. Tuomanen, P. Adrian, T. J. Mitchell, and J. N. Weiser. 2003. Serum immunoglobulin G response to candidate vaccine antigens during experimental human pneumococcal colonization. Infect. Immun. 71:5724-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDaniel, L. S., B. A. Ralph, D. O. McDaniel, and D. E. Briles. 1994. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb. Pathog. 17:323-337. [DOI] [PubMed] [Google Scholar]
- 20.Miyaji, E. N., D. M. Ferreira, A. P. Lopes, M. C. Brandileone, W. O. Dias, and L. C. Leite. 2002. Analysis of serum cross-reactivity and cross-protection elicited by immunization with DNA vaccines against Streptococcus pneumoniae expressing PspA fragments from different clades. Infect. Immun. 70:5086-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nabors, G. S., P. A. Braun, D. J. Herrmann, M. L. Heise, D. J. Pyle, S. Gravenstein, M. Schilling, L. M. Ferguson, S. K. Hollingshead, D. E. Briles, and R. S. Becker. 2000. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743-1754. [DOI] [PubMed] [Google Scholar]
- 22.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onwubiko, C., C. Shires, L. R. Quin, E. Swiatlo, and L. S. McDaniel. 2007. Characterization of Streptococcus pneumoniae isolated from children with otitis media. FEMS Immunol. Med. Microbiol. 50:119-125. [DOI] [PubMed] [Google Scholar]
- 24.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Kayhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 25.Ren, B., M. A. McCrory, C. Pass, D. C. Bullard, C. M. Ballantyne, Y. Xu, D. E. Briles, and A. J. Szalai. 2004. The virulence function of Streptococcus pneumoniae surface protein A involves inhibition of complement activation and impairment of complement receptor-mediated protection. J. Immunol. 173:7506-7512. [DOI] [PubMed] [Google Scholar]
- 26.Ren, B., A. J. Szalai, S. K. Hollingshead, and D. E. Briles. 2004. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect. Immun. 72:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren, B., A. J. Szalai, O. Thomas, S. K. Hollingshead, and D. E. Briles. 2003. Both family 1 and family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect. Immun. 71:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roche, H., B. Ren, L. S. McDaniel, A. Hakansson, and D. E. Briles. 2003. Relative roles of genetic background and variation in PspA in the ability of antibodies to PspA to protect against capsular type 3 and 4 strains of Streptococcus pneumoniae. Infect. Immun. 71:4498-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadowy, E., A. Skoczynska, J. Fiett, M. Gniadkowski, and W. Hryniewicz. 2006. Multilocus sequence types, serotypes, and variants of the surface antigen PspA in Streptococcus pneumoniae isolates from meningitis patients in Poland. Clin. Vaccine Immunol. 13:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaper, M., S. K. Hollingshead, W. H. Benjamin, Jr., and D. E. Briles. 2004. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin. Infect. Immun. 72:5031-5040. (Erratum, 72:7379.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simell, B., T. Jaakkola, M. Lahdenkari, D. Briles, S. Hollingshead, T. M. Kilpi, and H. Kayhty. 2006. Serum antibodies to pneumococcal neuraminidase NanA in relation to pneumococcal carriage and acute otitis media. Clin. Vaccine Immunol. 13:1177-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simell, B., T. M. Kilpi, and H. Kayhty. 2002. Pneumococcal carriage and otitis media induce salivary antibodies to pneumococcal capsular polysaccharides in children. J. Infect. Dis. 186:1106-1114. [DOI] [PubMed] [Google Scholar]
- 33.Simell, B., M. Korkeila, H. Pursiainen, T. M. Kilpi, and H. Kayhty. 2001. Pneumococcal carriage and otitis media induce salivary antibodies to pneumococcal surface adhesin A, pneumolysin, and pneumococcal surface protein A in children. J. Infect. Dis. 183:887-896. [DOI] [PubMed] [Google Scholar]
- 34.Simell, B., M. Melin, M. Lahdenkari, D. E. Briles, S. K. Hollingshead, T. M. Kilpi, and H. Kayhty. 2007. Antibodies to pneumococcal surface protein A families 1 and 2 in serum and saliva of children and the risk of pneumococcal acute otitis media. J. Infect. Dis. 196:1528-1536. [DOI] [PubMed] [Google Scholar]
- 35.Soininen, A., H. Pursiainen, T. Kilpi, and H. Kayhty. 2001. Natural development of antibodies to pneumococcal capsular polysaccharides depends on the serotype: association with pneumococcal carriage and acute otitis media in young children. J. Infect. Dis. 184:569-576. [DOI] [PubMed] [Google Scholar]
- 36.Suzumoto, M., M. Hotomi, D. S. Billal, D. E. Briles, S. K. Hollingshead, N. Yasui, and N. Yamanaka. 2003. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. G-276.
- 37.Syrjänen, R. K., T. M. Kilpi, T. H. Kaijalainen, E. E. Herva, and A. K. Takala. 2001. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J. Infect. Dis. 184:451-459. [DOI] [PubMed] [Google Scholar]
- 38.Tart, R. C., L. S. McDaniel, B. A. Ralph, and D. E. Briles. 1996. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J. Infect. Dis. 173:380-386. [DOI] [PubMed] [Google Scholar]
- 39.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vela Coral, M. C., N. Fonseca, E. Castaneda, J. L. Di Fabio, S. K. Hollingshead, and D. E. Briles. 2001. Pneumococcal surface protein A of invasive Streptococcus pneumoniae isolates from Colombian children. Emerg. Infect. Dis. 7:832-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White, P., A. Hermansson, C. Svanborg, D. Briles, and K. Prellner. 1999. Effects of active immunization with a pneumococcal surface protein (PspA) and of locally applied antibodies in experimental otitis media. ORL J. Otorhinolaryngol. Relat. Spec. 61:206-211. [DOI] [PubMed] [Google Scholar]
- 42.Wu, H. Y., M. H. Nahm, Y. Guo, M. W. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839-846. [DOI] [PubMed] [Google Scholar]
- 43.Yother, J., and D. E. Briles. 1992. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J. Bacteriol. 174:601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]