Abstract
PspA is a structurally variable surface protein important to the virulence of pneumococci. PspAs are serologically cross-reactive and exist as two major families. In this study, we determined the distribution of PspA families 1 and 2 among pneumococcal strains isolated from the middle ear fluid (MEF) of children with acute otitis media and from nasopharyngeal specimens of children with pneumococcal carriage. We characterized the association between the two PspA families, capsular serotypes, and multilocus sequence types (STs) of the pneumococcal isolates. MEF isolates (n = 201) of 109 patients and nasopharyngeal isolates (n = 173) of 49 children were PspA family typed by whole-cell enzyme immunoassay (EIA). Genetic typing (PCR) of PspA family was done for 60 isolates to confirm EIA typing results. The prevalences of PspA families 1 and 2 were similar among pneumococci isolated from MEF (51% and 45%, respectively) and nasopharyngeal specimens (48% each). Isolates of certain capsule types as well as isolates of certain STs showed statistical associations with either family 1 or family 2 PspA. Pneumococci from seven children with multiple pneumococcal isolates appeared to express serologically different PspA families in different isolates of the same serotype; in three of the children the STs of the isolates were the same, suggesting that antigenic changes in the PspA expressed may have taken place. The majority of the isolates (97%) belonged to either PspA family 1 or family 2, suggesting that a combination including the two main PspA families would make a good vaccine candidate.
Streptococcus pneumoniae (pneumococcus) is a frequent colonizer of the upper respiratory tract. The prevalence of nasopharyngeal (NP) carriage increases during the first months of life and starts to decrease after the age of 3 to 5 years (14). Acute otitis media (AOM) is a common disease among infants and young children, peaking at the age of 6 to 18 months (25, 47). Pneumococcal strains causing AOM are believed to be the same ones that are most frequently isolated in carriage (16).
Conjugate vaccines are approximately 57% efficacious against AOM caused by serotypes included in the vaccine formulation (12, 24, 39). However, the overall reduction in pneumococcal AOM can remain low (12, 24) due to replacement with nonvaccine serotypes. Pneumococcal proteins used as vaccine antigens could provide protection against pneumococci regardless of the capsular serotype. Results from animal models and the variation among pneumococcal strains in gene content and expression suggest that, to efficiently prevent pneumococcal carriage and infection with a protein vaccine, more than one protection-eliciting protein should be included in the vaccine (2, 5, 35, 36).
PspA is a surface antigen important to the virulence of pneumococcus (30), and it has been found in practically all clinical isolates of pneumococcus to date (10, 21). The pspA gene is remarkably variable at the level of DNA sequence, and the amino acid similarity of the surface-exposed N-terminal region of PspA proteins can be as little as 40% (22). The high degree of variability is consistent with PspA being a virulence protein and a target for host defense against pneumococci (22). Extensive cross-reactivity exists between PspAs of different pneumococcal strains (3, 4). Based on nucleotide and amino acid identity, pspA genes and encoded PspA proteins are classified together into six clades, in three families: family 1 (clades 1 and 2), family 2 (clades 3, 4, and 5), and family 3 (clade 6) (22). The extent of cross-reactivity of PspA clades follows roughly the degree of amino acid sequence homology (34) and is maximal within strains of the same PspA family (32). The family can be recognized serologically, but the clade must be identified by the sequence (22).
Despite great variation in the sequences of PspA, antibodies against PspA can be cross-protective; immunization of mice with one PspA protein can elicit protection against infection with strains that express other PspAs and different capsular types, although the level of protection may differ between strains (3, 7, 29, 35, 46). In other cases cross-protection by PspA antibodies may be limited to the same PspA family or sometimes even to the same clade (31, 40). Intranasal immunization of mice with PspA induces mucosal and systemic immune responses and protects against systemic infection as well as NP carriage of pneumococci (50). In human colonization studies both systemic and mucosal antibodies to PspA were associated with protection against experimental pneumococcal carriage (27, 28). Local antibodies to PspA may also have potential in protecting from AOM both in animals and in humans (6, 49). Our recent results suggest that salivary anti-PspA immunoglobulin A may have a role in protecting against AOM (43).
Our objective in this study was to determine the PspA family of pneumococcal isolates from the Finnish Otitis Media (FinOM) Cohort Study children with NP carriage or AOM (25). Both sample types were selected for analysis in order to assess the coverage of PspA 1 and 2 among isolates that potentially cause AOM. All isolates have been serotyped with capsule-type-specific antisera (25, 45), and the AOM isolates were previously multilocus sequence typed (16). Our second objective was to set up a serological method for PspA family typing and to compare the results to genetic characterization of the PspA family in a subset of samples. We now report the distribution of PspA families in general and within the most prevalent capsular serotypes and multilocus sequence types (STs).
MATERIALS AND METHODS
Study population and clinical samples.
The study population consisted of 329 children who had participated in the FinOM Cohort Study in 1994 to 1997, described in detail elsewhere (12, 25). Written informed consent was obtained at the time of study enrollment from the parents of all children participating in the FinOM Cohort Study. The study protocol was evaluated before the start of the trial by the National Public Health Institute ethics committee and by local health authorities (ethics committee and health board of Tampere). In short, the children were followed from 2 to 24 months of age in a special study clinic. Pneumococcal carriage was determined by 10 consecutive NP swab cultures from 2 to 24 months of age (45). At any time during the follow-up, when the child had a respiratory infection, an NP aspirate was taken and cultured for pneumococci. NP swabs and aspirates are here collectively referred to as NP. Myringotomy with aspiration of middle ear fluid (MEF) was performed and cultured in the case of diagnosed AOM (25).
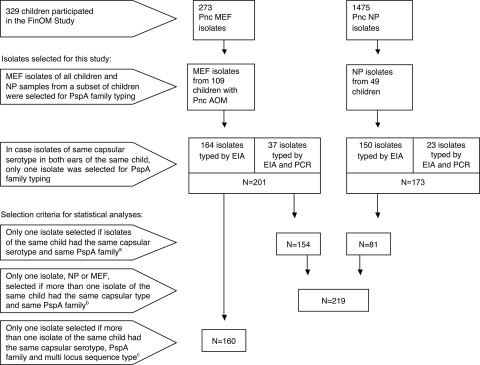
Analysis of PspA family was performed on pneumococci isolated from MEF of all children with AOM and on NP isolates from a subset of 49 children (Fig. 1). In case pneumococci of the same capsular serotype were isolated from both ears at the same visit, the MEF sample from only one of the ears was selected for PspA family typing, assuming that isolates of the same serotype found in both ears are likely to be the same strain (15).
FIG. 1.
Pneumococcal (Pnc) isolates selected from the FinOM study for PspA typing and samples selected for statistical analyses. Footnotes: a, frequencies of PspA families 1 and 2 were compared separately among NP and MEF strains; b, distribution of PspA families within capsular serotypes was analyzed among NP and MEF strains combined; c, distribution of PspA families within multilocus STs was analyzed among MEF strains; sequence data were available for MEF strains only.
Bacterial methods: preparation of bacterial lysates for PspA family typing.
Pneumococcal isolates stored in STGG (skim milk, tryptone, glucose, glycerol) medium (23) at −70°C were cultured on tryptic soy broth agar supplemented with 7.5% sheep blood (TSAB plates) overnight at +37°C in 5% CO2. Approximately 50 colonies from each plate were scraped and inoculated in 3 ml of Todd-Hewitt broth (Difco) supplemented with 0.5% yeast extract and cultured at +37°C in 5% CO2 overnight. Cells were then lysed by adding 600 μl (1/6 volume) of lysis buffer (0.1% sodium deoxycholate, 0.01% sodium dodecyl sulfate, 0.15 M sodium citrate). In case an isolate could not be PspA typed from the bacterial lysate made from liquid culture, a new lysate was made from blood agar culture by collecting the bacterial growth on the plate with a cotton swab and adding it to the lysis buffer diluted 1/6 in phosphate-buffered saline (PBS). The mixture was incubated at room temperature for 30 min to allow lysis to occur. The bacterial lysates were stored at +4°C before use.
Serological methods. (i) Capsular serotyping.
The pneumococcal isolates have previously been serotyped by using antiserum pools and type- and group-specific antisera from Statens Serum Institut, Copenhagen, Denmark. Pneumococcal isolates were serotyped by latex agglutination (for the neutral serotypes 7 and 14), counterimmunoelectrophoresis, and quellung reaction as a confirmation method when needed. The isolates of groups 6, 9, 18, 19, and 23 were subtyped by using pneumococcal factor antisera (25, 45).
(ii) Antisera for PspA family typing.
The PspA family of the pneumococcal isolates was assigned based upon their reactivity to polyclonal sera from rabbits immunized with PspAs from four different clades. A pool of immune sera for detection of PspA family 1 proteins came from two rabbits, one immunized with UAB069 (clade 1 protein from strain L82016) and the other with UAB055 (clade 2 from Rx1). The antiserum pool for detecting family 2 PspAs came from three rabbits immunized with UAB079 (clade 3 from V-032), UAB080 (clade 3 from V-024), or UAB100 (clade 4 from JCP#56). The antisera were prepared as described previously (48).
(iii) Whole-cell enzyme immunoassay (EIA) for PspA family typing.
The bacterial lysates were diluted 1:50 in PBS, pipetted in duplicate on the wells (100 μl/well) of two separate microtiter plates (MaxiSorp; Nalge Nunc International, Roskilde, Denmark), and attached by dry desiccation at +37°C overnight. Plates were washed with PBS containing 0.05% Tween 20, after which rabbit anti-PspA polyclonal antibodies in 1% bovine serum albumin in sterile PBS (B-PBS) were added in a volume of 100 μl/well. The plates were incubated for 1 h at +37°C and washed before addition of polyclonal biotin-conjugated goat anti-rabbit immunoglobulin G (Sigma Immunochemicals, St. Louis, MO) in B-PBS in a volume of 100 μl/well. Following incubation at +37°C for 1 h, the plates were washed, alkaline phosphatase-conjugated streptavidin (Southern Biotech, Birmingham, AL) in B-PBS was added (100 μl/well), and the plates were incubated at +37°C for 1 h. After the final wash, p-nitrophenyl phosphate substrate (Sigma Immunochemicals) was added and incubation was continued for 1 h at +37°C. The absorbance was read at 405 nm by photometer (Multiscan MCC/340). The reproducibility of the assay was followed by including in each plate control strains that were clearly positive for one PspA family only: one strain representing family 1 PspA and two strains with family 2 PspAs. In order to optimize the capacity of the serological PspA typing system to separate family 1- and family 2-positive isolates, reagents were titrated by using the above-mentioned three control strains. The results were interpreted by comparing the sample optical densities (ODs) across family 1 and family 2 plates and with the control samples. The PspA family was assigned based upon the antiserum that resulted in the highest OD. In order to receive a positive result, the sample ODs had to be twice as high as the ODs of the negative-control sample (e.g., the family 1 control strain on a family 2 plate). If an isolate reacted strongly (twice the OD of the negative control) with both family 1 and family 2 anti-PspA antisera or failed to react strongly enough with either serum, the PspA family was confirmed by PCR.
Genetic methods. (i) PCR for PspA family typing.
The PspA family types of 37 MEF isolates and 23 NP isolates were analyzed by PCR. Serologically nontypeable or ambiguous isolates and a set of serologically clearly PspA-typeable isolates were analyzed by the group of Susan Hollingshead and David Briles at the University of Alabama in Birmingham by using PspA family-specific primers (22, 48). Results were recorded as family 1, family 2, families 1 and 2, or not amplified. Any sample that was not amplified in the first-round PCR was rerun using a lower annealing temperature. If the isolate still failed to amplify, a family 3 test was done using family 3-specific primers (48). Finally, the presence of a pspA gene was tested by performing PCR with primers LSM12 and SKH2, which are not family specific but are thought to amplify all known pspA genes (48). In case no gene product was amplified with any of the primers designed to amplify the pspA gene, the isolate was designated as negative for PspA by these methods.
(ii) MLST.
The genotypes of the pneumococci recovered from cases of AOM in the FinOM study have previously been characterized by multilocus sequence typing (MLST) (16). In MLST, bacterial strains are identified by comparing the sequences of internal fragments of multiple housekeeping genes (26).
Statistical methods.
The distribution of family 1 and family 2 PspAs within pneumococcal serotypes and STs (or clonal complex groups) was analyzed with exact binomial test, and P values less than 0.05 were considered statistically significant. For comparison of PspA family distributions, isolates were carefully selected to reduce redundancy; in cases where more than one pneumococcal isolate was cultured from a child and the isolates had the same serotype and the same PspA family (and the same ST, in the case of MEF isolates), only one of the child's isolates was included in the statistical analysis (Fig. 1).
RESULTS
Comparison of serological and genetic PspA typing.
The PspA families of 201 pneumococcal MEF isolates and 173 NP isolates were examined using a serological typing assay; 355 of these isolates reacted with one or more family-specific PspA antisera. Of all the strains examined, 343 reacted with only a single family-specific serum whereas 31 isolates reacted with both sera or did not react strongly with either serum, thus giving ambiguous results for PspA family. In order to test the reliability of the serological PspA family typing assay set up for this study, a set of isolates with a serologically clear PspA family (n = 13) as well as all the isolates ambiguous with regard to PspA family (n = 31) were analyzed with PCR. Ambiguous typing results were those where the isolate was strongly reactive (at least twice the OD of the negative-control strain) with both families (n = 12), although these isolates often reacted more strongly with one family than the other; in other cases the isolates classified as ambiguous did not react with either of the PspA antisera in EIA (n = 13) or were only weakly positive (less than twice the OD of the negative control) for either PspA family (n = 6).
The results are presented in Table 1. The two methodologies for PspA family determination, serological and genetic, gave identical results for the 13 isolates that were clearly typeable by the serological method. Two of the isolates that were clearly positive for one family only in EIA were positive for both PspA families 1 and 2 by PCR. More than half of the isolates (8/13) that were undoubtedly negative for PspA in EIA were negative by PCR as well. For the rest of the serologically PspA-negative isolates, either family 1 (n = 1) or family 2 (n = 4) pspA genes could be found. Of the weakly positive isolates, four/six had the same PspA family by EIA and PCR, one had a different pspA family, and one was pspA negative in PCR. PCR confirmed that 11 of the 12 isolates reactive with antisera to both families had family 2 pspA genes, and one had a family 1 pspA gene. The PspA family of a few consecutive isolates was also confirmed with genotyping, if isolates of the same capsular serotype, isolated from the same child, appeared to have different PspAs according to the serological assay (n = 19). These results are presented in Table 2, along with data from MLST.
TABLE 1.
Results of PspA typing by serology (EIA) and genetics (PCR)
| Isolate status and type | Capsular serotype | Child identification no. | PspA family or families as determined by:
|
|
|---|---|---|---|---|
| EIA | PCR | |||
| Clearly positive for one PspA family (n = 13) | ||||
| MEF | 23F | 159 | 1 | 1 |
| MEF | 6B | 153 | 1 | 1 |
| NP | 19F | 150 | 1 | 1 |
| NP | 6A | 234 | 1 | 1 |
| NP | 6B | 228 | 1 | 1 |
| NP | 9N | 243 | 1 | 1 |
| MEF | 3 | 249 | 1 | 1, 2 |
| NP | 22 | 234 | 1 | 1, 2 |
| NP | 4 | 190 | 2 | 2 |
| NP | 11A | 168 | 2 | 2 |
| NP | 19F | 196 | 2 | 2 |
| NP | 6A | 175 | 2 | 2 |
| NP | 6B | 196 | 2 | 2 |
| Unclear for PspA family in EIA (n = 31) | ||||
| MEF | 19F | 315 | 0 | 0 |
| MEF | 19F | 315 | 0 | 0 |
| MEF | 6A | 148 | 0 | 0 |
| MEF | Rough | 28 | 0 | 0 |
| MEF | Rough | 26 | 0 | 0 |
| MEF | Rough | 176 | 0 | 0 |
| NP | 28 | 200 | 0 | 0 |
| NP | 28 | 200 | 0 | 0 |
| MEF | 6B | 209 | 0 | 1 |
| MEF | 14 | 54 | 0 | 2 |
| MEF | 7 | 284 | 0 | 2 |
| NP | Rough | 250 | 0 | 2 |
| NP | 9V | 155 | 0 | 2 |
| MEF | 28 | 33 | 1?a | 1 |
| MEF | 23F | 147 | 1? | 1 |
| MEF | 19F | 91 | 1? | 1 |
| MEF | Rough | 282 | 2? | 0 |
| MEF | 6B | 94 | 2? | 1 |
| MEF | 6A | 104 | 2? | 2 |
| MEF | 19F | 6 | 1, 2 | 2 |
| NP | 19A | 199 | 1, 2 | 2 |
| MEF | 33 | 128 | 1 < 2b | 1 |
| MEF | 15 | 97 | 1 < 2 | 2 |
| MEF | 14 | 28 | 1 < 2 | 2 |
| MEF | 19F | 95 | 1 < 2 | 2 |
| MEF | 19F | 217 | 1 < 2 | 2 |
| MEF | 19F | 217 | 1 < 2 | 2 |
| NP | 14 | 165 | 1 < 2 | 2 |
| NP | 14 | 165 | 1 < 2 | 2 |
| NP | 14 | 193 | 1 < 2 | 2 |
| NP | 14 | 226 | 1 < 2 | 2 |
Strains marked by a question mark were only weakly reactive in EIA.
PspA family 1 < 2 in EIA indicates that the strain was serologically reactive for both PspA families but that reactivity was stronger for PspA family 2.
TABLE 2.
Pneumococci of the same capsular serotype but different PspA in consecutive isolates from the same children
| Isolate type | Capsular serotype | Child identification no. | Age (days) | PspA family determined by:
|
STa | |
|---|---|---|---|---|---|---|
| EIA | PCR | |||||
| MEF | 23F | 61 | 491 | 2 | 2 | 37 |
| MEF | 23F | 61 | 522 | 1 | 2 | 37 |
| MEF | 23F | 61 | 638 | 1 | 1 | 37 |
| MEF | 19F | 95 | 365 | 1 | NDc | 199 |
| MEF | 19F | 95 | 376 | 1 < 2b | 2 | 199 |
| MEF | 19F | 95 | 416 | 2 | 2 | 199 |
| MEF | 23F | 105 | 474 | 2 | 2 | 37 |
| MEF | 23F | 105 | 479 | 1 | 2 | 37 |
| NP | 9V | 155 | 163 | 2 | 2 | ND |
| NP | 9V | 155 | 195 | 2 | ND | ND |
| NP | 9V | 155 | 216 | 0 | 2 | ND |
| MEF | 9V | 155 | 241 | 1 | 2 | 162 |
| NP | 9V | 155 | 268 | 2 | 2 | ND |
| NP | 23F | 165 | 246 | 1 | 2 | ND |
| MEF | 23F | 165 | 260 | 1 | 1 | 36 |
| MEF | 6B | 284 | 415 | 2 | 2 | 510 |
| MEF | 6B | 284 | 466 | 1 | 1 | 497 |
| MEF | 19F | 322 | 123 | 1 | 2 | 199 |
| MEF | 19F | 322 | 279 | 2 | 2 | ND |
Isolates with the same multilocus ST, isolated from the same child, represent the same strain.
Reactivities for families 1 and 2 were observed, but reactivity was stronger for family 2 PspA.
ND, no data.
When a PspA family was clearly identified by EIA, the result mostly confirmed that of PCR typing. We have therefore relied on the PCR results of the isolates that were analyzed with both methods and on the serological PspA family results for the rest of the isolates.
Distribution of PspA families 1 and 2 among pneumococci.
In order to compare the frequencies of PspA families 1 and 2, every effort was made not to include the same strain in the analyses more than once. In cases where more than one identical isolate (identical for both capsular type and PspA family) was obtained from the same child, the strain was included in the analysis only once (Fig. 1).
PspA families 1 (n = 78) and 2 (n = 69) were present at similar frequencies (50.6% and 44.8% of all strains, respectively) among the 154 AOM strains selected for analyses. Six AOM strains were PspA negative, and one had genes for both PspA families (confirmed by PCR). The two PspA families were equally common among NP strains: out of the 81 PspA-typed isolates, 39 had family 1 PspA and 39 had family 2 PspA. Two of the remaining NP strains were positive for both PspA families by PCR, and one was PspA negative by both PCR and serology. There were no age-dependent differences in the occurrence of either PspA family (data not shown).
We next looked at the associations of PspA families with capsular serotypes. Since the prevalence of PspA families was essentially the same among NP and AOM isolates, the isolates were pooled for further analyses. Here again, only one of several isolates of the same capsular serotype and PspA family per child was included (n = 219; Fig. 1). In case a child had several isolates, the MEF or NP strain that was isolated first was chosen for comparisons, MEF being the primary choice in cases in which both strains were isolated on the same date. Using these criteria 145 MEF strains and 74 NP strains were examined.
The 10 most prevalent serotypes (represented by at least five isolates) are listed individually in Table 3. The family 1- and family 2-positive isolates within the 10 serotypes cover 80% of the isolates (176/219) included in the analysis. Most serotypes included both family 1 and family 2 PspAs, but within some serotypes the distribution of the PspA families was unequal. Serotypes 9N and 23F were predominantly or exclusively associated with family 1, and serotypes 9V, 11A, and 14 were associated with family 2 PspA. Serogroups 6 and 19 contained family 1 and family 2 PspAs in equal numbers. Serotypes 6A, 19F (MEF), and 28 (NP) all included one strain with no PspA detected. Six strains (all MEF isolates) were nontypeable by the method used, and four of these rough strains were PspA negative; two had family 1 PspA. Two of the PspA-negative, rough strains were ST 448 and belong to a pneumococcal lineage previously reported to have lost its capsular locus (18); two were not genuine pneumococci (17).
TABLE 3.
Numbers of isolates with family 1 or family 2 PspA within the most common capsular types of Streptococcus pneumoniae in MEF and NP specimens
| Capsular serotype | No. of isolates with PspA family:
|
|
|---|---|---|
| 1 | 2 | |
| 19F | 22 | 16 |
| 23Fa | 34 | 3 |
| 6A | 11 | 12 |
| 6B | 12 | 11 |
| 14a | 2 | 12 |
| 11Aa | 1 | 12 |
| 18C | 3 | 6 |
| 19A | 4 | 4 |
| 9Va | 0 | 6 |
| 9N | 5 | 0 |
The distribution of PspA families was uneven and statistically significant for serotypes 23F (P < 0.0001), 14 (P = 0.013), 11A (P = 0.003), and 9V (P = 0.031, exact binomial test, two-tailed).
In order to study the possibility that the variation of PspA family 1 and 2 frequencies that was observed within capsular serotypes might be explained by diverse clonal lineages within a serotype, differing in PspA, MLST data were used to divide strains within serotypes into individual STs. MLST data were available only for MEF isolates, not NP isolates. Only one MEF isolate from each child was selected for analysis, unless the isolates differed in regards to either capsular serotype, PspA family, or ST (Fig. 1). A total of 160 strains were included. When the isolates of a capsule type were similarly distributed between the two PspA families, they usually also represented many different STs, being thus clonally more diverse. For example, serotypes 6A and 6B combined (PspA family 1, n = 17; family 2, n = 17) included 20 different STs and serotypes 19A and 19F (PspA family 1, n = 20; family 2, n = 18) had 18 STs. The five serotype 9V strains, all with family 2 PspA, had the same ST, indicating that they were a single clone. Ninety percent of the strains of serotype 23F had a family 1 PspA. While 23F included eight different STs, the number of STs was low in relation to the large number of serotype 23F strains (n = 30). Serotype 11A associated with family 2 PspA, however, included relatively many STs (five STs among 10 strains).
The distribution of PspA families among STs is presented in Table 4. Most STs included only one or two strains. Only 7 out of the 79 different STs (37, 62, 66, 124, 199, 482, and 485) contained isolates of both family 1 and family 2 PspAs. Three of the most common STs (36, 162, and 488) showed a significant association with one of the PspA families (P = 0.016, exact binomial test). Five STs (8, 66, 162, 199, and 490) included strains with more than one capsular serotype; in some but not all such STs, the PspA family was different in the members of the ST with different capsular types. STs differing from each other by only one of the seven sequences used in the MLST analysis are classified in the same clonal complex group (13). Both family 1 and family 2 PspAs were found within most (9/13) clonal complex groups, but often one PspA family was much more common than the other. The association of particular PspA families with particular clonal complex groups was statistically significant for clonal complex groups 1 (P = 0.031), 2 (P = 0.001), 3 (P = 0.039), and 8 (P = 0.008), suggesting that the PspAs that they express have not yet been randomized by natural genetic exchange.
TABLE 4.
Numbers of isolates with PspA families 1 and 2 within multilocus STs of pneumococci isolated from MEF
| Clonal complex groupa | ST | Capsular serotype | No. of isolates with PspA family:
|
|
|---|---|---|---|---|
| 1 | 2 | |||
| 1 | 15 | 19F | 2 | 0 |
| 1 | 423 | 19F | 2 | 0 |
| 1 | 484 | 19F | 1 | 0 |
| 1 | 534 | 19F | 1 | 0 |
| 2 | 37 | 23F | 8 | 3 |
| 2 | 504 | 23F | 4 | 0 |
| 2 | 507 | 23F | 3 | 0 |
| 2 | 535 | 23F | 1 | 0 |
| 3 | 62 | 11A | 1 | 5 |
| 3 | 500 | 11A | 0 | 1 |
| 3 | 509 | 11A | 0 | 1 |
| 3 | 513 | 11A | 0 | 1 |
| 4 | 66 | 19F | 0 | 1 |
| 4 | 66 | 23F | 1 | 1 |
| 4 | 66 | 9N | 1 | 0 |
| 4 | 71 | 15 | 0 | 1 |
| 4 | 525 | 9N | 1 | 0 |
| 5 | 110 | 18C | 0 | 2 |
| 5 | 113 | 18C | 0 | 2 |
| 6 | 124 | 14 | 2 | 4 |
| 6 | 134 | 14 | 0 | 1 |
| 6 | 307 | 14 | 0 | 1 |
| 7 | 138 | 6B | 0 | 4 |
| 7 | 176 | 6B | 2 | 0 |
| 7 | 510 | 6B | 0 | 1 |
| 8 | 156 | 14 | 0 | 1 |
| 8 | 162 | 19F | 0 | 2 |
| 8 | 162 | 9V | 0 | 5 |
| 9 | 199 | 15 | 0 | 3 |
| 9 | 199 | 19A | 1 | 0 |
| 9 | 199 | 19F | 1 | 2 |
| 9 | 483 | 19F | 0 | 1 |
| 10 | 309 | 19F | 4 | 0 |
| 10 | 523 | 19F | 1 | 0 |
| 11 | 520 | Rough | 2 | 0 |
| 11 | 531 | 22 | 1 | 0 |
| 12 | 479 | 6A | 0 | 1 |
| 12 | 486 | 6A | 0 | 1 |
| 12 | 488 | 6A | 0 | 7 |
| 12 | 490 | 6A | 0 | 1 |
| 12 | 490 | 6B | 1 | 0 |
| 12 | 512b | 6A | 0 | 0 |
| 12 | 518 | 6A | 2 | 0 |
| 13 | 494 | 28 | 1 | 0 |
| 13 | 546 | 28 | 1 | 0 |
| s | 1 | 23F | 1 | 0 |
| s | 8 | 11A | 0 | 1 |
| s | 8 | 19F | 0 | 1 |
| s | 36 | 23F | 7 | 0 |
| s | 87b | 19F | 0 | 0 |
| s | 100 | 33 | 2 | 0 |
| s | 147 | 6B | 1 | 0 |
| s | 177 | 19F | 0 | 1 |
| s | 180c | 3 | 0 | 2 |
| s | 191 | 7 | 0 | 1 |
| s | 205 | 4 | 0 | 2 |
| s | 207 | 6A | 0 | 1 |
| s | 236 | 19F | 1 | 0 |
| s | 448b | Rough | 0 | 0 |
| s | 460 | 6A | 3 | 0 |
| s | 482 | 19A | 1 | 2 |
| s | 485 | 19F | 1 | 7 |
| s | 492 | 6B | 1 | 0 |
| s | 496 | 18C | 1 | 0 |
| s | 497 | 6B | 2 | 0 |
| s | 498 | 35 | 0 | 1 |
| s | 506 | 6B | 1 | 0 |
| s | 514 | 6A | 0 | 1 |
| s | 515 | 23F | 1 | 0 |
| s | 526 | 19F | 3 | 0 |
| s | 529 | 6A | 1 | 0 |
| s | 530 | 20 | 1 | 0 |
| s | 532 | 6A | 1 | 0 |
| s | 533 | 31 | 2 | 0 |
| s | 537 | 6B | 1 | 0 |
| s | 538 | 6A | 1 | 0 |
| s | 540 | 19A | 1 | 0 |
| s | 542 | 18C | 0 | 1 |
Closely related STs are classified in the same clonal complex group; single clones (“s”) included only one kind of ST.
Three STs included strains with neither PspA family 1 nor family 2: ST 512 (one strain), ST 87 (one strain), and ST 448 (two strains).
ST 180 also included one capsule type 3 strain not listed here; the strain was family 1 positive by serology but had both family 1 and family 2 pspA genes detected in PCR; the other two ST 180 strains were family 2 positive by serology and were not PCR tested.
Consecutive isolates with the same serotype but different PspA type.
In the analyses described above, only one isolate with the same serotype and same PspA family was selected from each child. Pneumococci isolated from seven children on different occasions appeared to express serologically different PspAs even if the capsular serotype remained the same (Table 2). All but 2 of these 19 isolates were successfully PspA typed both by EIA and by PCR (Table 2). Multilocus sequence data were available for most MEF isolates. The consecutive isolates of three children (identification numbers 61, 165, and 284) had different PspA family types in both EIA and PCR assays. All isolates of child 61 belonged to the same ST, suggesting that the PspA family of a single strain had changed over time. The two consecutive isolates of child 165 were obtained within a short interval from different locations (NP and MEF), but since ST data are available only for the MEF isolate, we cannot tell whether the isolates are the same strain. Child 284 had two different strains (two different STs), which explains the “change” in PspA family. The course of events for child 95 was similar to that with child 61, where the PspA changed within a long-term-carriage strain, although this time we do not have the PCR data on the first isolate. The same pspA family was detected by PCR in the consecutive isolates of children 105, 155, and 322; different PspA families of these consecutive isolates were observed only at the serological level.
DISCUSSION
In this study we found that the vast majority of pneumococci isolated from the MEFs or NP samples of Finnish children less than 2 years old represented PspA families 1 and 2. The two PspA families were equally prevalent among the isolated strains. PspA family distribution varied between serotypes: serotypes 9N, 9V, 11A, 14, and 23F were mostly associated with one or the other of the PspA families whereas the distribution was more or less equal for the two PspA families among the strains representing serotypes 6A, 6B, 19A, and 19F. The number of different STs in relation to the number of isolates representing the capsular serotype was generally high in the capsular serotypes, with similar numbers of strains expressing each of the two PspA families. Some, but not all, single or clonally related STs were associated with a single PspA family. In seven children consecutive pneumococcal isolates of the same serotype seemed to express different PspA families; in some such cases the isolates were in fact different strains, but some isolates had altered serological properties of the PspA or even a different pspA gene.
In this study pneumococci were serologically typed by EIA to the level of PspA family by using pooled family 1 (rabbits immunized with clade 1 and clade 2 PspAs)- and family 2 (clades 3 and 4)-specific antisera. The methods for serological PspA typing described in previous studies have been based on dot blot methodology and family-specific antisera (1, 33, 48) or EIA using separate antisera raised against clade 2, clade 3, or clade 4 PspAs (21). PCR and serological techniques gave concordant results in 100% of cases in studies of 40 invasive isolates from Colombian children (48) and with 149 invasive isolates from Argentinean children (33). PspA family was scored nontypeable by serology in 44/1,847 invasive isolates from adults over 50 years of age in a multinational study; 35 of the 44 isolates had either PspA family 1, family 2, or both by molecular methods (21). While invasive and NP isolates collected in Brazil were characterized with both serology and PCR, isolates with discordant results between the two methods were excluded from further analysis and thus cannot be compared to our study (1).
In order to test the reliability of the serological assay for defining the PspA family, clearly typeable isolates as well as isolates with ambiguous PspA typing results were also examined by PCR. The two methodologies gave for the most part similar results; the respective pspA gene was amplified from 100% of the 13 serologically clearly typeable isolates. With respect to the ambiguous isolates, there were some discrepancies between the two assays, however. Nearly all isolates that reacted with both PspA antisera were family 2 positive in PCR, which indicates that the family 2 PspAs of these isolates were more cross-reactive with family 1 than were most family 2 PspAs. Cross-reactivity of family 1 antiserum with family 2 PspA protein has been reported in previous studies as well (1). No such cross-reactive isolates had genes for both PspA families in that study or ours. However, an additional family 2 pspA gene was found in two isolates that were serologically positive only for family 1. Isolates with pspA genes from both families 1 and 2 have been reported in previous studies: 4% of 149 invasive isolates in Argentina (33) and 20.7% of 29 MEF isolates in the United States (37) but only 0.7% of 1,844 invasive isolates from elderly patients in a multinational study (21). These differences in results could reflect differences in the populations of strains examined but may also result from small differences in assay protocols or the care with which anomalies were pursued. Although most PspAs can be fitted into one of the major PspA families, there are many PspAs that have portions of their sequences showing characteristics of more than one PspA family (22). The serological determination is dependent on those epitopes that are best recognized by each typing serum. The PCR typing is based on relatively short primer sequences within the alpha-helical region of the pspA gene. Thus, it is possible and expected that in some cases genes that encode several major epitopes of one family can have some epitopes or a primer site that is a better fit with another PspA family.
Nearly all the pneumococcal isolates characterized in this study had PspAs from either family 1 or family 2. Neither PspA family dominated over the other among the NP or MEF isolates. Both highly similar and somewhat different distributions of PspA families 1 and 2 have been reported in different geographic locations and in different studies, but neither PspA family appears to be more common than the other in infections of different body sites (1, 20, 33, 37, 41, 44, 48). To date, only a few strains with family 3 PspA have been reported (21), and in our study pspA family 3 genes were not found in any of the serologically PspA-negative isolates. The number of isolates with undetected PspA was low: 13 of the 374 NP and MEF isolates (3.5%) were PspA negative by the serological method and nine (2.4%) remained negative after PCR. This is consistent with most previous studies, in which the proportions of PspA-negative pneumococcal isolates have generally ranged from 1 to 6% (1, 21, 33, 37, 41, 48), although proportions as low as 0% (20) and as high as 20% (38) have been reported for PspA negativity.
The PspAs of the (five) serologically PspA-negative but genetically pspA-positive strains may not have been expressed, or the protein may be serologically too distinct to be detected with antibodies to the five different PspA molecules used for making the anti-PspA antisera. pspA genes could not be amplified from nine strains, not even with non-family-specific primers that were expected to detect all known pspA genes (22). Six of the seven strains negative for pspA were MEF isolates, and four of these were unencapsulated. Based on MLST, two of the unencapsulated, pspA-negative strains were not true pneumococci, and two belonged to ST 448. The fact that PspA could not be detected with either serology or genetic methods in five pneumococcal strains suggests that these isolates were lacking the pspA gene completely. The proportion of nontypeable isolates appears to be higher among noninvasive than among invasive isolates; in a comparison of isolates collected in Brazil 17% of the NP isolates and 4.9% of invasive isolates were nontypeable with both serological and molecular methods (1). It is of interest that pneumococci without two important virulence factors, capsule and PspA, were isolated from MEF during AOM. The capsule apparently plays a lesser role in mucosal infections, and unencapsulated pneumococci are relatively common among MEF isolates (16). The finding that some pneumococci may cause AOM without either capsule or PspA should be considered in designing the composition of a pneumococcal vaccine against AOM. Combinations of several surface proteins essential to the virulence of the bacterium, in addition to PspA, are advisable for inclusion in future pneumococcal protein vaccines against AOM (4) and invasive disease (36) in order to achieve the best protection.
The PspA protein is a virulence factor under evolutionary pressure (22) and like the capsule (9) may recombine independently of the genetic background. Both PspA families could be found within the most common capsular serotypes, but some serotypes were associated strongly with one of the PspA families. The capsular serotypes most strongly associated with one PspA family were 9N, 9V, 11A, 14, and 23F, whereas serogroups 6 and 19 contained equal numbers of the two PspA families. PspA family 1 dominated serotype 23F in this study as in two previous studies (20, 37), but for other serotypes different distributions have been reported in different studies (20, 37, 48), which indicates that serotypes are not globally associated with certain PspAs. There can be variation in different regions, and some capsular serotypes associated with one PspA family only may be heavily clonal. In this study, serogroups 6 and 19, which contained equal proportions of the two PspA families, were in fact more clonally diverse (including more different STs) than many of the capsular serotypes associated with only one PspA family type. We found that an ST or related STs (STs of the same clonal complex group) were mostly associated with only one PspA family. However, although a single ST usually expresses only one capsular serotype, it may also be composed of multiple capsular types (8, 11, 16, 19, 42). In this study five STs expressed more than one capsular type and seven STs were associated with both PspA families. In contrast to our study, pneumococcal STs isolated from meningitis patients in Poland seemed to include only one or the other of the two PspAs (41).
The expression of PspA was found to be unstable in a human colonization study (27). When a clinical isolate with a truncated PspA was inoculated into the nasopharynx, a second mutation in the pspA gene restored the expression of a full-length protein in one of the six colonized individuals during the period of colonization. This finding indicates that there is naturally occurring variability in the expression of PspA. Signs of PspA recombination were observed in our study at the level of consecutive isolates from the same child. Three of the children had consecutive AOM isolates that had the same capsular type and ST but were clearly different in terms of PspA serology, indicating that their PspAs have in some way changed antigenically. PCR results suggested that the PspA of one of these strains was altered from family 1 to family 2 in consecutive isolates. It is of interest that serological changes in PspA appeared more than once in two particular STs, 37 and 199.
The two assays for analyzing PspA family, serological and genetic, provide two different perspectives; PCR shows the possible repertoire of pspA genes that the strain possesses, whereas reactions with PspA antisera reveal the antigenic properties of the PspA expressed by the strain. Of the two typing methods (PCR and serological), the latter is probably to be preferred since it would be expected to have a better association with cross-protection than the PCR data, which do not necessarily reflect the serologic epitopes.
The results from this study indicate that in NP carriage and AOM nearly all pneumococci express either PspA family 1 or family 2 proteins, with 97% of NP and MEF isolates expressing one or the other. The equal distribution of PspA families 1 and 2 and the rarity of family 3 suggest that a vaccine composed of one PspA from family 1 and one PspA from family 2 may make a good vaccine candidate for preventing pneumococcal disease. In previous studies we have shown that young children develop antibodies specifically to the same PspA family type to which they have been exposed by natural colonization, and these antibodies do not generally cross-react across families (30a). A vaccine including at least one PspA from each family in combination with other pneumococcal proteins should offer high coverage against the strains colonizing young children.
Acknowledgments
Eeva Koskenniemi is acknowledged for data management. We thank Pat Coan at UAB, Birmingham, AL, for technical assistance in PCR typing. We thank all the children and parents who participated in the FinOM Cohort Study.
The FinOM Studies were supported by Merck and Co. Inc., Sanofi Pasteur, Wyeth Vaccines, and the World Health Organization (GPV/VRD contract V23/181/116). This work was in part supported by the Meningitis Research Foundation (Project 13/00). D. E. Briles and S. K. Hollingshead were supported by grant AI21548 from the National Institutes of Health. William P. Hanage is a Royal Society University Research Fellow.
T. Kilpi has consulted on advisory boards for GSK Bio and Wyeth Vaccines and has had travel paid for by Sanofi Pasteur, GSK Bio, and Wyeth Vaccines. H. Käyhty has consulted on advisory boards for Sanofi Pasteur and GSK Bio; has had travel paid for by Sanofi Pasteur, GSK Bio, and Wyeth Vaccines as an invited speaker or expert at symposia; and has received honoraria from Sanofi Pasteur, GSK Bio, and Wyeth Vaccines. D. E. Briles and S. K. Hollingshead are consultants with Sanofi Pasteur and have done research in collaboration with Sanofi Pasteur that has been supported in part or in total by Sanofi Pasteur.
Footnotes
Published ahead of print on 27 August 2008.
REFERENCES
- 1.Brandileone, M. C., A. L. Andrade, E. M. Teles, R. C. Zanella, T. I. Yara, J. L. Di Fabio, and S. K. Hollingshead. 2004. Typing of pneumococcal surface protein A (PspA) in Streptococcus pneumoniae isolated during epidemiological surveillance in Brazil: towards novel pneumococcal protein vaccines. Vaccine 22:3890-3896. [DOI] [PubMed] [Google Scholar]
- 2.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briles, D. E., S. K. Hollingshead, J. King, A. Swift, P. A. Braun, M. K. Park, L. M. Ferguson, M. H. Nahm, and G. S. Nabors. 2000. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694-1701. [DOI] [PubMed] [Google Scholar]
- 4.Briles, D. E., S. K. Hollingshead, G. S. Nabors, J. C. Paton, and A. Brooks-Walter. 2000. The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae. Vaccine 19:S87-S95. [DOI] [PubMed] [Google Scholar]
- 5.Briles, D. E., S. K. Hollingshead, J. C. Paton, E. W. Ades, L. Novak, F. W. van Ginkel, and W. H. Benjamin, Jr. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339-348. [DOI] [PubMed] [Google Scholar]
- 6.Briles, D. E., S. K. Hollingshead, E. Swiatlo, A. Brooks-Walter, A. Szalai, A. Virolainen, L. S. McDaniel, K. A. Benton, P. White, K. Prellner, A. Hermansson, P. C. Aerts, H. Van Dijk, and M. J. Crain. 1997. PspA and PspC: their potential for use as pneumococcal vaccines. Microb. Drug Resist. 3:401-408. [DOI] [PubMed] [Google Scholar]
- 7.Briles, D. E., R. C. Tart, E. Swiatlo, J. P. Dillard, P. Smith, K. A. Benton, B. A. Ralph, A. Brooks-Walter, M. J. Crain, S. K. Hollingshead, and L. S. McDaniel. 1998. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA). Clin. Microbiol. Rev. 11:645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brueggemann, A. B., D. T. Griffiths, E. Meats, T. Peto, D. W. Crook, and B. G. Spratt. 2003. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 187:1424-1432. [DOI] [PubMed] [Google Scholar]
- 9.Coffey, T. J., M. C. Enright, M. Daniels, J. K. Morona, R. Morona, W. Hryniewicz, J. C. Paton, and B. G. Spratt. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 27:73-83. [DOI] [PubMed] [Google Scholar]
- 10.Crain, M. J., W. D. Waltman II, J. S. Turner, J. Yother, D. F. Talkington, L. S. McDaniel, B. M. Gray, and D. E. Briles. 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58:3293-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enright, M. C., and B. G. Spratt. 1998. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144:3049-3060. [DOI] [PubMed] [Google Scholar]
- 12.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, and P. H. Makela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 13.Feil, E. J., B. C. Li, D. M. Aanensen, W. P. Hanage, and B. G. Spratt. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghaffar, F., I. R. Friedland, and G. H. McCracken, Jr. 1999. Dynamics of nasopharyngeal colonization by Streptococcus pneumoniae. Pediatr. Infect. Dis. J. 18:638-646. [DOI] [PubMed] [Google Scholar]
- 15.Gray, B. M., G. M. Converse III, and H. C. Dillon, Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 16.Hanage, W. P., K. Auranen, R. Syrjanen, E. Herva, P. H. Makela, T. Kilpi, and B. G. Spratt. 2004. Ability of pneumococcal serotypes and clones to cause acute otitis media: implications for the prevention of otitis media by conjugate vaccines. Infect. Immun. 72:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanage, W. P., T. Kaijalainen, E. Herva, A. Saukkoriipi, R. Syrjanen, and B. G. Spratt. 2005. Using multilocus sequence data to define the pneumococcus. J. Bacteriol. 187:6223-6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanage, W. P., T. Kaijalainen, A. Saukkoriipi, J. L. Rickcord, and B. G. Spratt. 2006. A successful, diverse disease-associated lineage of nontypeable pneumococci that has lost the capsular biosynthesis locus. J. Clin. Microbiol. 44:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanage, W. P., T. H. Kaijalainen, R. K. Syrjanen, K. Auranen, M. Leinonen, P. H. Makela, and B. G. Spratt. 2005. Invasiveness of serotypes and clones of Streptococcus pneumoniae among children in Finland. Infect. Immun. 73:431-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heeg, C., C. Franken, M. van der Linden, A. Al-Lahham, and R. R. Reinert. 2007. Genetic diversity of pneumococcal surface protein A of Streptococcus pneumoniae meningitis in German children. Vaccine 25:1030-1035. [DOI] [PubMed] [Google Scholar]
- 21.Hollingshead, S. K., L. Baril, S. Ferro, J. King, P. Coan, and D. E. Briles. 2006. Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J. Med. Microbiol. 55:215-221. [DOI] [PubMed] [Google Scholar]
- 22.Hollingshead, S. K., R. Becker, and D. E. Briles. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaijalainen, T., E. Ruokokoski, P. Ukkonen, and E. Herva. 2004. Survival of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis frozen in skim milk-tryptone-glucose-glycerol medium. J. Clin. Microbiol. 42:412-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilpi, T., H. Ahman, J. Jokinen, K. S. Lankinen, A. Palmu, H. Savolainen, M. Gronholm, M. Leinonen, T. Hovi, J. Eskola, H. Kayhty, N. Bohidar, J. C. Sadoff, and P. H. Makela. 2003. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin. Infect. Dis. 37:1155-1164. [DOI] [PubMed] [Google Scholar]
- 25.Kilpi, T., E. Herva, T. Kaijalainen, R. Syrjanen, and A. K. Takala. 2001. Bacteriology of acute otitis media in a cohort of Finnish children followed for the first two years of life. Pediatr. Infect. Dis. J. 20:654-662. [DOI] [PubMed] [Google Scholar]
- 26.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCool, T. L., T. R. Cate, G. Moy, and J. N. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCool, T. L., T. R. Cate, E. I. Tuomanen, P. Adrian, T. J. Mitchell, and J. N. Weiser. 2003. Serum immunoglobulin G response to candidate vaccine antigens during experimental human pneumococcal colonization. Infect. Immun. 71:5724-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDaniel, L. S., G. Scott, J. F. Kearney, and D. E. Briles. 1984. Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J. Exp. Med. 160:386-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDaniel, L. S., J. Yother, M. Vijayakumar, L. McGarry, W. R. Guild, and D. E. Briles. 1987. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J. Exp. Med. 165:381-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Melin, M. M., S. K. Hollingshead, D. E. Briles, M. I. Lahdenkari, T. M. Kilpi, and H. M. Käyhty. 2008. Development of antibodies to PspA families 1 and 2 in children after exposure to Streptococcus pneumoniae. Clin. Vaccine Immunol. 15:1529-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyaji, E. N., W. O. Dias, M. Gamberini, V. C. Gebara, R. P. Schenkman, J. Wild, P. Riedl, J. Reimann, R. Schirmbeck, and L. C. Leite. 2001. PsaA (pneumococcal surface adhesin A) and PspA (pneumococcal surface protein A) DNA vaccines induce humoral and cellular immune responses against Streptococcus pneumoniae. Vaccine 20:805-812. [DOI] [PubMed] [Google Scholar]
- 32.Miyaji, E. N., D. M. Ferreira, A. P. Lopes, M. C. Brandileone, W. O. Dias, and L. C. Leite. 2002. Analysis of serum cross-reactivity and cross-protection elicited by immunization with DNA vaccines against Streptococcus pneumoniae expressing PspA fragments from different clades. Infect. Immun. 70:5086-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mollerach, M., M. Regueira, L. Bonofiglio, R. Callejo, J. Pace, J. L. Di Fabio, S. Hollingshead, and D. Briles. 2004. Invasive Streptococcus pneumoniae isolates from Argentinian children: serotypes, families of pneumococcal surface protein A (PspA) and genetic diversity. Epidemiol. Infect. 132:177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nabors, G. S., P. A. Braun, D. J. Herrmann, M. L. Heise, D. J. Pyle, S. Gravenstein, M. Schilling, L. M. Ferguson, S. K. Hollingshead, D. E. Briles, and R. S. Becker. 2000. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743-1754. [DOI] [PubMed] [Google Scholar]
- 35.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogunniyi, A. D., M. Grabowicz, D. E. Briles, J. Cook, and J. C. Paton. 2007. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect. Immun. 75:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onwubiko, C., C. Shires, L. R. Quin, E. Swiatlo, and L. S. McDaniel. 2007. Characterization of Streptococcus pneumoniae isolated from children with otitis media. FEMS Immunol. Med. Microbiol. 50:119-125. [DOI] [PubMed] [Google Scholar]
- 38.Pimenta, F. C., F. Ribeiro-Dias, M. C. Brandileone, E. N. Miyaji, L. C. Leite, and A. L. Sgambatti de Andrade. 2006. Genetic diversity of PspA types among nasopharyngeal isolates collected during an ongoing surveillance study of children in Brazil. J. Clin. Microbiol. 44:2838-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prymula, R., P. Peeters, V. Chrobok, P. Kriz, E. Novakova, E. Kaliskova, I. Kohl, P. Lommel, J. Poolman, J. P. Prieels, and L. Schuerman. 2006. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 367:740-748. [DOI] [PubMed] [Google Scholar]
- 40.Roche, H., B. Ren, L. S. McDaniel, A. Hakansson, and D. E. Briles. 2003. Relative roles of genetic background and variation in PspA in the ability of antibodies to PspA to protect against capsular type 3 and 4 strains of Streptococcus pneumoniae. Infect. Immun. 71:4498-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadowy, E., A. Skoczynska, J. Fiett, M. Gniadkowski, and W. Hryniewicz. 2006. Multilocus sequence types, serotypes, and variants of the surface antigen PspA in Streptococcus pneumoniae isolates from meningitis patients in Poland. Clin. Vaccine Immunol. 13:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandgren, A., K. Sjostrom, B. Olsson-Liljequist, B. Christensson, A. Samuelsson, G. Kronvall, and B. H. Normark. 2004. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J. Infect. Dis. 189:785-796. [DOI] [PubMed] [Google Scholar]
- 43.Simell, B., M. Melin, M. Lahdenkari, D. E. Briles, S. K. Hollingshead, T. M. Kilpi, and H. Kayhty. 2007. Antibodies to pneumococcal surface protein A families 1 and 2 in serum and saliva of children and the risk of pneumococcal acute otitis media. J. Infect. Dis. 196:1528-1536. [DOI] [PubMed] [Google Scholar]
- 44.Suzumoto, M., M. Hotomi, D. S. Billal, D. E. Briles, S. K. Hollingshead, N. Yasui, and N. Yamanaka. 2003. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. G-276.
- 45.Syrjänen, R. K., T. M. Kilpi, T. H. Kaijalainen, E. E. Herva, and A. K. Takala. 2001. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J. Infect. Dis. 184:451-459. [DOI] [PubMed] [Google Scholar]
- 46.Tart, R. C., L. S. McDaniel, B. A. Ralph, and D. E. Briles. 1996. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J. Infect. Dis. 173:380-386. [DOI] [PubMed] [Google Scholar]
- 47.Teele, D. W., J. O. Klein, and B. Rosner. 1989. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J. Infect. Dis. 160:83-94. [DOI] [PubMed] [Google Scholar]
- 48.Vela Coral, M. C., N. Fonseca, E. Castaneda, J. L. Di Fabio, S. K. Hollingshead, and D. E. Briles. 2001. Pneumococcal surface protein A of invasive Streptococcus pneumoniae isolates from Colombian children. Emerg. Infect. Dis. 7:832-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White, P., A. Hermansson, C. Svanborg, D. Briles, and K. Prellner. 1999. Effects of active immunization with a pneumococcal surface protein (PspA) and of locally applied antibodies in experimental otitis media. ORL J. Otorhinolaryngol. Relat. Spec. 61:206-211. [DOI] [PubMed] [Google Scholar]
- 50.Wu, H. Y., M. H. Nahm, Y. Guo, M. W. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839-846. [DOI] [PubMed] [Google Scholar]