Abstract
Highly homologous meningococcal porin A (PorA) proteins induce protective humoral immunity against Neisseria meningitidis group B infection but with large and consistent differences in the levels of serum bactericidal activity achieved. We investigated whether a poor PorA-specific serological outcome is associated with a limited size of the specific B-cell subpopulation involved. The numbers of PorA-specific splenic plasma cells, bone marrow (BM) plasma cells, and splenic memory B cells were compared between mice that received priming and boosting with the weakly immunogenic PorA (P1.7-2,4) protein and those that received priming and boosting with the highly immunogenic PorA (P1.5-1,2-2) protein. Immunoglobulin G (IgG) titers (except at day 42), bactericidal activity, and the avidity of IgG produced against P1.7-2,4 were significantly lower at all time points after priming and boosting than against P1.5-1,2-2. These differences, however, were not associated with a lack of P1.7-2,4-specific plasma cells. Instead, priming with both of the PorAs resulted in the initial expansion of comparable numbers of splenic and BM plasma cells. Moreover, P1.7-2,4-specific BM plasma cells, but not P1.5-1,2-2-specific plasma cells, expanded significantly further after boosting. Likewise, after a relative delay during the priming phase, the splenic P1.7-2,4-specific memory B cells largely outnumbered those specific for P1.5-1,2-2, upon boosting. These trends were observed with different vaccine formulations of the porins. Our results show for the first time that B-cell subpopulations involved in a successfully maturated antibody response against a clinically relevant vaccine antigen are maintained at smaller population sizes than those associated with poor affinity maturation. This bears consequences for the interpretation of immunological memory data in clinical vaccine trials.
Infection with Neisseria meningitidis serogroup B (MenB) is a major cause of bacterial meningitis or sepsis (37). Various “universal” MenB vaccines targeting multiple MenB strains are under development at the moment (34). In general, protection against infections acquired after vaccination is based mainly on two immunological pillars: the induction of an immediate protective immune response and the formation of long-term immunological memory to prevent future infections. For meningococcal infections, vaccine-induced immediate protection is currently defined as serum bactericidal activity (SBA) (15), which is mediated by class-switched, affinity-maturated antibodies produced by specific long-lived plasma cells (39). The second major exponent of vaccine-induced protection, immunological memory, is established as a specific pool of memory B cells, required for low-grade self-renewal and the replenishment of plasma cells under homeostatic conditions (7). Furthermore, memory B cells provide the potential to rapidly differentiate into novel antibody-producing plasma cells with even more adapted antibodies upon reexposure to antigen or infection (16, 24). Understanding the mechanism by which meningococcal vaccines generate and sustain these B-cell subpopulations is essential in explaining and predicting the long-term efficacy of vaccines which mediate their protection through functional antibodies.
The generation of long-lived plasma cells and memory B cells specific for complex protein-based vaccines is associated with T-cell help. Naïve B cells recruited during this process grow exponentially in secondary lymphoid organs, either in extrafollicular foci as plasmablasts or in follicles, where they form germinal centers (GC). This latter process is T-cell dependent. Both the extrafollicular and the GC responses yield plasma cells, with only the GC-derived plasma cells being long-lived. B-cell growth and plasma cell formation in extrafollicular foci are responsible for the fast production of specific antibodies, first, of the immunoglobulin M (IgM) isotype, followed by a class switch to IgG; however, this is not associated with affinity maturation (19, 31). In contrast, B cells emerging from GC reactions, either as plasma cells or as memory B cells, have undergone B-cell receptor diversification via somatic hypermutation and are selected based on a high affinity for the antigen (12, 26).
Hence, B cells involved in functional and sustained immunity against the immunodominant but variable major outer membrane protein PorA in outer membrane vesicle (OMV) MenB vaccines develop through GC reactions. The common PorA serosubtypes P1.7-2,4 and P1.5-1,2-2 share 93% of their amino acid contents, yet in clinical and preclinical vaccination studies, bactericidal antibody titers against the serosubtype P1.7-2,4 are consistently lower than those against the serosubtype P1.5-1,2-2 (11, 22). To investigate whether this poor antibody outcome is linked to a lack of specific B-cell expansion, we analyzed the development of relevant class-switched B-cell types in different immune compartments of mice early after they underwent priming and boosting with different PorA-based vaccines. The data reveal that limited production and affinity maturation of the P1.7-2,4-specific IgG antibodies are associated with a partial cellular delay in the primary immune response but, unexpectedly, with a cellular overexpansion after boosting.
MATERIALS AND METHODS
Vaccines.
OMV were prepared by extraction of bacteria with 0.5% deoxycholate (0.1 M Tris-HCl, 10 mM EDTA [pH 8.6]) and purified by differential centrifugation (14). Both of the OMV vaccines used for the immunizations were obtained from class 3- and 4-deficient variants of isogenic PorA strains, TR52 (P1.5-1,2-2) and TR4 (P1.7-2,4) (33), in an H44/76 background, differing only in the PorA gene. The PorA protein content, as determined by scanning a 12% sodium dodecyl sulfate-polyacrylamide gel stained with Coomassie brilliant blue, was 77% for P1.7-2,4 OMV and 74% for P1.5-1,2-2 OMV. Class 2, 3, and 4 proteins were absent, and a small amount of class 5 protein was present. OMV contained 4 to 7.5% wild-type (L3) lipopolysaccharide (LPS) relative to the total protein content.
Liposomes were prepared as previously described (3), using recombinant PorA (rPorA), incorporated as the antigen, and an LpxL1 mutant of LPS (44) as the adjuvant. Briefly, the rPorAs P1.7-2,4 and P1.5-1,2-2 (a gift from Wyeth Vaccines, Pearl River, NY) were precipitated and dissolved in Tris-buffered saline containing 150 mM n-octyl β-d-glucopyranoside (Sigma, United Kingdom) to a final concentration of 200 μg/ml. Meningococcal LPS was resuspended in 600 mM n-octyl β-d-glucopyranoside. A lipid film was obtained by solvent evaporation of dimyristoyl phosphatidylcholine (Rhône-Poulenc Rorer, Germany), dimyristoyl phosphatidylglycerol (a gift from Lipoïd GmbH, Germany), and cholesterol (Sigma, United Kingdom) in an 8:2:2 molar ratio. The film was solubilized in the PorA solution, forming mixed micelles (protein/lipid ratio of 25 μg/μmol), and LpxL1 was added (protein/adjuvant ratio of 2 [wt/wt]). Mixed micelles were rapidly diluted to form liposomes, which were pelleted by ultracentrifugation (160,000 × g, 1 h), resuspended in Tris-buffered saline, filtered through sterile 0.2-μm filters, and characterized as described by Arigita et al. (2).
Immunizations and sample collection.
Immunization experiments were approved by the local ethical committee for animal experiments. In duplicate sets of experiments, four groups of five specific-pathogen-free female BALB/c mice 6 to 8 weeks old (NVI, The Netherlands) were untreated or immunized subcutaneously (on days 0 and 28) with OMV, either with the P1.7-2,4 or the P1.5-1,2-2 serosubtype. One OMV dose consisted of 1.5 μg of PorA in a 0.3-ml solution containing 0.45 mg of AlPO4 as an adjuvant. Longitudinal serum samples from individual mice were collected at days 0 (preimmunization), 14, 28 (preboost), and 42, until mice were sacrificed per group per experiment, either at day 0, 14, 28, or 42, and BM cells (from two femurs per mouse) and spleens were collected. Immunizations were scheduled such that organs of all groups could be collected and used on the same day for assay consistency. Sera were stored at −20°C, and tissues were further processed directly.
Additional groups of mice (five mice/group) were immunized with either the P1.7-2,4 or the P1.5-1,2-2 OMV or with the LpxL1-adjuvanted PorA-liposome mixture containing 1.5 μg of either of the PorA types in phosphate-buffered saline (PBS) at day 0 and 28. Sera, femurs, and spleens were collected at day 42.
ELISA.
PorA-specific IgM and IgG total antibody titers in sera were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (22). Briefly, ELISA plates (Immulon 2; Nunc) were coated with OMV (3 μg protein/ml) in PBS (100 μl/well) of the class 3- and 4-deficient variants of isogenic strains TR52 (P1.5-1,2-2) and TR4 (P1.7-2,4) and the PorA-deficient strain HI-5 (43) as a negative control and incubated overnight at room temperature (RT). After being coated, the plates were washed and incubated for 80 min at 37°C with threefold serial dilutions of the serum samples in PBS plus 0.05% Tween 80. Then, plates were washed and incubated at 37°C with goat anti-mouse IgM or an IgG-horseradish peroxidase conjugate (Southern Biotechnology Associates Inc., Birmingham, AL), diluted 1:5,000 in PBS plus 0.05% Tween 80 and 0.5% skim milk powder (100 μl/well; Protifar; Nutricia, The Netherlands). After 80 min, plates were washed, and 100 μl of the peroxidase substrate (3,3′,5,5′-tetramethylbenzidine with 0.01% H2O2 in 0.11 M sodium acetate buffer [pH 5.5]) was added per well for 10 min at RT, followed by 100 μl of 2 M H2SO4 per well. IgG antibody titers were expressed as the average 10Log of the reciprocal serum dilutions giving 50% of the maximum optical density (ODmax) at 450 nm ± standard error of the mean (SEM) per time point after immunization.
Avidity ELISA.
The avidity of antibodies in sera was determined by two different variants of an ELISA elution method, using either an increasing concentration of the chaotropic agent sodium thiocyanate (NaSCN) (35) in combination with a fixed serum dilution (21) or a fixed NaSCN concentration (1.5 M) with increasing serum dilutions (45). Briefly, in the fixed serum dilution method, plates were incubated with 100 μl of serum dilution, previously determined to give ELISA results of 50% of the ODmax at 450 nm (an OD of approximately 1.1). Plates were washed, and replicate wells were incubated for 15 min at RT with 100 μl of increasing concentrations of NaSCN in PBS (0 to 5.0 M). Plates were washed and developed as described above. The avidity of the sera per group was calculated as the geometric mean of avidity (GMA) ± SEM, corresponding to the geometric mean of the molar concentration of NaSCN required for a 50% reduction of the maximal absorbance in the absence of NaSCN. In the fixed NaSCN concentration method, sera were serially diluted in duplicate. After the incubation, one ELISA plate was incubated with 1.5 M NaSCN for 15 min at RT, and the duplicate plate was incubated with PBS. Then plates were washed and developed as described above. The titer after NaSCN treatment is a percentage of the original titer, and results per group are expressed as the GMA index {GMAI; where GMAI = geometric mean [titer (+NaSCN)/titer (−NaSCN) × 100]}.
Bactericidal assay.
The SBA was tested against the isogenic PorA strains (TR52 and TR4) (33), expressing normal amounts of class 1, 2/3, 4, and 5, and against HI-5, a PorA-deficient strain of H44/76 as a negative control. Briefly, sera were diluted 1:5 in Gey's balanced salt solution (Sigma, United Kingdom) plus 0.5% bovine serum albumin, complement inactivated (30 min 56°C), and incubated in serial dilutions with bacteria (104 CFU/ml) for 20 min at RT in 96-well plates. After baby rabbit complement (20% of total volume) was added, the time zero samples were taken out and plated on agar, and the 96-well plates were further incubated at 37°C for 60 min before being plated on GC agar supplemented with 1% IsoVitaleX (Becton Dickinson). CFU of time zero samples and serum dilutions were counted after being incubated for 18 to 20 h at 37°C and 5% CO2 on GC agar supplemented with 1% IsoVitaleX. The serum bactericidal titer was calculated as the 2 Log of the reciprocal of the lowest serum dilution yielding ≥90% killing of the CFU counted on time zero plates ± SEM. When reciprocals of dilutions exceeded 640 (2Log of >9.32), the serum was diluted 1:200 before complement inactivation.
Murine cell suspensions.
BM cell suspensions were obtained by flushing both femoral bones per animal with complete medium, i.e., Iscove's medium (Gibco BRL), supplemented with 10% fetal calf serum (HyClone), β-mercaptoethanol, and penicillin-streptomycin-glutamine (Gibco BRL). Splenocyte suspensions were obtained by meshing spleens through 100-μm-pore-size nylon filters, subsequent treatment with lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA in distilled water, 2 min, 4°C), and washing in complete medium. BM cells and splenocyte suspensions were counted using a TT CASY cell counter (Schärfe system, Reutlingen, Germany). BM cell suspensions and splenocyte suspensions contained on average 12.6 ± 3.8 × 106 and 42 ± 9.2 × 106 cells, per animal, respectively. No significant differences between these cell counts were observed for time points in the vaccination schedule or those between vaccines.
For the detection of splenic and BM plasma cells, splenocyte and BM suspensions, pooled per group, were used in a PorA-specific B-cell enzyme-linked immunospot (ELISPOT) assay directly ex vivo. For the detection of splenic memory B cells, splenocyte suspensions were first differentiated in vitro to become antibody-secreting cells (ASC) before being used in the B-cell ELISPOT assay. Briefly, cells were pooled per group and cultured at 3 × 106 cells/ml for 5 days in complete medium in the presence of Pansorbin cells (Calbiochem), diluted 1:25,000, and 100 U/ml recombinant mouse interleukin-2 (Cetus, Emeryville, CA), as adapted from a method described by Nanan et al. (30), and washed before use.
B-cell ELISPOT assay.
A PorA-specific B-cell ELISPOT assay was developed based on previously described methods (28, 30) and optimized for the highly specific and sensitive detection of the immunodominant VR2 regions of P1.7-2,4 and P1.5-1,2-2 by using specific B-cell hybridomas as ASC (data not shown). Briefly, 96-well Multiscreen HTS plates (Millipore, Bedford, MA) were coated (overnight at 4°C with 0.1 M sodium carbonate buffer [pH 9.6], 100 μl/well) with specific P1.7-2,4 or P1.5-1,2-2 OMV under optimized coating conditions of 25 μg protein/ml or HI5 OMV as PorA-negative control (background) and with goat anti-mouse IgG (10 μg/ml; Southern Biotechnologies, Birmingham, AL) as a positive control. When indicated, as described in Results, rPorA coated at 25 μg/ml was used as a comparison. Plates were blocked (5% fetal calf serum in PBS, 30 min, 37°C) and washed three times with 200 μl PBS. Then, 5- to 10-fold serially diluted fresh or cultured murine cell suspensions (ranging from 1 × 107 to 1 × 105 cells/ml), pooled per group, were incubated in duplicate or triplicate wells overnight (100 μl/well), at 37°C in 5% CO2. Thereafter, cells were removed, and plates were washed three times with 200 μl of 0.05% Tween 20 in PBS and incubated for 2 to 4 h (37°C) with 1:10,000 alkaline phosphatase-conjugated goat anti-mouse IgG (Southern Biotechnologies, Birmingham, AL). Plates were washed three times with 0.05% Tween 20 in PBS and once with PBS before being developed with an alkaline phosphatase conjugate substrate kit (Bio-Rad, Hercules, CA) in 0.1 M Tris, 0.1 M NaCl (pH 9.5) buffer for 20 to 40 min at RT. Then, plates were washed with water and allowed to dry, and spots were counted. Spots above the background from at least two countable cell dilutions were used and expressed as the geometric mean of spots per 106 plated BM or splenic cells and then multiplied by a variable factor to obtain the averaged amount of femoral BM ASC or the total amount of splenic ASC per animal. To establish a flow diagram of specific B-cell populations per animal, two femurs were estimated to contain 12.6% of the total mouse BM cells (6).
Statistical analysis.
IgG titers were expressed as 10Log values of the geometric mean titer for groups of mice ± SEM. SBA titers are expressed as 2Log values of the averages of groups of mice. Differences between IgG and SBA titers and avidities from groups were considered significant at P values of <0.05, using a Student t test.
RESULTS
Early development of IgM and IgG titers specific for differently immunogenic PorAs.
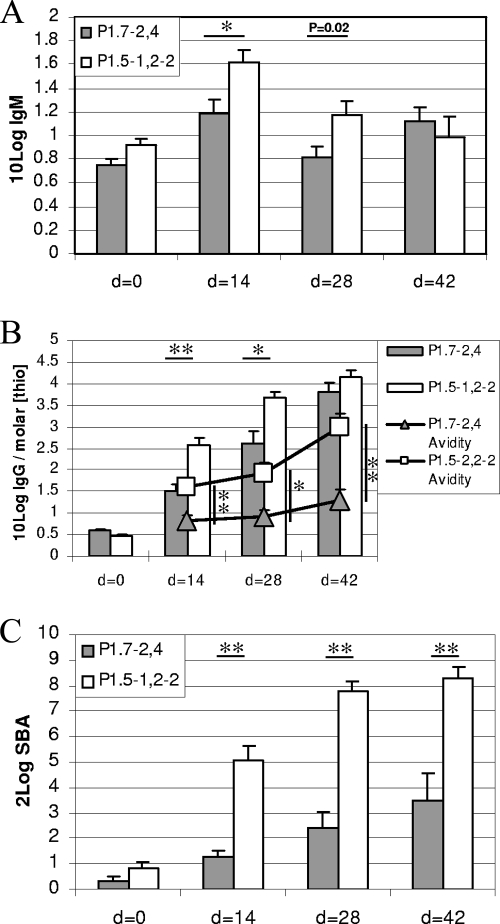
Previously, we found consistently lower and less functional PorA-specific IgG titers in mice at 6 weeks after immunization with P1.7-2,4 OMV (given at day 0 and day 28) than in mice immunized likewise with P1.5-1,2-2-specific OMV (22). To establish when these serological differences arose, we analyzed sera from individual mice at days 0 (preimmunization), 14, 28 (preboost), and 42 of the vaccination schedule in more detail for their development of specific IgM and IgG titers. Natural IgM antibodies could be detected against both PorAs in naïve mice. P1.5-1,2-2 IgM titers were 1.5 times (not significantly) higher than P1.7-2,4 IgM titers (Fig. 1A). Immunization with OMV vaccine induced a temporal threefold increase in IgM titer specific for P1.7-2,4, compared to a fivefold increase in IgM for P1.5-1,2-2, as measured at day 14, which resulted in a statistically higher IgM titer against P1.5-1,2-2 at days 14 and 28 (P < 0.01 and P = 0.02, respectively). Upon boosting, IgM levels specific for P1.7-2,4 but not for P1.5-1,2-2 increased again by a factor of 2, so that the difference was no longer statistically significant.
FIG. 1.
Serological responses against the P1.7-2,4 and P1.5-1,2-2 serosubtypes after OMV immunization on days 0 and 28. (A) IgM titers. (B) IgG titers with corresponding avidity indices in a dual y-axis plot. IgG titers are given as 10Log IgG (bars), and avidity is indicated as the molar concentration of NaSCN (molar [thio]) giving 50% reduction in IgG titer (symbols). (C) Serum bactericidal titers. SBA titers are expressed as the serum dilution resulting in 90% killing of the homologous strains. Data are from two experiments. Error bars indicate SEM. P values are indicated, where * is <0.01 and ** is <0.001.
IgG titers (10Log values) against P1.7-2,4 were significantly lower than those against P1.5-1,2-2 at day 14 (1.49 versus 2.58, respectively; P < 0.001) (Fig. 1B, bars) and day 28 (2.61 versus 3.67, respectively; P < 0.01). At day 42, the difference between the IgG titers for the two vaccines was no longer significant (3.81 versus 4.16, respectively), partly due to the smaller amount of cumulative serum samples at this time point per group (n = 10).
Functionality and avidity of induced PorA-specific IgG antibodies.
Sera of individual mice were tested in a bactericidal assay. SBA titers (2Log values) against P1.7-2,4 were significantly lower at all three time points after immunization than those established against P1.5-1,2-2 (P < 0.001) (Fig. 1C). Together the serological data suggest that the differences in immunogenicity between P1.7-2,4 and P1.5-1,2-2 are established early in the primary immune response. Hence, while class switching to IgG antibodies occurred in response to both PorA serosubtypes, anti-P1.5-1,2-2 IgG levels not only rose more rapidly but were more functional in SBA than anti-P1.7-2,4 IgG levels.
To compare the affinity maturations of the IgG antibodies, as measured by an increase in avidity of antibody-antigen interactions over time, individual serum samples were tested in a fixed serum dilution assay. The avidity of P1.7-2,4-specific IgG was significantly lower at all three time points (P < 0.001, P < 0.01, and P < 0.001, respectively) (Fig. 1B, triangles) than the IgG avidity established for P1.5-1,2-2 (Fig. 1A, squares). In addition, the avidity maturation for P1.7-2,4, but not for P1.5-1,2-2, was already arrested at day 14 in the primary immune response and required boosting to further increase.
Development of P1.7-2,4- and P1.5-1,2-2-specific plasma cells and memory B cells in the spleen.
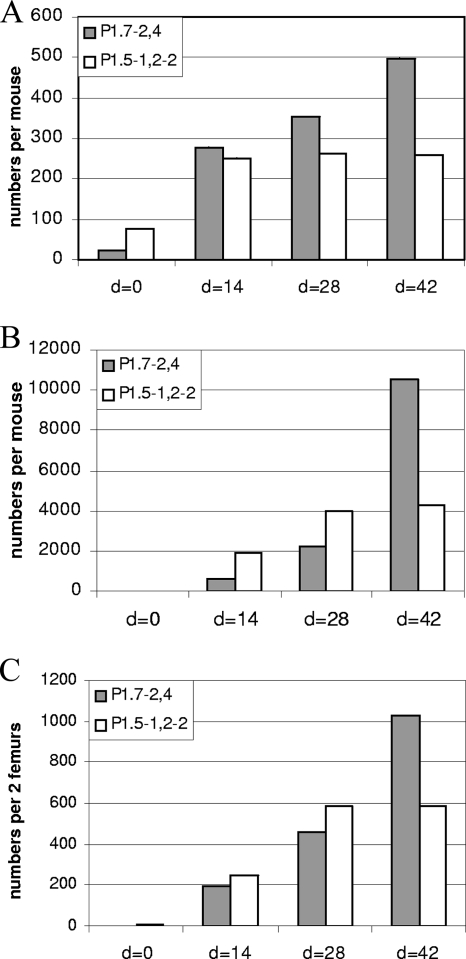
To assess whether the serological differences observed for the anti-PorA response correlated with differences in B-cell dynamics, we studied cell suspensions derived from spleen and BM cells, the presumed sites for short-lived plasma cells and memory B cells on the one hand and long-lived plasma cells on the other hand (38, 40), at different time points of the immune response, using an optimized PorA-specific B-cell ELISPOT assay. First, splenocyte suspensions were freshly isolated at days 0 (preimmunization), 14, 28 (preboost), and 42 of the OMV immunization schedule and were pooled per group for analysis. When directly tested with the ELISPOT assay, modest, comparable numbers of splenic ASC per animal were found for P1.7-2,4 and P1.5-1,2-2 at day 14 after priming. Then, the numbers increased slightly higher for P1.7-2,4 but remained stable for P1.5-1,2-2 (Fig. 2A).
FIG. 2.
B-cell responses against the P1.7-2,4 and P1.5-1,2-2 serosubtypes after OMV immunization on days 0 and 28. (A) Splenic plasma cells, which are defined as IgG-secreting cells from the spleen without the requirement for further ex vivo stimulation. (B) Splenic memory B cells, which are defined as IgG-secreting cells from the spleen after in vitro stimulation. (C) Bone marrow plasma cells, which are defined as IgG-secreting cells from the bone marrow without the requirement for further ex vivo stimulation. Data are from one of two experiments showing similar results.
When splenic memory B-cell numbers were assessed, by counting ASC after in vitro differentiation of splenocytes, larger expansions and more pronounced differences were observed between time points and PorA types (Fig. 2B). While specific antibody-secreting cultured splenocytes from nonvaccinated mice were hardly detectable, significant numbers of PorA-specific memory B cells per spleen were revealed at day 14 after priming, albeit at a threefold lower level for P1.7-2,4 than for P1.5-1,2-2 (588 versus 1,884, respectively, in a representative experiment of two replicates). At day 28 after immunization, these numbers had increased further for both antigens but relatively faster for P1.7-2,4 (to 2,241 versus 3,949, respectively). At day 42, 2 weeks after the booster vaccination, the number of P1.7-2,4-specific splenic memory B cells had exponentially augmented further to 10,554, while those specific for P1.5-1,2-2 remained unchanged.
Arrival of P1.7-2,4- and P1.5-1,2-2-specific plasma cells in the bone marrow.
Numbers of PorA-specific ASC in BM cell suspensions steadily increased with time. In one representative experiment of two, numbers of P1.7-2,4- and P1.5-1,2-2-specific ASC per two femurs were induced from an undetectable level at day 0 to 197 versus 246, respectively, at day 14 (Fig. 2C). Two weeks later, these numbers had increased at the same rate (460 versus 586, respectively). At day 42, however, following boosting, the number of femoral P1.7-2,4-specific ASC per animal had doubled (1,025), while the number of P1.5-1,2-2-specific ASC remained unaltered (585). Hence, while functional antibody titers still increased and maturated for P1.5-1,2-2 after boosting, this was not paralleled by a further expansion of BM plasma cells or splenic plasma cells, rather suggesting an ongoing refinement or replacement of the P1.5-1,2-2-specific antibody-secreting cellular compartment. In contrast, boosting induced further expansion of the less productive and lower-affinity P1.7-2,4-specific BM cells and splenic plasma cells and of P1.7-2,4-specific splenic memory B cells by a factor ranging from 2 to 5, thereby greatly outnumbering their counterparts involved in the P1.5-1,2-2 response.
Hyperproliferation at day 42 of the B-cell response to the P1.7-2,4 antigen is independent of the vaccine formulation.
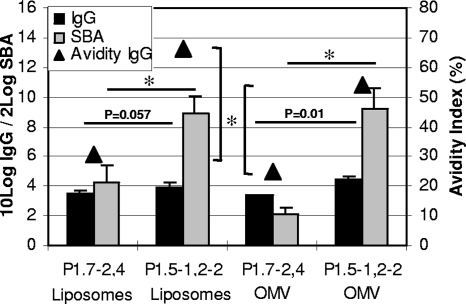
To investigate whether the larger number of B cells involved in the boosting phase of the P1.7-2,4 response could also be observed irrespective of the vaccine formulation of the P1.7-2,4 antigen, we compared the numbers of day 42 IgG titers, BM plasma cells, and splenic memory B-cells of mice immunized with OMV or with PorA-liposomes, containing equal amounts of the P1.7-2,4 or P1.5-1,2-2 proteins per dose as OMV and LpxL1, a genetically modified form of neisserial LPS, as an adjuvant. The P1.7-2,4 IgG titers were lower than the P1.5-1,2-2 IgG titers, in both the PorA-liposome- and the OMV-vaccinated groups (P = 0.057 and P = 0.01, respectively) (Fig. 3, black bars).
FIG. 3.
Serological responses against the P1.7-2,4 and P1.5-1,2-2 serosubtypes after liposome or OMV immunization on days 0 and 28. Avidity is indicated as the percentage of titers that remains when ELISA plates are incubated with 1.5 M of NaSCN. SBA titers are expressed as the serum dilution resulting in 90% killing of the homologous strains. Data are from one experiment with five mice per group. Error bars indicate SEM. P values are indicated, where * is <0.01 and ** is <0.001.
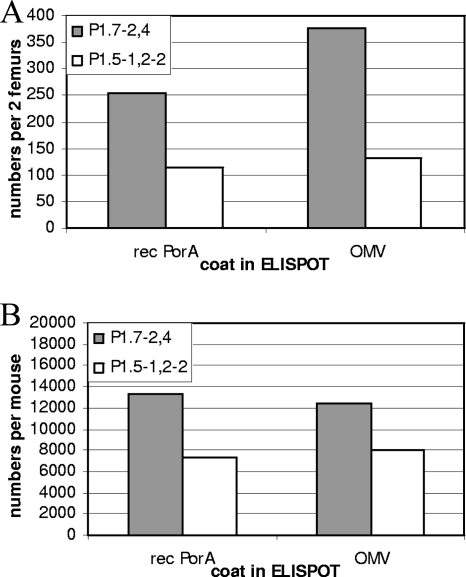
Trends in bactericidal activity and avidity were also in accordance with those in earlier findings, with SBA titers and avidity indices against P1.7-2,4 significantly lower for both the PorA-liposome- and the OMV-vaccinated groups at day 42 (SBA, P < 0.01 and P < 0.01, respectively) (Fig. 3). ELISPOT assay results showed that BM plasma cells from liposome-immunized mice were 2 to 3 times more numerous for P1.7-2,4 than for P1.5-1,2-2 at day 42, independent of whether OMV or rPorA was used as the coating antigen in ELISPOT assays (Fig. 4A). The average number of specific splenic memory B cells per mouse was also higher at day 42 for P1.7-2,4 than for P1.5-1,2-2 (Fig. 4B). This indicates that the overexpansion of P1.7-2,4-specific memory B cells and plasma cells at this stage of the anti-PorA response is caused by intrinsic properties of the P1.7-2,4 antigen and not by formulation differences.
FIG. 4.
B-cell responses at day 42 against the P1.7-2,4 and P1.5-1,2-2 serosubtypes after OMV or liposome immunization on days 0 and 28. (A) Bone marrow plasma cells, which are defined as IgG-secreting cells from the bone marrow, without the requirement for further ex vivo stimulation. (B) Splenic memory B cells, which are defined as IgG-secreting cells from the spleen after in vitro stimulation. Data are from one experiment.
DISCUSSION
P1.7-2,4 is the predominant disease-causing PorA serosubtype of MenB in western Europe and New Zealand (5, 25), suggesting a weak natural immunity against this porin. It is also the main antigen of the MeNZB vaccine, the OMV vaccine introduced to protect against the strain circulating in New Zealand (32). Bactericidal antibodies against this serosubtype are induced less easily than against others (11, 22). In this study, we investigated for the first time the specific B-cell dynamics underlying the serological response to this relevant vaccine antigen in comparison to its more immunogenic natural homologue P1.5-1,2-2, using a mouse model. Poorer immunogenicity of the P1.7-2,4 serosubtype, as characterized by relatively impaired secretion, function, and affinity maturation of IgG antibodies after OMV priming and boosting, was not associated with a relative lack of specific B cells arising against P1.7-2,4. Instead, an unexpected hyperproliferation of P1.7-2,4-specific B-cell types occurred. Similar serological endpoints and cellular trends were obtained when mice were vaccinated with adjuvanted PorA-liposomes, indicating that the differences between the P1.7-2,4 and the P1.5-1,2-2 B-cell responses are caused by intrinsic properties of the porins and not by their OMV context.
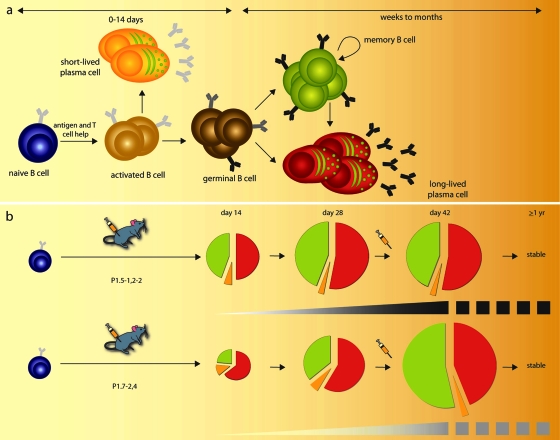
Studies of vaccine-induced development of B-cell populations involved in humoral protection against pathogens are scarce. Our data reveal for the first time that the ultimate size of specific B-cell populations involved in vaccine responses may not correlate linearly with the quality of the antibody response (summarized as a model of PorA-specific B-cell development in Fig. 5). The specific B-cell response against the immunogen P1.5-1,2-2, associated with the most functional and high-affinity antibody responses, differs not only in absolute but also in relative cell numbers from that of the P1.7-2,4-specific B-cell response. When they were extrapolated to total numbers per animal, the splenic memory B cells and BM plasma cells specific for P1.5-1,2-2 developed at a stable 1:1 ratio throughout the immune response, while the ratio of P1.7-2,4-specific memory B cells to BM plasma cells increased over time. Ultimately, expansion of the P1.5-1,2-2 B-cell types was arrested after day 28, while the P1.7-2,4 B-cell types continued to proliferate, eventually outnumbering their P1.5-1,2-2-specific counterparts by a factor of 2. These qualitative and quantitative differences between the two immune responses observed 2 weeks after booster vaccination were stable for at least 1 year (T. Luijkx, unpublished data). What the two immune responses had in common was that the BM and not the spleen was the primary anatomical site of PorA-specific ASC in vaccinated mice, as was also recently reported for another PorA serosubtype, P1.19,15, by Cruz et al. (9).
FIG. 5.
PorA-specific B-cell differentiation. (a) In response to antigen and specific T-cell help, naïve B cells clonally expand and form clusters of activated B cells. These cells either differentiate into short-lived plasma cells or they initiate germinal-center reactions in secondary lymphoid tissues, where they proliferate and undergo affinity maturation and selection. GC B cells generate both long-lived plasma cells that home to the BM and produce affinity-maturated antibodies and memory B cells with affinity-maturated B-cell receptors. Memory B cells are self-renewing and replenish the pool of long-lived plasma cells to maintain long-term antibody production. (b) Naïve B cells specific for either P1.5-1,2-2 (upper panel) or P1.7-2,4 (lower panel) give rise to different B-cell dynamics (pie charts) and affinity maturation of antibodies (gray-to-black shading, where black represents high avidity) after immunization. Pie charts represent ratios and relative numbers of specific splenic short-lived plasma cells (orange), splenic memory B cells (green), and BM plasma cells (red) at different time points of the immunization schedule. Cell numbers, ratios, and avidity indices at day 42 are stable for at least 57 weeks (T. Luijkx, unpublished data).
The emergence of high-affinity IgG levels after vaccination with complex protein antigens reflects the development of a T-cell-dependent GC reaction. In such secondary lymphoid structures, naïve B cells with initially low affinities for antigen clonally expand and somatically hypermutate their Ig genes before being selected based on competition for antigen (10, 17, 41). End-differentiated plasma cells emerging from these GC reactions from the second week after initial antigen exposure have been proposed to represent those cells that have undergone this process of affinity maturation most successfully (23, 26). Hence, assuming an unlimited naïve B-cell repertoire for any given antigen, the typical differences observed between the P1.7-2,4- and the P.15-1,2-2-specific B-cell responses in our vaccination model should be accounted for by selective checkpoints during GC reactions. Although events critical for the outcome of GC reactions are still being explored, at least three antigen-related parameters should be taken into account. First, a number of studies have implied that secretion of IgM is a prerequisite for optimal induction and formation of GC reactions and IgG production (8, 13). Low natural IgM antibody titers were detected against both PorAs, P1.5-1,2-2 IgM titers not being significantly higher than P1.7-2,4 IgM titers (8.3 versus 5.4, respectively) in naïve mice. Our data indicate that if the level of natural IgM secretion can determine poor or successful PorA-specific GC development and final functional outcome, then differences in titers smaller than a factor of 1.5 are discriminative. Second, another important event required for naïve B cells to enter and leave GC reactions is cognate T-cell help (26) (Fig. 5a). Over 90% of identical meningococcal porins diverge mostly at their surface-exposed hypervariable loops (VR1 and VR2), leaving considerable membrane-embedded conserved parts of the protein available for shared T-cell epitope generation. Preliminary studies indicate that T cells from PorA-immunized mice (H-2d and H-2b), as well as human T-cell lines specific for conserved as well as variable PorA epitopes, are consistently less reactive against P1.7-2,4 than against P1.5-1,2-2 (C. van Els, unpublished data). This could indicate that the extent of P1.7-2,4 T-cell help could be a limiting factor in the outcome of P1.7-2,4-specific GC reactions. More research, however, is needed to elucidate the role of PorA T-cell specificities in the humoral response. Third, at different stages of the GC reaction, the local presence of intact antigen is required: both entry into the GC cycle and survival of GC B cells are affinity-based competitive mechanisms driven by antigen (4, 20, 27). Follicular dendritic cells have been implicated in providing a local depot of antigen by capturing immune complexes via their FcγR and complement receptors (42), although this mechanism has been debated (17, 18). Affinity maturation of the P1.7-2,4-specific IgG B-cell response was impaired, and boosting with antigen was required for further maturation, indicating that the local depot of antigen might have been limited. Studies to investigate the persistence of local antigen in P1.7-2,4 and P1.5-1,2-2 GC reactions in tissue sections are ongoing.
Hence, although further studies are needed to clarify which factors have limited the affinity maturation of the P1.7-2,4 response, our study demonstrated a hitherto unknown association between this arrest and an unrestrained, rather than inhibited, secondary proliferation of specific B cells. This is reminiscent of the GC congestion observed for genetically manipulated mice that lack factors that regulate somatic hypermutation or terminal differentiation (1, 29). Hence, our data suggest for the first time that poor affinity maturation and unrestrained secondary B-cell proliferation might be physiologically relevant linked phenomena in response to vaccine antigens in wild-type hosts. Following boosting, the numbers of plasma cells that relocated to the BM and those of splenic memory B cells specific for P1.7-2,4 were at least twice the numbers specific for P1.5-1,2-2. These striking differences were maintained for a minimum of 57 weeks (T. Luijkx, unpublished data). This implies that upon vaccination, the long-term B-cell niches in BM and spleen might be dominated by those specificities associated with the poorest functional antibody titers. Consequently, if old B-cell specificities in lymphoid survival niches are gradually replaced by new ones, as proposed by Radbruch and colleagues (36), then antibody responses mediated by high-affinity but less-dominant plasma cell populations might wane more rapidly than those mediated by low-affinity but more-abundant plasma cell populations.
Preexisting antibody and B-cell memory against multiple meningococcal outer membrane porins are important correlates of protection against infection with serogroup B meningococci. A better understanding of factors that drive successful affinity maturation and regulation of B-cell population size, also against poor immunogens, is important to improve vaccine-induced immune responses.
Acknowledgments
We thank G. Zlotnick from Wyeth Vaccines for donating rPorA, CDF personnel for excellent biotechnical support, C. Arigita for advice on liposomes, L. van Alphen for helpful discussion, and W. Witkamp for illustrations.
Footnotes
Published ahead of print on 3 September 2008.
REFERENCES
- 1.Angelin-Duclos, C., G. Cattoretti, K. I. Lin, and K. Calame. 2000. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J. Immunol. 165:5462-5471. [DOI] [PubMed] [Google Scholar]
- 2.Arigita, C., G. F. Kersten, T. Hazendonk, W. E. Hennink, D. J. Crommelin, and W. Jiskoot. 2003. Restored functional immunogenicity of purified meningococcal PorA by incorporation into liposomes. Vaccine 21:950-960. [DOI] [PubMed] [Google Scholar]
- 3.Arigita, C., T. Luijkx, W. Jiskoot, M. Poelen, W. E. Hennink, D. J. Crommelin, P. van der Ley, C. Van Els, and G. F. Kersten. 2005. Well-defined and potent liposomal meningococcal B vaccines adjuvated with LPS derivatives. Vaccine 23:5091-5098. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and F. Rousset. 1991. Growing human B lymphocytes in the CD40 system. Nature 353:678-679. [DOI] [PubMed] [Google Scholar]
- 5.Bart, A., J. Dankert, and A. van der Ende. 1999. Antigenic variation of the class I outer membrane protein in hyperendemic Neisseria meningitidis strains in The Netherlands. Infect. Immun. 67:3842-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benner, R., W. Hijmans, and J. J. Haaijman. 1981. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin. Exp. Immunol. 46:1-8. [PMC free article] [PubMed] [Google Scholar]
- 7.Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199-2202. [DOI] [PubMed] [Google Scholar]
- 8.Boes, M. 2000. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 37:1141-1149. [DOI] [PubMed] [Google Scholar]
- 9.Cruz, S. C., A. C. Cruz, J. M. Oliveira, A. M. Soares, C. A. Gioia, and L. G. Milagres. 2007. Generation of long-lived plasma cells to serogroup B Neisseria meningitidis after murine immunisation with an outer membrane protein vaccine. Vaccine 25:5046-5052. [DOI] [PubMed] [Google Scholar]
- 10.Dal Porto, J. M., A. M. Haberman, M. J. Shlomchik, and G. Kelsoe. 1998. Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J. Immunol. 161:5373-5381. [PubMed] [Google Scholar]
- 11.de Kleijn, E. D., R. De Groot, J. Labadie, A. B. Lafeber, D. G. van den Dobbelsteen, L. Van Alphen, H. van Dijken, B. Kuipers, G. W. van Omme, M. Wala, R. Juttmann, and H. C. Rumke. 2000. Immunogenicity and safety of a hexavalent meningococcal outer-membrane-vesicle vaccine in children of 2-3 and 7-8 years of age. Vaccine 18:1456-1466. [DOI] [PubMed] [Google Scholar]
- 12.Dorner, T., and A. Radbruch. 2007. Antibodies and B cell memory in viral immunity. Immunity 27:384-392. [DOI] [PubMed] [Google Scholar]
- 13.Ehrenstein, M. R., T. L. O'Keefe, S. L. Davies, and M. S. Neuberger. 1998. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc. Natl. Acad. Sci. USA 95:10089-10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Froholm, A. K. Lindbak, B. Mogster, E. Namork, and U. Rye. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 14:67-79. [PubMed] [Google Scholar]
- 15.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gourley, T. S., E. J. Wherry, D. Masopust, and R. Ahmed. 2004. Generation and maintenance of immunological memory. Semin. Immunol. 16:323-333. [DOI] [PubMed] [Google Scholar]
- 17.Haberman, A. M., and M. J. Shlomchik. 2003. Reassessing the function of immune-complex retention by follicular dendritic cells. Nat. Rev. Immunol. 3:757-764. [DOI] [PubMed] [Google Scholar]
- 18.Hannum, L. G., A. M. Haberman, S. M. Anderson, and M. J. Shlomchik. 2000. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J. Exp. Med. 192:931-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob, J., R. Kassir, and G. Kelsoe. 1991. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J. Exp. Med. 173:1165-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, Y. J., D. E. Joshua, G. T. Williams, C. A. Smith, J. Gordon, and I. C. MacLennan. 1989. Mechanism of antigen-driven selection in germinal centres. Nature 342:929-931. [DOI] [PubMed] [Google Scholar]
- 21.Luijkx, T., H. van Dijken, C. Van Els, and G. van den Dobbelsteen. 2006. Heterologous prime-boost strategy to overcome weak immunogenicity of two serosubtypes in hexavalent Neisseria meningitidis outer membrane vesicle vaccine. Vaccine 24:1569-1577. [DOI] [PubMed] [Google Scholar]
- 22.Luijkx, T. A., H. van Dijken, H. J. Hamstra, B. Kuipers, P. van der Ley, L. Van Alphen, and G. van den Dobbelsteen. 2003. Relative immunogenicity of PorA subtypes in a multivalent Neisseria meningitidis vaccine is not dependent on presentation form. Infect. Immun. 71:6367-6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLennan, I. C. 1994. Germinal centers. Annu. Rev. Immunol. 12:117-139. [DOI] [PubMed] [Google Scholar]
- 24.Manz, R. A., A. E. Hauser, F. Hiepe, and A. Radbruch. 2005. Maintenance of serum antibody levels. Annu. Rev. Immunol. 23:367-386.:367-386. [DOI] [PubMed] [Google Scholar]
- 25.Martin, D. R., S. J. Walker, M. G. Baker, and D. R. Lennon. 1998. New Zealand epidemic of meningococcal disease identified by a strain with phenotype B:4:P1.4. J. Infect. Dis. 177:497-500. [DOI] [PubMed] [Google Scholar]
- 26.McHeyzer-Williams, L. J., and M. G. McHeyzer-Williams. 2005. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23:487-513. [DOI] [PubMed] [Google Scholar]
- 27.Melchers, F., and W. Lernhardt. 1985. Three restriction points in the cell cycle of activated murine B lymphocytes. Proc. Natl. Acad. Sci. USA 82:7681-7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno, R. L., J. S. Sampson, S. Romero-Steiner, B. Wong, S. E. Johnson, E. Ades, and G. M. Carlone. 2004. A murine model for the study of immune memory in response to pneumococcal conjugate vaccination. Vaccine 22:3069-3079. [DOI] [PubMed] [Google Scholar]
- 29.Muramatsu, M., K. Kinoshita, S. Fagarasan, S. Yamada, Y. Shinkai, and T. Honjo. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102:553-563. [DOI] [PubMed] [Google Scholar]
- 30.Nanan, R., D. Heinrich, M. Frosch, and H. W. Kreth. 2001. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine 20:498-504. [DOI] [PubMed] [Google Scholar]
- 31.Nossal, G. J., and C. Riedel. 1989. Sudden appearance of anti-protein IgG1-forming cell precursors early during primary immunization. Proc. Natl. Acad. Sci. USA 86:4679-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oster, P., D. Lennon, J. O'Hallahan, K. Mulholland, S. Reid, and D. Martin. 2005. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 23:2191-2196. [DOI] [PubMed] [Google Scholar]
- 33.Peeters, C. C., H. C. Rumke, L. C. Sundermann, E. M. Rouppe van der Voort, J. Meulenbelt, M. Schuller, A. J. Kuipers, P. van der Ley, and J. T. Poolman. 1996. Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine 14:1009-1015. [DOI] [PubMed] [Google Scholar]
- 34.Price, A. A. 2007. Meningococcal vaccines. Curr. Pharm. Des. 13:2009-2014. [DOI] [PubMed] [Google Scholar]
- 35.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86:83-87. [DOI] [PubMed] [Google Scholar]
- 36.Radbruch, A., G. Muehlinghaus, E. O. Luger, A. Inamine, K. G. Smith, T. Dorner, and F. Hiepe. 2006. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 6:741-750. [DOI] [PubMed] [Google Scholar]
- 37.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease 59. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 38.Slifka, M. K., and R. Ahmed. 1998. B cell responses and immune memory. Dev. Biol. Stand. 95:105-115. [PubMed] [Google Scholar]
- 39.Slifka, M. K., R. Antia, J. K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8:363-372. [DOI] [PubMed] [Google Scholar]
- 40.Slifka, M. K., M. Matloubian, and R. Ahmed. 1995. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 69:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, K. G., A. Light, G. J. Nossal, and D. M. Tarlinton. 1997. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 16:2996-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tew, J. G., J. Wu, M. Fakher, A. K. Szakal, and D. Qin. 2001. Follicular dendritic cells: beyond the necessity of T-cell help. Trends Immunol. 22:361-367. [DOI] [PubMed] [Google Scholar]
- 43.Tommassen, J., P. Vermeij, M. Struyve, R. Benz, and J. T. Poolman. 1990. Isolation of Neisseria meningitidis mutants deficient in class 1 (PorA) and class 3 (PorB) outer membrane proteins. Infect. Immun. 58:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Ley, P., L. Steeghs, H. J. Hamstra, J. ten Hove, B. Zomer, and L. Van Alphen. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 69:5981-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vermont, C. L., H. H. Van Dijken, C. J. van Limpt, R. De Groot, L. Van Alphen, and G. P. Van Den Dobbelsteen. 2002. Antibody avidity and immunoglobulin G isotype distribution following immunization with a monovalent meningococcal B outer membrane vesicle vaccine. Infect. Immun. 70:584-590. [DOI] [PMC free article] [PubMed] [Google Scholar]