Abstract
The Eastern oyster, Crassostrea virginica, inhabits shallow coastal waters that frequently experience periods of low dissolved oxygen (hypoxia) and elevated CO2 (hypercapnia) levels. Bacteria are extremely abundant in these environments and accumulate in large numbers in filter-feeding oysters, which can act as passive carriers of human pathogens. Although hypercapnic hypoxia (HH) can affect certain specific immune mechanisms, its direct effect on the inactivation, degradation and elimination of bacteria in oysters is unknown. This research was conducted to determine whether exposure to HH reduces the ability of C. virginica to inactivate and eliminate Vibrio campbellii following its injection into the adductor muscle. Oysters were held in fully air-saturated (normoxic; partial O2 pressure [PO2] = 20.7 kPa, CO2 < 0.06 kPa, pH 7.8 to 8.0) or HH (PO2 = 4 kPa, CO2 = 1.8 kPa, pH 6.5 to 6.8) seawater at 25°C for 4 h before being injected in the adductor muscle with 105 live Vibrio campbellii bacteria and remained under these conditions for the remainder of the experiment (up to 24 h postinjection). Real-time PCR was used to quantify the number of intact V. campbellii bacteria, while selective plating was used to quantify the number of injected bacteria remaining culturable in whole-oyster tissues, seawater, and feces/pseudofeces at 0, 1, 4, and 24 h postinjection. We found that oysters maintained under normoxic conditions were very efficient at inactivating and degrading large numbers of injected bacteria within their tissues. Moreover, a small percentage (∼10%) of injected bacteria were passed into the surrounding seawater, while less than 1% were recovered in the feces/pseudofeces. In contrast, HH increased the percentage of culturable bacteria recovered from the tissues of oysters, suggesting an overall decrease in bacteriostasis. We suggest that poor water quality may increase the risk that oysters will harbor and transmit bacterial pathogens hazardous to human and ecosystem health.
The Eastern oyster, Crassostrea virginica (Gmelin), is an ecologically and economically important shellfish species broadly distributed along the Atlantic coast of the United States and the Gulf of Mexico (20). As with other filter-feeding marine bivalves, oysters can accumulate large numbers of viruses, such as hepatitis A virus, and bacteria, such as Vibrio cholerae, Vibrio vulnificus, and Vibrio parahaemolyticus, when they are present in the surrounding water (16, 25, 49, 53) and may act as passive carriers of human pathogens (14). To help defend themselves against viral and microbial infections, oysters have efficient cellular and humoral immune defense mechanisms (14, 36). However, poor water quality can impair some of these mechanisms, thereby increasing the risk that oysters will harbor and transmit pathogens that are harmful to humans, oysters, and the environment (8, 10, 16, 23, 33, 36, 49).
Hemocytes, cells which circulate in oyster hemolymph, form the cellular component of the innate immune response and play a key role in the internal defense. Hemocytes are responsible for phagocytosis of nonself particles (28, 31, 37), agglutination and encapsulation of foreign materials (21, 57), production of reactive oxygen species (ROS) (8, 24), and lysis of foreign cells. Essential to these processes is the ability of hemocytes to migrate across epithelial borders to the exterior, where foreign particles are ingested and/or eliminated (20, 30). In addition, mollusks also employ a broad range of humoral immune defense molecules, such as antimicrobial peptides, lectins, lysozymes, and serine proteases, that work in combination with the phagocytic hemocytes to inactivate (stop growth) and degrade microbial pathogens and nonself particles (5, 14).
Several mechanisms for the removal of bacteria, viruses, and other foreign particles from bivalve mollusks have been described (30, 57). Pioneering work by Stauber (52) revealed that India ink particles injected intracardially into oysters are phagocytosed and removed from the tissues as the phagocytic cells migrate across epithelial layers to the exterior. Subsequently Tripp (57) showed that yeast cells and bacterial spores are eliminated from the tissues of C. virginica in much the same manner as India ink particles. Initially, foreign particles clump in efferent vessels, and large numbers of hemocytes accumulate around the aggregates. Thereafter, most of the particles are engulfed by hemocytes, removed from circulation, and finally externalized by migration to the exterior or digested by the phagocytic cell (57). Furthermore, Timoney and Abston (56) demonstrated that hard clams, Mercenaria mercenaria, exposed to Escherichia coli and Salmonella enterica serovar Typhimurium eliminated test bacteria primarily via fecal and pseudofecal excretion, although the removal of S. enterica serovar Typhimurium was less complete (56). The intraphagocytic digestion of injected bacteria as well as the differences in the abilities of invertebrate hemocytes to successfully recognize, ingest, and degrade specific microorganisms has been documented by others (6, 28, 29). Collectively, these studies suggest that mollusks have more than one mechanism for the removal of bacteria and foreign particles. Moreover, inactivation, degradation, and elimination of injected bacteria vary with ambient water temperature (30, 53), as well as stress and stress-induced neuroendocrine fluctuations (36, 38), acid-base status, and levels of dissolved oxygen (8, 10, 33).
Hypoxia is fast becoming a major factor leading to the degradation of marine estuarine habitats worldwide (9, 12, 19). Estuarine habitats are well known for the development of low environmental oxygen levels, which occur naturally on a daily and seasonal basis (12). However, the frequency, extent, and duration of these hypoxic events are steadily increasing due to excessive anthropogenic input of nutrients and organic matter into coastal waters (17, 19). In addition, hypoxia is almost always accompanied by elevated levels of carbon dioxide (hypercapnia) produced by respiration, causing a decrease in water pH (12). Hypoxia and hypercapnic hypoxia (HH) can have a significant impact on benthic, sedentary organisms, such as oysters, triggering a broad range of physiological, biochemical, and genetic responses (2, 12, 13, 17). Environmental hypoxia adversely impacts immune mechanisms of marine invertebrates (10, 33), such as phenoloxidase (PO) activity (54) and in vitro hemolymph antimicrobial activity (47), and has been shown to reduce circulating hemocyte numbers (39) and suppress the production of ROS (8). Furthermore, hypoxia slows the elimination of culturable bacteria from tissues of the Atlantic blue crab, Callinectes sapidus (33), and the Pacific white shrimp, Litopenaeus vannamei (9).
Bacteria are extremely abundant in marine estuarine environments, ranging from approximately 109 bacteria liter−1 in water to 1012 bacteria liter−1 in sediment (22), and species of genera such as Pseudomonas and Vibrio, in particular, appear to dominate both the external surfaces and the internal organs of marine bivalves (48). Of the numerous human diseases that can be transmitted by oysters, Vibrio cholerae, Vibrio vulnificus, and Vibrio parahaemolyticus are significant causes of severe systemic infections, morbidity, and mortality (35, 46, 48). However, little is known about the inactivation, degradation, and elimination of Vibrio in C. virginica and how these processes might be altered by environmental hypoxia. Therefore, in the present study, we examined the ability of oysters to inactivate, degrade, and externalize Vibrio campbellii and how these processes are influenced by exposure to HH.
MATERIALS AND METHODS
Animal specimen collection and maintenance.
Adult C. virginica (Gmelin) specimens were collected from Schooners Creek in Charleston Harbor and immediately transferred to holding tanks at the Hollings Marine Laboratory, Charleston, SC. Oysters were collected from the low- and mid-intertidal zone at low tide and had shell heights ranging from 112 to 160 mm. All oysters were thoroughly cleaned to remove all mud and epibionts before being placed in the holding tanks. Specimens were held in well-aerated recirculating seawater systems maintained at 30-ppt salinity and 22°C with a 12-h light cycle. Oysters were held for a minimum of 3 days. Animals were fed an algal suspension (Shellfish Diet 1800; Reed Mariculture) every second day, but food was withheld for at least 24 h before experiments.
Preparation of experimental animals.
One day prior to experimentation, oysters were prepared for hemolymph sampling and intramuscular injection by drilling a 1-mm hole in the shell directly above the adductor muscle. Tripp (57) indicated that the adductor muscle was a suitable site for injecting foreign material since it is bathed by extensive blood channels that drain directly into systemic vessels, thus allowing injected material to rapidly reach systemic circulation. Two layers of dental rubber dam were glued over the drilled hole with cyanoacrylate glue to prevent leakage of the inocula and hemolymph following injection of bacteria and withdrawal of hemolymph, respectively. Thereafter, oysters were placed in 5-liter plastic beakers containing 4 liters of well-aerated natural seawater equilibrated to 25°C and 30-ppt salinity.
Preparation of the bacterial solution for injection.
The bacterial pathogen used in this study was a strain of V. campbellii, 90-69B3, transfected with the stable Vibrio-derived plasmid pEVS146 coding for kanamycin (Kan) and chloramphenicol (Cm) antibiotic resistance and the expression of green fluorescent protein (Eric Stabb, University of Georgia). The multicopy plasmid pEVS146 has no detectable effect on the growth rate of V. campbellii and is stably transmitted, being lost at a rate of approximately 10−4 per bacterial generation when bacteria are grown in the absence of antibiotics (11). Moreover, data from our laboratory have shown that multiple copies of marker genes in each bacterium allows for sensitive PCR-based detection of low numbers of V. campbellii in a variety of tissues (10, 11, 33, 41), making this a suitable model pathogen for investigating the fate of injected bacteria in oysters. The bacterium is also a known crustacean pathogen (10, 11, 26, 27), originally isolated from diseased shrimp by D. Lightner and L. Mahone (11), that has been isolated from the Pacific oyster, Crassostrea giga (50).
For each assay, V. campbellii bacteria were streaked onto tryptic soy agar supplemented with 2.5% NaCl and the antibiotics Kan (100 μg/ml) and Cm (5 μg/ml) and incubated overnight at 25°C. Following incubation, a small amount of the streaked bacteria was transferred into 5 ml of sterile HEPES-buffered saline (10 mM HEPES buffer plus 2.5% NaCl) and vortexed thoroughly to resuspend the bacteria. This solution was further diluted as required with HEPES-buffered saline to obtain an optical density of 0.1 at 540 nm, which has previously been determined to be equal to 1 × 108 V. campbellii CFU ml−1 (44). This stock suspension of bacteria was further diluted with HEPES-buffered saline to 1 × 106 Vibrio CFU ml−1. This dilution of bacteria was used for injecting oysters for the bacterial clearance assay.
Basic experimental protocol.
Oysters were held in 5-liter plastic beakers (four oysters per beaker) containing 4 liters of either well-aerated (normoxic) seawater (partial O2 pressure [PO2] = 20.7 kPa; CO2 < 0.06 kPa; pH 7.8 to 8.0) or hypoxic and hypercapnic seawater (PO2 = 4 kPa; CO2 = 1.8 kPa; pH 6.5 to 6.8). Animals were held under these two conditions for 4 h prior to receiving the V. campbellii injection. Following injection, animals were transferred to individual holding containers with 500 ml seawater preequilibrated to either normoxic or HH condition and remained under these conditions for the remainder of the experiment (up to 24 h postinjection). Specific oxygen and carbon dioxide levels for the HH treatment were maintained by gassing the water continuously with a mixture of N2, O2, and CO2 gases. All gases were mixed and delivered using a Pegas 4000MF gas-mixing pump (Columbus Instruments). The oxygen pressure in the water was continuously monitored using an oxygen electrode (YSI model 58; Yellow Springs, OH), while the pH of the water was routinely measured over the course of the experiment as an indirect measure of CO2 concentration. A CO2 level of 1.8 kPa corresponds to a pH of approximately 6.9 to 7.2. At the steady state, this system maintained constant O2 and CO2 pressures. Oysters were routinely monitored throughout the experiment to ensure that they were open and ventilating. Oysters were discarded when they did not open during the 4 h preexposure period and again at least 15 min following the Vibrio injection.
In a typical experiment, oysters that had been preexposed to either normoxic or HH seawater for 4 h were transferred to a bench top in the laboratory and maintained at a constant temperature of 25°C. The oyster weight, length, and width were recorded. Hemolymph was then sampled from the adductor muscle of each oyster immediately before injection with V. campbellii, and the total number of hemocytes was determined by counting (total hemocyte count [THC] ml−1 of hemolymph) according to the protocol described below. Following hemolymph withdrawal, oysters were injected with 100 μl of a stock solution of 1 × 106 V. campbellii bacteria ml−1 using a 100-μl glass Hamilton syringe fitted with a 26-gauge by -inch needle, directly into the adductor muscle through the injection port. The injection port was thoroughly cleaned with 100% ethanol immediately before and after injection, and a small drop of cyanoacrylate glue was placed over the site of injection as an additional precaution to prevent leakage of the inocula. Animals in which leakage occurred were discarded. Oysters were then transferred to individual holding containers with 500 ml seawater preequilibrated to either normoxic or HH conditions (25°C, 30 ppt salinity).
At 0, 1, 4, and 24 h after injection with bacteria, oysters were removed from the holding containers, dried thoroughly with paper towels, and shucked. Preliminary experiments that quantified bacteria in C. virginica following V. campbellii injection indicated that these time points would be optimal for discerning the impacts of HH on the ability of oysters to clear bacteria. Furthermore, Mikulski et al. (44) reported that HH had no direct impact on the growth and survival of V. campbellii (then putatively identified as V. parahaemolyticus) incubated under these HH conditions for up to 48 h. All tissue and fluid samples were collected and weighed together to the nearest 0.01 g. Tissue weight was recorded separately to the nearest 0.01 g. All shells and instruments (i.e., oyster knife, forceps, weighing boats) were rinsed with 200 ml of HEPES-buffered saline solution, supplemented with Antifoam A (0.05%) and Tween 20 (0.1%), and collected into a 500-ml Nalgene bottle to ensure that all bacteria were recovered. Preliminary experiments indicated that supplementing the wash and homogenization buffer with Antifoam A and Tween dramatically improved recovery of bacteria and did not interfere with quantitative real-time PCR (Q-PCR). The entire procedure for collecting and weighing the tissue and fluid samples took less than 1 minute. Tissue and body fluid samples were then added to the wash buffer and homogenized for 1.5 min using a Polytron PT 2100 tissue homogenizer. Aliquots (1 ml) of each homogenate were transferred to three separate 1.5-ml microcentrifuge tubes and stored at −70°C until they were used for Q-PCR to quantify intact (undegraded) bacteria, while aliquots of the remaining homogenate of each oyster were plated onto selective plates to quantify culturable bacteria according to the protocols described below. The procedures for homogenizing and plating samples took less than 4 min to complete. A total of eight oysters were sampled at each time point for each treatment, with oyster processing staggered to ensure that the shucking, weighing, homogenization, and plating procedures were first completed on one oyster before proceeding with the next.
Immediately after completing the homogenization and plating procedures, all visible feces and pseudofeces were removed from the holding containers with a sterile forceps and transferred to 1.5-ml microcentrifuge tubes. Tubes were centrifuged briefly at 10,000 × g for 5 s to pellet the samples. Supernatants were carefully removed and returned to their respective holding containers before adding 1 ml HEPES-buffered saline to each tube and vortexing thoroughly to resuspend the pelleted samples. Thereafter, seawater (500 ml) from each of the holding containers was carefully transferred to individual 500-ml Nalgene bottles. Tween 20 (0.1%) was added to each bottle and thoroughly mixed by inversion before filtering the seawater samples through 0.22-μm Millipore filters. Bacteria trapped on the filters were resuspended in 10 ml HEPES-buffered saline in 15-ml centrifuge tubes by vortexing thoroughly for approximately 1.5 min. Duplicate 0.25-ml aliquots of the resuspended fecal/pseudofecal samples and duplicate 1-ml aliquots of the filtered seawater samples were transferred to separate 1.5-ml microcentrifuge tubes and stored at −70°C until they were used for Q-PCR, while aliquots of the remaining samples were plated onto selective plates according to the protocols described below.
Quantification of THC.
Using an ice-cold 1-ml syringe fitted with a 26-gauge -inch needle, approximately 0.15 ml of hemolymph was withdrawn from the adductor muscle and immediately transferred into a 1.5-ml microcentrifuge tube that had been placed on ice. A 100-μl aliquot of this hemolymph sample was transferred to a second 1.5-ml microcentrifuge tube containing 100 μl of neutral buffered formaldehyde to fix the cells and prevent them from lysing. After mixing, an aliquot of the hemocyte suspension was transferred to a hemocytometer for direct counting under a light microscope. Three separate aliquots of the hemocyte suspension were counted, and the data were recorded as the mean (± the standard error [SE]) THC ml−1 of hemolymph for each oyster.
Quantification of culturable V. campbellii by selective plating.
Selective plating was used to quantify the total numbers of culturable V. campbellii bacteria remaining in the tissue, water, and fecal/pseudofecal samples of each oyster at each time point and treatment. Immediately following homogenization, triplicate 0.5- to 3-ml aliquots (depending on the time point tested) of the homogenate, duplicate 0.25-ml aliquots of the resuspended fecal/pseudofecal samples, and duplicate 2-ml aliquots of the filtered seawater samples were transferred to separate 15-ml centrifuge tubes containing 0.9 ml molten marine agar (55°C), supplemented with 200 μg ml−1 Kan and 50 μg ml−1 Cm, and gently mixed by inversion. Each marine agar overlay was then poured onto separate thiosulfate-citrate-bile-sucrose agar plates, supplemented with 200 μg ml−1 Kan and 50 μg ml−1 Cm. Plates were incubated at 25°C for 24 h, at which point the bacterial colonies were counted and the numbers were recorded. The selective plating assay can be used to accurately quantify as few as three culturable V. campbellii bacteria in 1 ml of tissue homogenate. Assuming that 20 g of tissue is homogenized in 200 ml of HEPES-buffered saline solution, this equates to approximately 30 culturable bacteria g−1 tissue. Aliquots of tissue homogenate from saline-injected control oysters were plated as negative controls to ensure that there was no growth on plates except for the injected V. campbellii.
Quantification of total (intact) V. campbellii bacteria by Q-PCR.
Q-PCR was used to quantify the total numbers of undegraded, i.e., intact, V. campbellii bacteria remaining in the tissue, seawater, and fecal/pseudofecal samples of C. virginica. As described by Burgents et al. (10, 11), quantification using Q-PCR was limited to plasmids associated with intact bacteria and not to plasmids from lysed or degraded bacteria. Therefore, intact bacterial numbers represent both culturable and nonculturable bacteria remaining in tissue, seawater and fecal samples which have remained sufficiently intact to retain plasmids. Consequently, the percentage of intact bacteria remaining culturable (percent culturable) in each sample at each time point was calculated by dividing the number of intact bacteria, as determined by PCR, by the number of culturable bacteria, as determined by selective plating, according to the formulae described by Macey et al. (41).
Tissue, seawater, and fecal/pseudofecal samples for Q-PCR were prepared according to Burgents et al. (11) with some minor modifications. Samples were centrifuged at 10,000 × g for 10 min at 4°C. The resulting supernatants were removed, and the pellets for the seawater and fecal/pseudofecal samples were resuspended in 100-μl volumes of InstaGene matrix (Bio-Rad catalog no. 732-6030), whereas the pellets from the tissue samples were resuspended in 300- to 800-μl aliquots of InstaGene matrix. This was done to account for slight differences in tissue weights and to obtain an equal tissue-weight-to- InstaGene-matrix ratio for each sample. InstaGene is a particulate matrix made up of negatively charged microscopic beads that chelate metal ions which are required as catalysts or cofactors in enzymatic reactions. The samples were thoroughly vortexed to resuspend the cell pellets and incubated for 20 min at 56°C on an orbital shaker set to rotate at 130 rpm. This incubation step helps soften plasma membranes, release clumps of cells from one another, and denature enzymes such as DNases. Following incubation, the samples were vortexed briefly before incubating the samples for a further 30 min at 95°C to lyse all of the cells. The lysed samples were allowed to cool at room temperature for 5 min, vortexed briefly and centrifuged at 18,000 × g for 5 min at 4°C. The resulting supernatants, containing the plasmids from lysed V. campbellii, were analyzed by Q-PCR. A 129-bp fragment of the Kan resistance gene was amplified using 400 nM forward primer RTKnF 5′-TGATGCGCTGGCAGTGTT-3′, 400 nM reverse primer RTKnR 5′-CTCGCATCAACCAAACCGTTA-3′, and 200 nM TaqMan probe 5′-TGCGCCGGTTGCATTCGATTCCTGT-3′, 5′ labeled with 6-carboxyfluorescein and 3′ labeled with Black Hole Quencher-1 (Integrated DNA Technologies, Inc., Coralville, IA). The reaction mixtures (50 μl) were prepared using the Qiagen QuantiTect probe PCR kit (catalog no. 204443). Amplification was monitored using Applied Biosystem's 7500 sequence detection system and consisted of 15 min of incubation at 95°C to activate the hot-start polymerase, followed by 50 cycles of denaturation at 95°C for 30 s and a combined annealing and elongation step at 60°C for 45 s.
A standard curve for the tissue samples was constructed by spiking known numbers of culturable V. campbellii bacteria into tissue samples obtained from saline-injected control oysters, allowing us to accurately compare the numbers of intact and culturable bacteria in the tissue of oysters, as described previously by Burgents et al. (11). Since each V. campbellii cell contains between 10 and 15 copies of plasmid pEVS146 bearing the Kan resistance gene (Eric Stabb, University of Georgia), we established that the Q-PCR assay can be used to accurately quantify as few as one V. campbellii cell per 50-μl reaction mixture volume. This equates to approximately 571 intact bacteria g−1 tissue, assuming that 1 ml of tissue homogenate (20 g tissue homogenized in 200 ml HEPES-buffered saline solution) was resuspended in 400 μl InstaGene matrix and that 7 μl of the supernatant was included in the Q-PCR volume. Similarly, a standard curve for the seawater and fecal/pseudofecal samples was constructed by spiking known numbers of V. campbellii bacteria into HEPES-buffered saline solution. Seawater, fecal/pseudofecal, and homogenate samples from saline-injected control oysters, along with nontemplate controls (PCR-grade H2O), were analyzed as negative controls to ensure that there was no amplification except for the injected V. campbellii bacteria. Aliquots of tissue from a single Vibrio-injected oyster were also analyzed during each PCR to ensure consistency between Q-PCRs.
Statistical analysis.
SigmaStat 3.1 software was used to perform all statistical analyses. Data for THC ml−1 hemolymph were log transformed prior to statistical analysis. To determine whether THCs ml−1 hemolymph differed between oysters preexposed to normoxia and HH for 4 h, a one-way analysis of variance (ANOVA) was performed. To determine whether the number of intact and culturable V. campbellii or percent culturable V. campbellii bacteria in the tissue changed as a function of time after injection with bacteria, a two-way ANOVA was performed. Tests for normality (Kolmogorov-Smirnov test) failed for these data sets; therefore, a two-way ANOVA on rank-transformed data was used to test for significant differences. To determine whether the percent recovery of intact and culturable V. campbellii bacteria in the seawater and fecal/pseudofecal samples changed as a function of time after injection with bacteria, a two-way ANOVA was performed on rank-transformed data. The Holm-Sidak method was used for post hoc multiple comparisons between individual time points. Significance was assigned to P values of <0.05 for t test, two-way ANOVA, and Holm-Sidak analysis.
RESULTS
Effects of prior exposure to HH on THC.
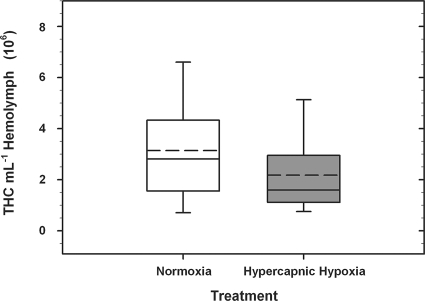
The densities of the hemocytes in the hemolymph of oysters ranged between 2.93 × 105 and 8.32 × 106 hemocytes ml−1 hemolymph for oysters exposed to fully air-saturated seawater (n = 24) and between 5.78 × 105 and 7.16 × 106 hemocytes ml−1 hemolymph for oysters exposed to HH seawater (n = 23) for 4 h (Fig. 1). In a pairwise comparison, there was no significant difference in THCs in the hemolymph of oysters from the two treatment groups (one-way ANOVA; P = 0.155).
FIG. 1.
Box plots of THC ml−1 hemolymph in Crassostrea virginica exposed to either fully air-saturated (normoxic) or HH seawater for 4 h. Data represent the mean (horizontal dashed line) and median (horizontal solid line) values (±SE [whiskers]) of the 23 and 24 oysters in the normoxic and HH treatment groups, respectively.
Impact of HH on the recovery of culturable V. campbellii in tissue.
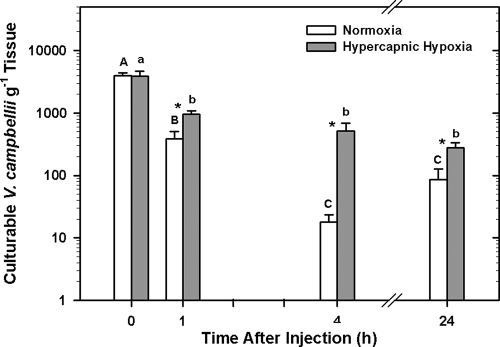
Exposure to HH had a significant effect on the number of culturable bacteria recovered from the tissue and body fluid samples of oysters injected with bacteria (two-way ANOVA; P < 0.001) (Fig. 2). Fewer culturable V. campbellii bacteria g−1 tissue were detected at 1, 4, and 24 h in oysters maintained in fully air-saturated water than in oysters preexposed and maintained for the same time periods in HH (Holm-Sidak method; P = 0.013, P = 0.001, and P < 0.001, respectively).
FIG. 2.
Mean log10 number (±SE; n = 8 animals) of culturable Vibrio campbellii bacteria g−1 tissue recovered from Crassostrea virginica at 0, 1, 4, and 24 h after receiving an injection containing 1 × 105 Vibrio campbellii bacteria. Oysters were exposed to either fully air-saturated (normoxic) or HH seawater for 4 h before receiving the injection containing bacteria and remained under these conditions for the duration of the experiment (up to 24 h postinjection). Significant differences between normoxia- and HH-exposed animals at each time point were assessed by analyzing rank-transformed culturable bacterial data by two-way ANOVA using the Holm-Sidak method and are indicated by asterisks, whereas significant differences between each time point within normoxia- and HH-exposed animals are indicated by different upper- and lowercase letters, respectively.
In both treatment groups, the numbers of culturable V. campbellii bacteria g−1 tissue decreased significantly over time (two-way ANOVA; P < 0.001). Within the normoxia treatment group, the number of culturable bacteria in oyster tissue decreased significantly between 0 and 1 h and 1 and 4 h (Holm-Sidak method; P < 0.001 and P = 0.004, respectively), but not between 4 and 24 h. In contrast, numbers of culturable V. campbellii bacteria in the HH treatment group decreased only between 0 and 1 h after injection with bacteria (Holm-Sidak; P = 0.002). No significant differences could be detected in the numbers of V. campbellii bacteria g−1 tissue at any of the other time points tested.
Impact of HH on the recovery of intact (undegraded) V. campbellii in tissue.
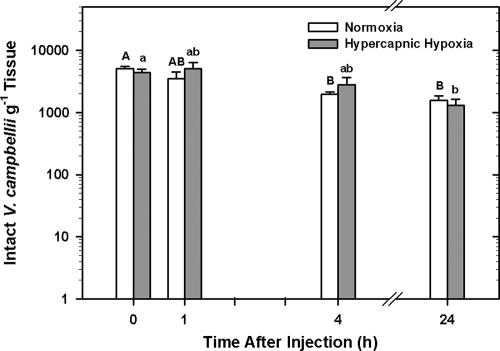
Exposure to HH did not alter the ability of oysters to eliminate intact bacteria from their tissues, compared to that of oysters exposed to fully air-saturated water (two-way ANOVA; P = 0.902). However, there was a significant effect of time on the elimination of intact bacteria in both treatment groups (two-way ANOVA; P < 0.001). Approximately 3 to 5 min following injection (0 h time point), 84.4% ± 8.7% (5,039 ± 472 V. campbellii cells g−1 tissue) and 81.4% ± 10.5% (4,358 ± 581 V. campbellii cells g−1 tissue) of the injected bacteria could be recovered from the normoxia and HH treatment groups, respectively (Fig. 3). By 24 h after injection, the number of intact bacteria in oyster tissues had declined in both treatment groups, with recovery of 31.3% ± 5.9% (two-way ANOVA; P = 0.001) for normoxia and 23.6% ± 6.6% (P = 0.002) for HH.
FIG. 3.
Mean log10 number (±SE; n = 8 animals) of total (intact) Vibrio campbellii bacteria g−1 tissue recovered from Crassostrea virginica at 0, 1, 4, and 24 h after receiving an injection containing 1 × 105 Vibrio campbellii bacteria. Oysters were exposed to either fully air-saturated (normoxic) or HH seawater for 4 h before receiving the injection containing bacteria and remained under these conditions for the remainder of the experiment (up to 24 h postinjection). Significant differences between normoxia- and HH-exposed animals at each time point were assessed by analyzing rank-transformed culturable bacterial data by two-way ANOVA using the Holm-Sidak method and are indicated by asterisks, whereas significant differences between each time point within normoxia- and HH-exposed animals are indicated by different upper- and lowercase letters, respectively.
Percentage of culturable V. campbellii bacteria.
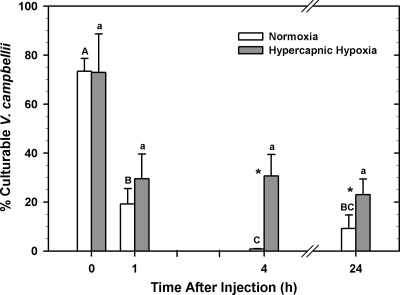
In both treatment groups, the percentage of intact V. campbellii bacteria that could be cultured decreased significantly with time (two-way ANOVA; P < 0.001) (Fig. 4). Furthermore, treatment significantly impacted the culturability of intact V. campbellii (two-way ANOVA; P = 0.003). Pairwise comparisons indicated that exposure to HH increased the culturability of intact V. campbellii at 4 and 24 h after injection with bacteria (Holm-Sidak method; P < 0.001 and P = 0.027, respectively).
FIG. 4.
Mean percentages (±SE; n = 8 animals), determined by using real-time PCR, of intact Vibrio campbellii bacteria g−1 tissue that were culturable using selective plating at 0, 1, 4, and 24 h after injecting 1 × 105 V. campbellii bacteria into the adductor muscle of Crassostrea virginica. Oysters were exposed to either fully air-saturated (normoxic) or HH seawater for 4 h before receiving the injection containing bacteria and remained under these conditions for the remainder of the experiment (up to 24 h postinjection). Significant differences in the percentages of culturable V. campbellii bacteria between normoxia- and HH-exposed oysters at each time point were assessed by analyzing rank-transformed data by two-way ANOVA with the Holm-Sidak method and are indicated by asterisks, whereas significant differences between each time point within normoxia- and HH-exposed animals are indicated by different upper- and lowercase letters, respectively.
Recovery of intact and culturable V. campbellii bacteria in seawater and feces/pseudofeces.
At 4 and 24 h after injection with V. campbellii, intact bacteria were detected in the holding water of oysters exposed to normoxia (1.66% and 1.43% of the injected dose, respectively) (Table 1). However, culturable bacteria were detected in the seawater of normoxia-treated oysters only at the 24 h time point (0.3% of the injected dose). In contrast, both intact and culturable bacteria were detected in the holding water of HH-exposed oysters at 1, 4, and 24 h after injection (Table 1). There was no significant effect of treatment or time on the numbers of bacteria detected in the seawater samples (two-way ANOVA; P > 0.05).
TABLE 1.
Recovery of intact and culturable V. campbellii in seawater (500 ml)
| Time after injection (h) | % Recovery (mean ± SE) of indicated V. campbellii from oysters exposed to:
|
|||
|---|---|---|---|---|
| Normoxia
|
HH
|
|||
| Intact | Culturable | Intact | Culturable | |
| 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0.600 ± 0.652 | 0.187 ± 0.122 |
| 4 | 1.660 ± 3.104 | 0.004 ± 0.003 | 8.210 ± 14.548 | 1.048 ± 0.464 |
| 24 | 1.430 ± 2.220 | 0.217 ± 0.208 | 1.241 ± 0.147 | 0.013 ± 0.008 |
Intact and culturable bacteria were detected in feces and pseudofeces particulates of normoxia-treated oysters at 1, 4, and 24 h after injection of V. campbellii (Table 2). However, very few of the injected bacteria were recovered from feces/pseudofeces. By 24 h after injection, less than 0.01% and 0.15% of the dose were recovered as culturable and intact V. campbellii, respectively. Fecal and pseudofecal samples were obtained from less than 10% of oysters exposed to HH conditions (data not shown), suggesting that HH may have an effect on digestive processes.
TABLE 2.
Recovery of intact and culturable V. campbellii in feces/pseudofeces
| Time after injection (h) | % Recovery (mean ± SE) of indicated V. campbellii
|
|
|---|---|---|
| Intact | Culturable | |
| 0 | 0 | 0 |
| 1 | 0.042 ± 0.020 | 0.019 ± 0.019 |
| 4 | 0.059 ± 0.032 | 0 |
| 24 | 0.123 ± 0.053 | 0.006 ± 0.042 |
DISCUSSION
HH dramatically reduced the ability of oysters to render V. campbellii nonculturable over a 24-h period (Fig. 2). Thus, HH appears to strongly suppress the immune mechanisms that prevent the growth of bacteria (bacteriostasis) in oysters. In contrast, HH did not alter the ability of oysters to degrade or remove bacteria from their tissues. The numbers of intact bacteria in both treatments decreased over the test period (Fig. 3); however, there are still significant numbers of intact (undegraded) bacteria present in oyster tissues at 24 h after bacterial challenge. These findings are consistent with the results of Holman et al. (33) and Burgents et al. (10), who showed that HH impairs the inactivation of live, culturable bacteria in the tissues of the crustaceans C. sapidus and Litopenaeus vannamei but does not impede the degradation or removal of intact bacteria.
Circulating hemocytes are regarded as primary mediators of the innate immune response in oysters and are responsible for recognition, binding, and internalization of foreign particles (14). As a result, THCs are often used as a general indicator of invertebrate immune status, where changes in circulating hemocyte numbers, above or below basal levels, may indicate stress (38, 39). Alvarez et al. (3) demonstrated that oysters exposed to anoxia (6% air saturation) for 72 h had significantly lower hemocyte counts than oysters held in fully air-saturated water. Similarly, circulating hemocyte numbers were significantly lower in the shrimp Penaeus stylirostris and the freshwater prawn Macrobrachium rosenbergii exposed to severe hypoxia (<30% air saturation) for 24 h and 120 h, respectively (15, 39). In contrast, we found no effect on circulating hemocyte counts after exposing oysters to severe hypoxia (20% air saturation) and hypercapnia (2% CO2) for 4 h (Fig. 1), suggesting that circulating hemocyte numbers may not have contributed to the increased recovery of culturable bacteria in oysters exposed to HH. Oysters inhabiting the low- and mid-intertidal zones of estuarine environments are well adapted to endure short durations of severe hypoxia (13), so it may be that long-term exposure to severe hypoxia (>4 h) is required to induce a significant change in circulating hemocyte numbers in C. virginica.
Low dissolved oxygen levels have been found to adversely affect specific humoral immune functions (7, 8, 39, 54), which may account for the reduced clearance of culturable V. campbellii observed in the present study. In vitro studies found that environmental hypoxia can reduce PO activity (54) and hemolymph antimicrobial activity (47) in blue crabs, Callinectes sapidus, and suppress the generation of ROS in the shrimp Penaeus stylirostris (39). PO activity has been documented for several molluscan species (4, 32, 40, 45), although sensitivities of PO and antimicrobial peptides to oxygen in molluscan species are unreported. However, Boyd and Burnett (8) have shown that production of ROS decreased by 67% in oyster hemocytes incubated under in vitro conditions of dissolved gas and pH that occur in the hemolymph of hypoxia-exposed oysters compared to that in control cells incubated under in vivo hemolymph conditions of oysters held in normoxia. This decrease was shown to be due to the specific and independent effects of lower oxygen levels (64% of normoxia) and low pH (44% of normoxia) in the hemolymph of oysters exposed to hypoxia. In contrast, Alvarez et al. (3) found that hypoxia had no effect on in vitro hemocyte phagocytic activity in C. virginica. However, unlike in the Boyd and Burnett (8) study, hemocytes in the Alvarez et al. (3) study were not incubated at the physiological oxygen pressures that exist in oyster hemolymph during hypoxia. Nonetheless, it is likely that other immune mechanisms or physiological processes are affected by low dissolved oxygen and pH levels in oysters that may lead to the reduced elimination of culturable bacteria observed in this study.
The present study is unique in that both intact and culturable V. campbellii bacteria were quantified simultaneously in whole oysters and their holding water for as long as 24 h following injection with live bacteria. The pool of intact bacteria in oyster tissues is comprised of culturable bacteria plus bacteria whose growth has been inhibited (nonculturable) but that have not been degraded or externalized. Moreover, determining the percentage of intact bacteria that remained culturable, using selective plating, allowed us to determine the amount of bacteriostasis occurring within whole-oyster tissues with time. The rapid reduction in the number of culturable bacteria in oyster tissues mirrors the results of others and confirms previous investigations examining depuration of fecal pollutants, such as Escherichia coli, Salmonella spp., and Shigella spp., as well as Vibrio spp., such as Vibrio cholerae and Vibrio vulnificus (35, 46). Previous studies have suggested that a vast majority of injected bacteria or particles are engulfed by circulating hemocytes and are removed by externalization across epithelia, possibly without being degraded (52, 57). However, Hartland and Timoney (30) observed that bacterial numbers in the seawater surrounding C. virginica and M. mercenaria inoculated with bacteria were lower than expected if the majority of the inoculated bacteria were being transported across epithelial borders by phagocytes into the surrounding seawater, suggesting intracellular and/or extracellular degradation. By quantifying intact bacteria by using the combination of culture and Q-PCR approaches, we have confirmed that a relatively small percentage (<10%) of injected bacteria do appear in the holding water of oysters injected with V. campbellii. In contrast to Timoney and Abston (56), who demonstrated that the greatest concentrations of E. coli and S. enterica serovar Typhimurium inoculated into M. mercenaria are eliminated in feces and pseudofeces, we recovered very few (<1%) injected bacteria in feces and pseudofeces. Other laboratories have suggested that another mechanism is employed by oysters for eliminating bacteria, namely degradation of bacteria by enzymatic action (6, 30, 57). Our results confirm these observations, demonstrating that approximately 90% of injected V. campbellii bacteria are inactivated and degraded within oysters over 24 h. However, this could be a reflection of the different route of exposure, strain of bacteria, and the invertebrate species employed in this study.
It was interesting to note that injected bacteria are rapidly converted from a culturable to a nonculturable status in the tissues of oysters exposed to fully air-saturated seawater, with less than 10% of the injected V. campbellii bacteria remaining culturable by 24 h (Fig. 4). This observation may indicate a strategy employed by oysters to rapidly prevent growth and spread of bacteria to surrounding tissues. The ability to rapidly inactivate bacteria could be attributed to the high intensity and titer of agglutinins in the plasma of C. virginica (21), which are thought to enhance phagocytosis, ROS production, and lysosomal degradation of foreign particles (23). However, we demonstrated that oysters exposed to HH are less capable of inactivating bacteria, with approximately 22% of the injected V. campbellii bacteria remaining culturable by 24 h, compared with 10% for oysters maintained in well-aerated water (Fig. 4), suggesting that one or more of these immune processes are sensitive to low dissolved oxygen and/or pH levels.
Although the results of previous studies (3, 8, 54) provide compelling evidence that inhibition of immune mechanisms may be responsible for the greater number of culturable V. campbellii bacteria recovered from tissues of oysters exposed to HH, other direct or indirect effects of low dissolved oxygen and pH levels on the physiology of oysters cannot be overlooked as a possible source for the effects observed in this study. Following exposure to low levels of dissolved oxygen, the typical response of most marine benthic invertebrates is a reduction in heart rate and oxygen consumption (18, 51), which occurs commonly in species with a high capacity for anaerobic respiration, such as the Eastern oyster (13). Furthermore, exposure to low levels of dissolved oxygen has also been shown to cause changes in the partitioning of cardiac output into the arterial distribution system (1) and in the perfusion of tissues, such as respiratory surfaces (55). These changes may alter the accumulation and distribution of hemocytes and/or bacteria in oysters, resulting in the reduced inactivation of culturable V. campbellii in whole-oyster tissues observed in the present study. Indeed, previous research has shown that specific tissues and organs, as well as fixed phagocytic cells within these tissues and organs, play an important role in the inactivation and degradation of injected bacteria and viruses (11, 34, 42, 43, 58). If the rate and distribution of hemolymph are changed, this could have a profound impact on these processes.
The present study demonstrates that oysters maintained under optimal environmental conditions are very efficient at inactivating and degrading large numbers of injected bacteria within their tissues. Furthermore, most injected bacteria are eliminated by being degraded within the tissues of oysters, with only a small percentage of the bacteria being released into the surrounding seawater. This study shows that hypoxia and low pH adversely affect the ability of oysters to render bacteria nonculturable. This finding, together with the observation that a large proportion of the bacteria eliminated into the surrounding seawater are culturable, may be important from a human health and ecological perspective. We suggest that oysters inhabiting hypoxic environments may pose a greater risk for harboring and transmitting bacteria that are potentially harmful to humans, oysters, and the environment.
Acknowledgments
This report is based upon work supported by a grant (DBI-0552828) from the U.S. National Science Foundation and support from the U.S. Department of Defense ASSURE program and the NOAA Center of Excellence in Oceans and Human Health at the Hollings Marine Laboratory, as part of the National Centers for Coastal Ocean Science.
This is contribution no. 326 of the Grice Marine Laboratory.
Footnotes
Published ahead of print on 1 August 2008.
REFERENCES
- 1.Airriess, C. N., and B. R. McMahon. 1994. Cardiovascular adaptations enhance tolerance of environmental hypoxia in the crab, Cancer magister. J. Exp. Biol. 190:23-41. [DOI] [PubMed] [Google Scholar]
- 2.Allen, S. M., and L. E. Burnett. 2008. The effects of intertidal air exposure on the respiratory physiology and the killing activity of hemocytes in the Pacific oyster, Crassostrea gigas (Thunberg). J. Exp. Mar. Biol. Ecol. 357:165-171. [Google Scholar]
- 3.Alvarez, M. R., F. E. Friedl, C. M. Hudson, and R. L. O'Neil. 1992. Effects of hypoxic and hyperoxic conditions on hemocyte activity and abiotic particle retention by the Eastern oyster, Crassostrea virginica (Gmelin, 1791). J. Shellfish Res. 11:383-386. [Google Scholar]
- 4.Asokan, R., M. Arumugam, and P. Mullainadhan. 1997. Activation of prophenoloxidase in the plasma and haemocytes of the marine mussel Perna viridis Linnaeus. Dev. Comp. Immunol. 21:1-12. [DOI] [PubMed] [Google Scholar]
- 5.Bachère, E., Y. Gueguen, M. Gonzalez, J. de Lorgeril, J. Garnier, and B. Romestand. 2004. Insights into the anti-microbial defense of marine invertebrates: the penaeid shrimps and the oyster Crassostrea gigas. Immunol. Rev. 198:149-168. [DOI] [PubMed] [Google Scholar]
- 6.Bayne, C. J. 1973. Molluscan internal defense mechanisms: the fate of C14-labelled bacteria in the land snail Helix pomatia (L.). J. Comp. Physiol. 86:17-25. [Google Scholar]
- 7.Boleza, K. A., L. E. Burnett, and K. G. Burnett. 2001. Hypercapnic hypoxia compromises bactericidal activity of fish anterior kidney cells against opportunistic environmental pathogens. Fish Shellfish Immunol. 11:593-610. [DOI] [PubMed] [Google Scholar]
- 8.Boyd, J. N., and L. E. Burnett. 1999. Reactive oxygen intermediate production by oyster hemocytes exposed to hypoxia. J. Exp. Biol. 202:3135-3143. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer, M., P. Larkin, N. Brown-Peterson, C. King, S. Manning, and N. Denslow. 2004. Effects of hypoxia on gene and protein expression in the blue crab, Callinectes sapidus. Mar. Environ. Res. 58:787-792. [DOI] [PubMed] [Google Scholar]
- 10.Burgents, J. E., K. G. Burnett, and L. E. Burnett. 2005. Effects of hypoxia and hypercapnic hypoxia on the localization and the elimination of Vibrio campbellii in Litopenaeus vannamei, the Pacific white shrimp. Biol. Bull. 208:159-168. [DOI] [PubMed] [Google Scholar]
- 11.Burgents, J. E., L. E. Burnett, E. V. Stabb, and K. G. Burnett. 2005. Localization and bacteriostasis of Vibrio introduced into the Pacific white shrimp, Litopenaeus vannamei. Dev. Comp. Immunol. 29:681-691. [DOI] [PubMed] [Google Scholar]
- 12.Burnett, L. E. 1997. The challenges of living in hypoxic and hypercapnic aquatic environments. Am. Zool. 37:633-640. [Google Scholar]
- 13.Burnett, L. E., and W. B. Stickle. 2001. Physiological responses to hypoxia, p. 101-114. In N. N. Rabalais and R. E. Turner (ed.), Coastal hypoxia: consequences for living resources and ecosystems, coastal and estuarine studies, vol. 57. American Geophysical Union, Washington, DC. [Google Scholar]
- 14.Canesi, L., G. Gallo, M. Gavioli, and C. Pruzzo. 2002. Bacteria-hemocyte interactions and phagocytosis in marine bivalves. Microsc. Res. Tech. 57:469-476. [DOI] [PubMed] [Google Scholar]
- 15.Cheng, W., C.-H. Liu, J.-P. Hsu, and J.-C. Chen. 2002. Effect of hypoxia on the immune response of giant freshwater prawn Macrobrachium rosenbergii and its susceptibility to pathogen Enterococcus. Fish Shellfish Immunol. 13:351-365. [DOI] [PubMed] [Google Scholar]
- 16.Chu, F.-L. E., A. K. Volety, R. C. Hale, and Y. Huang. 2002. Cellular responses and disease expression in oysters (Crassostrea virginica) exposed to suspended field contaminated sediments. Mar. Environ. Res. 53:17-35. [DOI] [PubMed] [Google Scholar]
- 17.David, E., A. Tanguy, K. Pichavant, and D. Moraga. 2005. Response of the Pacific oyster Crassostrea gigas to hypoxia exposure under experimental conditions. FEBS J. 272:5635-5652. [DOI] [PubMed] [Google Scholar]
- 18.deFur, P. L., and C. P. Mangum. 1979. The effects of environmental variables on the heart rates of invertebrates. Comp. Biochem. Physiol. A 62:283-294. [Google Scholar]
- 19.Diaz, R. J. 2001. Overview of hypoxia around the world. J. Environ. Qual. 30:275-281. [DOI] [PubMed] [Google Scholar]
- 20.Fisher, W. S. 2004. Antimicrobial activity of copper and zinc accumulated in Eastern oyster amebocytes. J. Shellfish Res. 23:321-351. [Google Scholar]
- 21.Fisher, W. S., and A. R. DiNuzzo. 1991. Agglutination of bacteria and erythrocytes by serum from six species of marine molluscs. J. Invertebr. Pathol. 57:380-394. [DOI] [PubMed] [Google Scholar]
- 22.Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541-548. [DOI] [PubMed] [Google Scholar]
- 23.Genthner, F. J., A. K. Volety, L. M. Oliver, and W. S. Fisher. 1999. Factors influencing in vitro killing of bacteria by hemocytes of the Eastern oyster (Crassostrea virginica). Appl. Environ. Microbiol. 65:3015-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez, M., B. Romestand, J. Fievet, A. Huvet, M. C. Lebart, Y. Gueguen, and E. Bachère. 2005. Evidence in oyster of a plasma extracellular superoxide dismutase which binds LPS. Biochem. Biophys. Res. Commun. 338:1089-1097. [DOI] [PubMed] [Google Scholar]
- 25.Griffin, D. W., K. A. Donaldson, J. H. Paul, and J. B. Rose. 2003. Pathogenic human viruses in coastal waters. Clin. Microbiol. Rev. 16:129-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hameed, A. S., P. V. Rao, J. J. Farmer, F. W. Hickman-Brenner, and G. R. Fanning. 1996. Characteristics and pathogenicity of a Vibrio campbellii-like bacterium affecting hatchery-reared Penaeus indicus (Milne Edwards, 1837) larvae. Aquac. Res. 27:853-863. [Google Scholar]
- 27.Hameed, A. S. S. 1995. Susceptibility of three Penaeus species to a Vibrio campbellii-like bacterium. J. World Aquac. Soc. 26:315-319. [Google Scholar]
- 28.Harris-Young, L., M. L. Tamplin, W. S. Fisher, and J. W. Mason. 1993. Effects of physicochemical factors and bacterial colony morphotype on association of Vibrio vulnificus with hemocytes of Crassostrea virginica. Appl. Environ. Microbiol. 59:1012-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris-Young, L., M. L. Tamplin, J. W. Mason, H. C. Aldrich, and J. K. Jackson. 1995. Viability of Vibrio vulnificus in association wih hemocytes of the American oyster (Crassotrea virginica). Appl. Environ. Microbiol. 61:52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartland, B. J., and J. F. Timoney. 1979. Vivo clearance of enteric bacteria from the hemolymph of the hard clam and the American oyster. Appl. Environ. Microbiol. 37:517-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hégaret, H., G. H. Wikfors, and P. Soudant. 2003. Flow cytometric analysis of haemocytes from Eastern oysters, Crassostrea virginica, subjected to a sudden temperature elevation: II. Haemocyte functions: aggregation, viability, phagocytosis, and respiratory burst. J. Exp. Mar. Biol. Ecol. 293:249-265. [Google Scholar]
- 32.Hellio, C., A. Bado-Nilles, B. Gagnaire, T. Renault, and H. Thomas-Guyon. 2007. Demonstration of a true phenoloxidase activity and activation of a ProPO cascade in Pacific oyster, Crassostrea gigas (Thunberg) in vitro. Fish Shellfish Immunol. 22:433-440. [DOI] [PubMed] [Google Scholar]
- 33.Holman, J. D., K. G. Burnett, and L. E. Burnett. 2004. Effects of hypercapnic hypoxia on the clearance of Vibrio campbellii in the Atlantic blue crab, Callinectes sapidus Rathbun. Biol. Bull. 206:188-196. [DOI] [PubMed] [Google Scholar]
- 34.Johnson, P. T. 1987. A review of fixed phagocytic and pinocytotic cells of decapod crustaceans, with remarks on hemocytes. Dev. Comp. Immunol. 11:679-704. [DOI] [PubMed] [Google Scholar]
- 35.Kelly, M. T., and A. Dinuzzo. 1985. Uptake and clearance of Vibrio vulnificus from Gulf Coast oysters (Crassostrea virginica). Appl. Environ. Microbiol. 50:1548-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lacoste, A., F. Jalabert, S. K. Malham, A. Cueff, and S. A. Poulet. 2001. Stress and stress-induced neuroendocrine changes increase the susceptibility of juvenile oysters (Crassostrea gigas) to Vibrio splendidus. Appl. Environ. Microbiol. 67:2304-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacoste, A., S. K. Malham, A. Cueff, and S. A. Poulet. 2001. Noradrenaline modulates oyster hemocyte phagocytosis via a beta-adrenergic receptor-cAMP signaling pathway. Gen. Comp. Endocrinol. 122:252-259. [DOI] [PubMed] [Google Scholar]
- 38.Lacoste, A., S. K. Malham, F. Gelebart, A. Cueff, and S. A. Poulet. 2002. Stress-induced immune changes in the oyster Crassostrea gigas. Dev. Comp. Immunol. 26:1-9. [DOI] [PubMed] [Google Scholar]
- 39.Le Moullac, G., C. Soyez, D. Saulnier, D. Ansquer, J. C. Avarre, and P. Levy. 1998. Effect of hypoxic stress on the immune response and the resistance to vibriosis of the shrimp Penaeus stylirostris. Fish Shellfish Immunol. 8:621-629. [Google Scholar]
- 40.Luna-González, A., A. N. Maeda-Martínez, F. Vargas-Albores, F. Ascencio-Valle, and M. Robles-Mungaray. 2003. Phenoloxidase activity in larval and juvenile homogenates and adult plasma and haemocytes of bivalve molluscs. Fish Shellfish Immunol. 15:275-282. [DOI] [PubMed] [Google Scholar]
- 41.Macey, B. M., C. K. Rathburn, L. K. Thibodeaux, L. E. Burnett, and K. G. Burnett. 2008. Clearance of Vibrio campbellii injected into the hemolymph of Callinectes sapidus, the Atlantic blue crab: the effects of prior exposure to bacteria and environmental hypoxia. Fish Shellfish Immunol. doi: 10.1016/j.fsi.2008.02.009. [DOI] [PubMed]
- 42.Martin, G. G., J. E. Hose, G. Minka, and S. Rosenberg. 1996. Clearance of bacteria injected into the hemolymph of the ridgeback prawn, Sicyonia ingentis (Crustacea: Decapoda): role of hematopoietic tissue. J. Morphol. 227:227-233. [DOI] [PubMed] [Google Scholar]
- 43.Martin, G. G., D. Poole, C. Poole, J. E. Hose, M. Arias, L. Reynolds, N. McKrell, and A. Whang. 1993. Clearance of bacteria injected into the hemolymph of the penaeid shrimp, Sicyonia ingentis. J. Invertebr. Pathol. 62:308-315. [Google Scholar]
- 44.Mikulski, C. M., L. E. Burnett, and K. G. Burnett. 2000. The effects of hypercapnic hypoxia on the survival of shrimp challenged with Vibrio parahaemolyticus. J. Shellfish Res. 19:301-311. [Google Scholar]
- 45.Muñoz, P., J. Meseguer, and M. Á. Esteban. 2006. Phenoloxidase activity in three commercial bivalve species. Changes due to natural infestation with Perkinsus atlanticus. Fish Shellfish Immunol. 20:12-19. [DOI] [PubMed] [Google Scholar]
- 46.Murphree, R. L., and M. L. Tamplin. 1995. Uptake and retention of Vibrio cholerae O1 in the Eastern oyster, Crassostrea virginica. Appl. Environ. Microbiol. 61:3656-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noga, E. J., D. P. Engel, T. W. Arroll, S. McKenna, and M. Davidian. 1994. Low serum antibacterial activity coincides with increased prevalence of shell disease in blue crabs Callinectes sapidus. Dis. Aquat. Organ. 19:121-128. [Google Scholar]
- 48.Olafsen, J. A., H. V. Mikkelsen, H. M. Giæver, and G. Hφvik Hansen. 1993. Indigenous bacteria in hemolymph and tissues of marine bivalves at low temperatures. Appl. Environ. Microbiol. 59:1848-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliver, L. M., and W. S. Fisher. 1999. Appraisal of prospective bivalve immunomarkers. Biomarkers 4:510-530. [DOI] [PubMed] [Google Scholar]
- 50.Shen, X., Y. Cai, W. Fang, R. Gu, and D. Gao. 2005. Identification of Vibrio campbellii isolated from cultured pacific oyster. Acta Microbiol. Sin. 45:177-180. [PubMed] [Google Scholar]
- 51.Shumway, S. E., and R. K. Koehn. 1982. Oxygen consumption in the American oyster Crassostrea virginica. Mar. Ecol. Prog. Ser. 9:59-68. [Google Scholar]
- 52.Stauber, L. A. 1950. The fate of India ink injected intracardially into the oyster, Ostrea virginica Gmelin. Biol. Bull. 98:227-241. [DOI] [PubMed] [Google Scholar]
- 53.Tamplin, M. L., and G. M. Capers. 1992. Persistence of Vibrio vulnificus in tissues of Gulf Coast oysters, Crassostrea virginica, exposed to seawater disinfected with UV light. Appl. Environ. Microbiol. 58:1506-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanner, C. A., L. E. Burnett, and K. G. Burnett. 2006. The effects of hypoxia and pH on phenoloxidase activity in the Atlantic blue crab, Callinectes sapidus. Comp. Biochem. Physiol. A 144:218-223. [DOI] [PubMed] [Google Scholar]
- 55.Taylor, A. C., and A. R. Brand. 1975. A comparative study of the respiratory responses of the bivalves Arctica islandica (L.) and Mytilus edulis L. to declining oxygen tension. Proc. R. Soc. Lond. B 190:443-456. [DOI] [PubMed] [Google Scholar]
- 56.Timoney, J. F., and A. Abston. 1984. Accumulation and elimination of Escherichia coli and Salmonella typhimurium by hard clams in an in vitro system. Appl. Environ. Microbiol. 47:986-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tripp, M. R. 1960. Mechanisms of removal of injected microorganims from the American oyster, Crassostrea virginica. (Gmlin). Biol. Bull. 119:273-282. [Google Scholar]
- 58.van de Braak, C. B., M. H. Botterblom, N. Taverne, W. B. van Muiswinkel, J. H. Rombout, and W. P. van der Knaap. 2002. The roles of haemocytes and the lymphoid organ in the clearance of injected Vibrio bacteria in Penaeus monodon shrimp. Fish Shellfish Immunol. 13:293-309. [DOI] [PubMed] [Google Scholar]