Abstract
The possibility that graphite electrodes can serve as the direct electron donor for microbially catalyzed reductive dechlorination was investigated with Geobacter lovleyi. In an initial evaluation of whether G. lovleyi could interact electronically with graphite electrodes, cells were provided with acetate as the electron donor and an electrode as the sole electron acceptor. Current was produced at levels that were ca. 10-fold lower than those previously reported for Geobacter sulfurreducens under similar conditions, and G. lovleyi anode biofilms were correspondingly thinner. When an electrode poised at −300 mV (versus a standard hydrogen electrode) was provided as the electron donor, G. lovleyi effectively reduced fumarate to succinate. The stoichiometry of electrons consumed to succinate produced was 2:1, the ratio expected if the electrode served as the sole electron donor for fumarate reduction. G. lovleyi effectively reduced tetrachloroethene (PCE) to cis-dichloroethene with a poised electrode as the sole electron donor at rates comparable to those obtained when acetate serves as the electron donor. Cells were less abundant on the electrodes when the electrodes served as an electron donor than when they served as an electron acceptor. PCE was not reduced in controls without cells or when the current supply to cells was interrupted. These results demonstrate that G. lovleyi can use a poised electrode as a direct electron donor for reductive dechlorination of PCE. The ability to colocalize dechlorinating microorganisms with electrodes has several potential advantages for bioremediation of subsurface chlorinated contaminants, especially in source zones where electron donor delivery is challenging and often limits dechlorination.
A wide diversity of microorganisms can electrochemically interact with electrodes, directly donating or accepting electrons from electrode surfaces (17, 18, 23). In most previous studies, the microorganisms donated electrons to the anodes of microbial fuel cells, which served as an electron acceptor. However, when an electrode is poised at a sufficiently low potential, Geobacter sulfurreducens and Geobacter metallireducens can accept electrons from graphite electrodes, using the electrons for the reduction of electrochemically more positive electron acceptors, such as fumarate, nitrate, or U(VI) (10, 11). Other, as-yet-undefined microorganisms may function in a similar manner (23).
Previous studies have suggested that Geobacter species directly transfer electrons to electrode surfaces without a requirement for soluble electron carriers (7, 24; K. P. Nevin, B.-C. Kim, R. H. Glaven, J. P. Johnson, T. L. Woodard, B. A. Methé, R. J. DiDonato, Jr., S. F. Covalla, A. E. Franks, A. Liu, and D. R. Lovley, submitted for publication), and it is expected that electron transfer is also direct when electrons flow from an electrode to Geobacter cells (10, 11). This contrasts with observations made with a number of organisms, including Shewanella (14, 19, 30), Pseudomonas (22), and Geothrix (8) species, that produce electron shuttles to promote electrode-microbe electron transfer.
Tetrachloroethene (PCE) and trichloroethene (TCE) are prevalent groundwater contaminants due to their widespread commercial, industrial, and military use (20). PCE and TCE form dense non-aqueous-phase liquids (DNAPLs), and such sources can feed dissolved-phase contaminant plumes for decades (1, 28). Bioremediation through stimulation of microbial reductive dechlorination of PCE and TCE to the nontoxic end product ethene can be achieved by addition of fermentable organic substrates to indirectly provide the fermentation products, hydrogen and acetate, as electron donors to dechlorinating microorganisms (12, 15, 16). However, this approach also stimulates the growth of unwanted, nondechlorinating microorganisms and the production of methane, a potent greenhouse gas. Furthermore, delivery and sustained supply of the electron donor(s) to DNAPL source zones are engineering challenges (16) and are considered major limitations for achieving microbially enhanced DNAPL dissolution (4).
It was previously suggested (10) that just as electrodes may serve as the electron donor for microbial reduction of the groundwater contaminant U(VI) (11), electrodes might be a suitable electron donor to promote microbially catalyzed reductive dechlorination. Initial studies evaluated this possibility using a mixed culture capable of dechlorinating TCE (5). Unfortunately, the electrode did not serve as an electron donor for dechlorination, even though it was poised at a very low potential (−500 mV) versus a standard hydrogen electrode (5). However, when the electron shuttle methyl viologen was added, TCE was dechlorinated, primarily to cis-1,2-dichloroethene (cis-DCE) (5). There was negligible reductive dechlorination of TCE with the poised electrode in the presence of methyl viologen in the absence of the mixed culture. These and other results suggested that the mixed culture was capable of accepting electrons from electrode-reduced methyl viologen for reductive dechlorination.
In an attempt to promote direct electron transfer from the electrode to the dechlorinating microorganisms, methyl viologen was adsorbed onto a glassy carbon electrode (5). TCE dechlorination began immediately. This contrasts with the lag period that would be expected if the cells had to first attach to the electrode surface in order to utilize it as an electron donor. Although it was suggested that there was “negligible” dissolution of methyl viologen from the electrode and into the culture (5), such dissolution was not directly verified, and it seems likely that methyl viologen would leach from such a surface. Furthermore, it was not determined whether cells attached to the electrode or were planktonic. Thus, definitive evidence that there was direct electron transfer from the electrode to the dechlorinating microorganisms was not obtained. Moreover, employing methyl viologen, a highly toxic compound, as a mediator for bioremediation is untenable.
In order to evaluate the possibility that there is direct electron transfer from electrodes to dechlorinating microorganisms under more defined and environmentally friendly conditions, we performed experiments with Geobacter lovleyi, which reductively dechlorinates PCE and TCE to cis-DCE with acetate as the electron donor (29). It was hypothesized that G. lovleyi might directly interact with electrodes in a manner similar to that observed for previously investigated Geobacter species. We report here that G. lovleyi can both donate electrons to and accept electrons from graphite electrodes and that an electrode is as effective an electron donor as acetate for PCE dechlorination.
MATERIALS AND METHODS
Source of bacterium, growth medium, and culture conditions.
G. lovleyi strain SZ (= ATCC BAA-1151) was maintained in a previously described medium (7) under strictly anaerobic conditions in the presence of a mixture of N2 and CO2 (80:20, vol/vol). In batch cultures, acetate (10 mM) served as the electron donor, and either fumarate (20 mM) or PCE (initial aqueous concentration, 200 to 400 μM) served as the electron acceptor.
Electrode experiments.
Studies with an electrode as the electron acceptor or donor were carried out at 25°C in potentiostat-poised, dual-chambered systems as previously described (7, 10). The total volume of each chamber was 265 ml, and each chamber was filled with 250 ml of medium. The working electrode was poised at either +500 or −300 mV (versus a standard hydrogen electrode), depending on whether the electrode served as the electron donor or the electron acceptor.
For growth with the electrode as the electron acceptor, log-phase, acetate-grown cells were inoculated (10% [vol/vol] inoculum) into the working electrode (poised graphite) chamber of the two-chamber system with both fumarate (20 mM) and the poised graphite electrode (+500 mV versus a standard hydrogen electrode) initially available as electron acceptors. When the optical density at 600 nm of the culture reached 0.1 to 0.2, the medium was replaced with medium containing acetate (10 mM) but no fumarate. Under these conditions, the poised electrode was the only available electron acceptor for acetate oxidation. Acetate was replenished as needed by exchanging the medium in the working electrode chamber.
Growth with the electrode serving as the donor for either fumarate or PCE reduction was also established in a stepwise fashion by first supplying acetate and the electrode as electron donors and eventually adapting the cells to utilize the electrode as the sole electron donor in the absence of acetate. For fumarate-reducing electrodes the medium was replaced twice before stoichiometric measurements were obtained. The adaptation for PCE reduction included an intermediate step in which lactate (2 mM) was added as a carbon source (29). As indicated below, controls were included, in which the supply of electrons from the electrode to the cells was discontinued by disconnecting the two-chamber electrode system from the potentiostat or PCE was added to the electrode system in the absence of cells.
Confocal laser scanning and electron microscopy.
The biofilms that grew when the electrode served as the electron acceptor were stained with a LIVE/DEAD BacLight viability kit (Molecular Probes), and images were obtained by confocal laser scanning laser microscopy as previously described (24). Images of the biofilms that grew when the electrode served as the electron donor were obtained by scanning electron microscopy as previously described (7, 10).
Analytical methods.
Current measurements were obtained as previously described with a Power Laboratory 4SP unit and CHART 4.0 software (AD Instruments) (7, 10). The numbers of electrons transferred were calculated as previously described (10) using the following conversions: 1 C = 1 A·s, 1 C = 6.24 × 1018 electrons, and 1 mol = 6.23 × 1023 electrons (96,500 C mol−1). Volatile fatty acids were analyzed by high-performance liquid chromatography (Shimadzu LC-10AT liquid chromatograph) using a UV-Vis detector (Shimadzu SPD-10A VP) at a wavelength of 210 nm. The column was an Aminex HPX-87H column (Bio-Rad), and the eluent was 8 mM H2SO4. PCE, TCE, and cis-DCE were separated on a VOCOL capillary column (60 m by 0.25 mm; Supelco) and were detected with a flame ionization detector using a Perkin Elmer Clarus 600 gas chromatograph. Helium was the carrier gas, and the injector split flow rate was 25 ml/min (column flow rate, 0.89 ml/min; split flow ratio, 28.1:1). The inlet temperature was set to 200°C, the oven temperature was 140°C, and the detector temperature was 250°C. Headspace samples (50 μl) were removed from the working electrode chamber through sampling ports using a gas-tight glass syringe and were injected manually into the gas chromatograph. Standard curves were generated using a methanol stock solution containing known amounts of each compound in headspace vials with the same headspace-to-aqueous phase ratio as the working electrode chamber (3, 9). The values reported below are averages for duplicate headspace samples for each time point. The concentration in the aqueous phase was calculated with Henry's law constants for each compound at 24.8°C (9). Hydrogen was analyzed with a Carbosieve S-II column (Supelco) at room temperature using N2 as the carrier gas; the column was attached to a reduction gas analyzer (RGD2; Trace Analytical) (10).
RESULTS AND DISCUSSION
Current production.
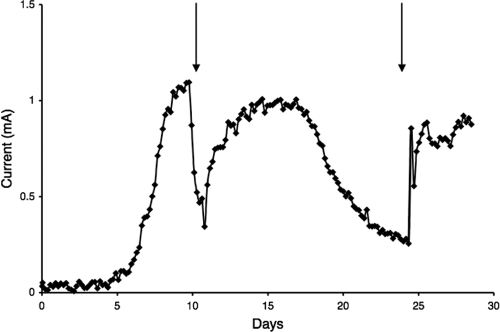
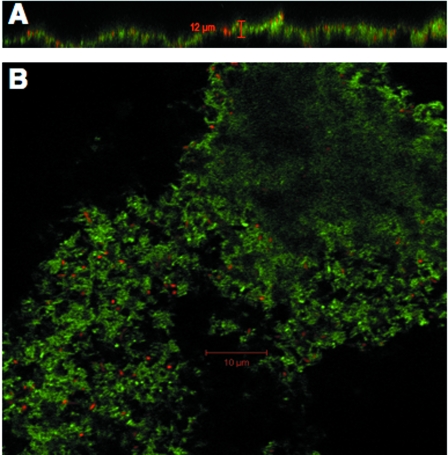
Although many Geobacteraceae can transfer electrons to or from electrodes (6, 7, 13), this ability is not universal within the family (25). Therefore, the ability of G. lovleyi to produce current was evaluated first. G. lovleyi was capable of oxidizing acetate with an electrode serving as the sole electron acceptor (Fig. 1). However, the maximum current, ca. 1 mA, was significantly less than the ca. 15 mA that G. sulfurreducens typically produces in the same system (24; Nevin et al., submitted). The anode biofilms reached heights of approximately 12 μm (Fig. 2). Thus, these biofilms were substantially thinner than the 50-μm-thick biofilms that G. sulfurreducens forms on similar anodes (21a, 24), which is consistent with the lower current that G. lovleyi produces. Cells stained predominately green with LIVE/DEAD stain, suggesting that most of the biofilm cells were metabolically active. These results demonstrated that G. lovleyi could electrochemically interact with a graphite electrode. Physiological differences that lead to the relatively low current production with this species require further investigation.
FIG. 1.
Current production by G. lovleyi with acetate (10 mM) serving as the electron donor and a poised electrode serving as the electron acceptor. The arrows indicate when fresh acetate medium was added. The data are data for a representative of duplicate current-producing cultures.
FIG. 2.
Confocal laser scanning microscopy of G. lovleyi grown on a graphite electrode that served as the electron acceptor. (A) Cross-sectional view of the biofilm. The black area at the bottom is the graphite electrode, and the black area at the top is the growth medium. (B) View looking down onto the biofilm. Individual cells are visible. Cells were treated with a LIVE/DEAD BacLight bacterial viability kit before images were obtained.
Electrode-enabled fumarate reduction.
G. lovleyi also reduced fumarate to succinate with the electrode serving as the sole electron donor (Fig. 3). The ratio of the number of cumulative electrons transferred (1.68 mmol) to the amount of succinate produced (0.82 mmol) was ca. 2:1, the ratio expected for the two-electron reduction of fumarate to succinate (Fig. 3), and the coulombic efficiency of the electrode system for this reaction was 98%. This contrasts with the 1:1 stoichiometry observed in studies with G. sulfurreducens (10). G. sulfurreducens may be able to derive some reducing power from fumarate (10), a trait that G. lovleyi apparently does not share.
FIG. 3.
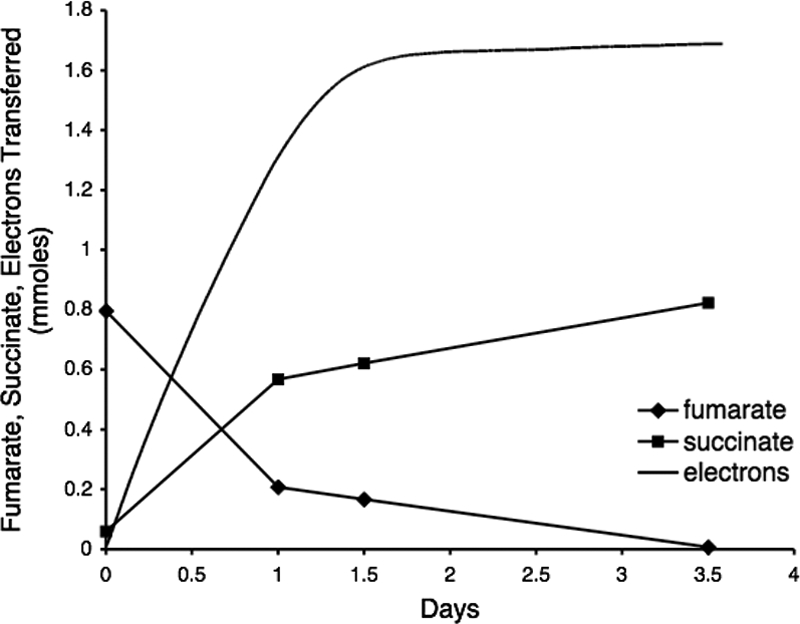
Consumption of electrons by G. lovleyi coupled with the reduction of fumarate to succinate with an electrode serving as the sole electron donor. The data are representative of duplicate fumarate-reducing cultures.
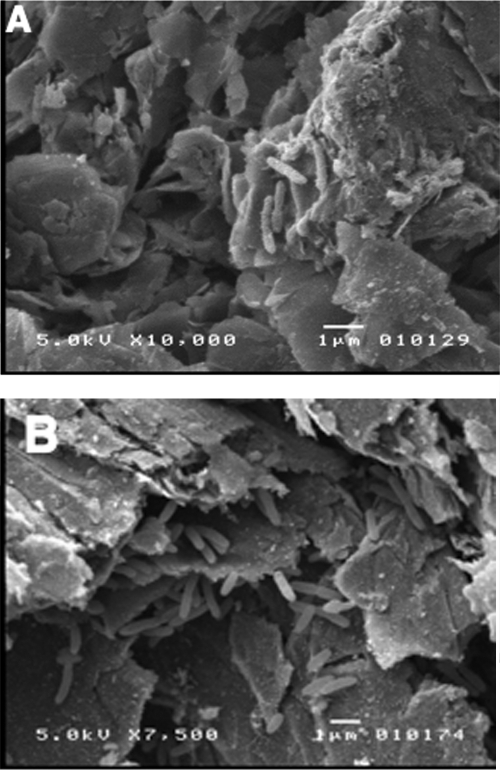
As expected from previous studies with G. sulfurreducens (10), a substantial G. lovleyi biofilm did not form when the electrode served as the electron donor, and thus, confocal laser scanning microscopy of the electrode surface was not very informative. Scanning electron microscopy revealed cells in close association with the electrode but scattered on the electrode surface (Fig. 4A).
FIG. 4.
Scanning electron micrographs of G. lovleyi grown on a graphite electrode surface with the electrode as the sole electron donor and either fumarate as the electron acceptor (A) or PCE as the electron acceptor (B).
Dechlorination with an electrode as the electron donor.
In order to determine if reductive dechlorination was possible with an electrode serving as the electron donor, G. lovleyi was again grown in the presence of a poised electrode but with PCE as the electron acceptor. After maximum dechlorination rates (ca. 25 μmol/day) were established, the medium in the working electrode chamber was replaced with fresh medium containing 100 μmol of PCE and with the lactate carbon source omitted (Fig. 5). Dechlorination began immediately, before the added PCE was completely dissolved, thus compromising measurement of the PCE concentration. There was an immediate and steady accumulation of cis-DCE that was accompanied by the slight transitory formation of TCE and the loss of PCE. The rate of cis-DCE production with the electrode serving as the electron donor was comparable to maximum rates observed when acetate was supplied as an electron donor (29). No TCE or cis-DCE accumulated when the poised electrode system was not inoculated with G. lovleyi, and electrodes colonized with G. lovleyi did not support dechlorination when the supply of electrons to the electrode was discontinued (data not shown).
FIG. 5.
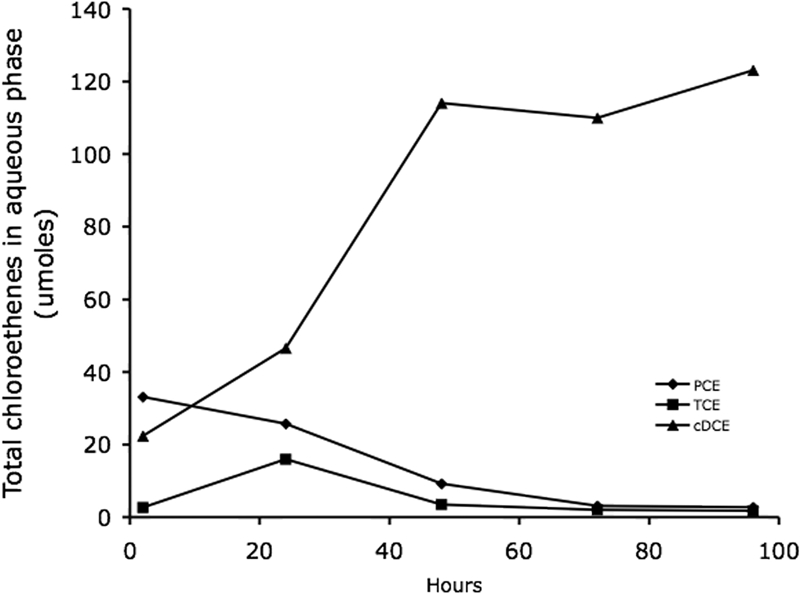
Dechlorination of PCE by G. lovleyi with an electrode serving as the sole electron donor. The data are data for a representative of triplicate PCE-reducing cultures. cDCE, cis-1,2-dichloroethene.
It was not possible to accurately measure current consumption during PCE reductive dechlorination because the level of PCE that the culture could tolerate was too low. The conversion of PCE to cis-DCE in 72 h required a current consumption rate of 11 to 29 μA/s, which is within the range of the background current for our monitoring system (±30 to 40 μA). As noted in a previous study (10), at the potential at which the electrodes were poised there was little, if any, reduction of protons to produce hydrogen (≤2 nmol hydrogen produced per h). Therefore, current-dependent reduction of PCE was attributed to G. lovleyi directly accepting electrons from the electrode surface and transferring the electrons to PCE.
Scanning electron microscopy of the electrode surfaces colonized by G. lovleyi actively dechlorinating PCE revealed cells scattered on the electrode surface (Fig. 4B). The low cell density was expected based upon the low rates of current consumption.
Implications.
The results described here demonstrate that an electrode is an effective electron donor for reductive dechlorination of PCE by G. lovleyi. This finding expands the known forms of respiration that electrodes can support as an electron donor and has practical implications for the in situ bioremediation of anaerobic subsurface environments contaminated with chlorinated pollutants.
Electrode-dependent, microbially catalyzed reductive dechlorination, without a requirement for modification of the electrode with toxic electron shuttles (5), offers a potentially attractive alternative strategy for stimulating reductive dechlorination. With dechlorinating microorganisms adhering to electrodes, it is potentially feasible to specifically colocalize the electron donor and the dechlorinating microorganisms in specific locations in the subsurface and control the flux of electrons into the site in order to fine-tune the rate of biostimulation. This approach could eliminate the substantial growth of undesired, competing microorganisms and the production of deleterious end products that are often associated with the addition of organic electron donors to the subsurface (2, 12).
Abiotic dechlorination of chlorinated solvents is possible with electrodes poised at very low potentials, but this nonenzymatic approach nonspecifically reduces protons and other redox-active components typically found in the subsurface and has undesirable consequences. The reduction of protons to hydrogen gas increases the groundwater pH (26, 27), which can disrupt biological, chemical, and physical soil functions. The hydrogen produced can stimulate the growth of nondechlorinating microorganisms, resulting in the accumulation of unwanted biomass and end products that result in deterioration of environmental quality. Furthermore, nondiscriminatory reduction of redox-active components in the subsurface with electrodes poised at a low potential wastes energy. The very low current demands of the microbial process could readily be supplied by solar panels, providing a sustainable bioremediation option.
Although G. lovleyi dehalogenates PCE only to cis-DCE, conversion of PCE to cis-DCE near source zones can be very effective for enhanced PCE dissolution, and there can be subsequent treatment of the more soluble compound cis-DCE with more traditional bioremediation strategies (3, 4, 21). Furthermore, the finding that G. lovleyi directly accepts electrons from graphite electrodes for reductive dechlorination suggests that other organisms capable of complete dechlorination of PCE or dechlorination of other environmental contaminants might be enriched with electrodes serving as the electron donor. Efforts to recover such organisms in culture are currently under way.
Acknowledgments
This work was supported by the office of Naval Research (award number N00014-07-1-0966).
We thank the staff of the University of Massachusetts Central Microscopy Facility for their contributions to this research.
Footnotes
Published ahead of print on 25 July 2008.
REFERENCES
- 1.Adamson, D. T., D. Y. Lyon, and J. B. Hughes. 2004. Flux and product distribution during biological treatment of tetrachloroethene dense non-aqueous-phase liquid. Environ. Sci. Technol. 38:2021-2028. [DOI] [PubMed] [Google Scholar]
- 2.Air Force Center for Environmental Excellence. 2004. Principles and practices of enhanced anaerobic bioremediation of chlorinated solvents. U.S. DoD Air Force Center for Environmental Excellence and ESTCP. http://www.afcee.brooks.af.mil/products/techtrans/Bioremediation/BIOREMresources.asp458.
- 3.Amos, B. K., J. A. Christ, L. M. Abriola, K. D. Pennell, and F. E. Löffler. 2007. Experimental evaluation and mathematical modeling of microbially enhanced tetrachloroethene (PCE) dissolution. Environ. Sci. Technol. 41:963-970. [DOI] [PubMed] [Google Scholar]
- 4.Amos, B. K., E. J. Suchomel, K. D. Pennell, and F. E. Löffler. 2008. Correlating microbial activity and distribution with enhanced contaminant dissolution from a NAPL source zone. Water Res. 42:5718-5726. [DOI] [PubMed] [Google Scholar]
- 5.Aulenta, F., A. Catervi, M. Majone, S. Panero, P. Reale, and S. Rossetti. 2007. Electron transfer from a solid-state electrode assisted by methyl viologen sustains efficient microbial reductive dechlorination of TCE. Environ. Sci. Technol. 41:2554-2559. [DOI] [PubMed] [Google Scholar]
- 6.Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483-485. [DOI] [PubMed] [Google Scholar]
- 7.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bond, D. R., and D. R. Lovley. 2005. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl. Environ. Microbiol. 71:2186-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gossett, J. M. 1987. Measurement of Henry's law constants for C1 and C2 chlorinated hydrocarbons. Environ. Sci. Technol. 21:202-208. [Google Scholar]
- 10.Gregory, K. B., D. R. Bond, and D. R. Lovley. 2004. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 6:596-604. [DOI] [PubMed] [Google Scholar]
- 11.Gregory, K. B., and D. R. Lovley. 2005. Remediation and recovery of uranium from contaminated subsurface environments with electrodes. Environ. Sci. Technol. 39:8943-8947. [DOI] [PubMed] [Google Scholar]
- 12.He, J., Y. Sung, M. E. Dollhopf, B. Z. Fathepure, J. M. Tiedje, and F. E. Löffler. 2002. Acetate versus hydrogen as direct electron donors to stimulate the microbial reductive dechlorination process at chloroethene-contaminated site. Environ. Sci. Technol. 36:3945-3952. [DOI] [PubMed] [Google Scholar]
- 13.Holmes, D. E., J. S. Nicoll, D. R. Bond, and D. R. Lovley. 2004. Potential role of a novel psychrotolerant Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp. nov., in electricity production by the marine sediment fuel cell. Appl. Environ. Microbiol. 70:6023-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanthier, M., K. B. Gregory, and D. R. Lovley. 2008. Electron transfer to electrodes with high planktonic biomass in Shewanella oneidensis fuel cells. FEMS Microbiol. Lett. 278:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lendvay, J. M., F. E. Löffler, M. Dollhopf, M. R. Aiello, G. Daniels, B. Z. Fathepure, M. Gebhard, R. Heine, R. Helton, J. Shi, R. Krajmalnik-Brown, C. L. Major, J. R., M. J. Barcelona, E. Petrovskis, R. Hickey, J. M. Tiedje, and P. Adriaens. 2003. Bioreactive barriers: a comparison of bioaugmentation and biostimulation for chlorinated solvent remediation. Environ. Sci. Technol. 37:1422-1431. [Google Scholar]
- 16.Löffler, F. E., and E. A. Edwards. 2006. Harnessing microbial activities for environmental cleanup. Curr. Opin. Biotechnol. 17:274-284. [DOI] [PubMed] [Google Scholar]
- 17.Lovley, D. R. 2006. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 4:497-508. [DOI] [PubMed] [Google Scholar]
- 18.Lovley, D. R. 2006. Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr. Opin. Biotechnol. 17:327-332. [DOI] [PubMed] [Google Scholar]
- 19.Marsili, E., D. B. Baron, I. D. Shikhare, D. Coursolle, J. A. Gralnick, and D. R. Bond. 2008. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 105:3968-3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moran, M. J., J. S. Zogorski, and P. J. Squillace. 2007. Chlorinated solvents in groundwater of the United States. Environ. Sci. Technol. 41:74-81. [DOI] [PubMed] [Google Scholar]
- 21.Mravik, S. C., R. K. Sillan, A. L. Wood, and G. W. Sewell. 2003. Field evaluation of the solvent extraction residual biotreatment technology. Environ. Sci. Technol. 37:5040-5049. [DOI] [PubMed] [Google Scholar]
- 21a.Nevin, K. P., H. Richter, S. F. Covalla, J. P. Johnson, T. L. Woodard, A. L. Orloff, H. Jia, M. Zhang, and D. R. Lovley. Power output and coulombic efficiencies from biofilms of Geobacter sulfurreducens comparable to mixed community microbiol fuel cells. Environ. Microbiol., in press. [DOI] [PubMed]
- 22.Pham, T. H., N. Boon, P. Aelterman, P. Clauwaert, L. De Schamphelaire, L. Vanhaecke, K. De Maeyer, M. Hoftë, W. Verstraete, and K. Rabaey. 2008. Metabolites produced by Pseudomonas sp. enable a Gram-positive bacterium to achieve extracellular electron transfer. Appl. Microbiol. Biotechnol. 77:1119-1129. [DOI] [PubMed] [Google Scholar]
- 23.Rabaey, K., J. Rodríguez, L. L. Blackall, J. Keller, P. Gross, D. Batstone, W. Verstraete, and K. H. Nealson. 2007. Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J. 1:9-18. [DOI] [PubMed] [Google Scholar]
- 24.Reguera, G., K. P. Nevin, J. S. Nicoll, S. F. Covalla, T. L. Woodard, and D. R. Lovley. 2006. Biofilm and nanowire production leads to increased current in Geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 72:7345-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richter, H., M. Lanthier, K. P. Nevin, and D. R. Lovley. 2007. Lack of electricity production by Pelobacter carbinolicus indicates that the capacity for Fe(III) oxide reduction does not necessarily confer electron transfer ability to fuel cell anodes. Appl. Environ. Microbiol. 73:5347-5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimomura, T., and R. A. Sanford. 2005. Reductive dechlorination of tetrachloroethene in a sand reactor using a potentiostat. J. Environ. Qual. 34:1435-1438. [DOI] [PubMed] [Google Scholar]
- 27.Skadberg, B., S. L. Geoly-Horn, V. Sangamalli, and J. R. V. Flora. 1999. Influence of pH, current and copper on the biological dechlorination of 2,6-dichlorophenol in an electrochemical cell. Water Res. 33:1997-2010. [Google Scholar]
- 28.Stroo, H. F., M. Unger, C. H. Ward, M. C. Kavanaugh, K. Vogel, A. Leeson, J. A. Marqusee, and B. P. Smith. 2003. Remediating chlorinated solvent source zones. Environ. Sci. Technol. 37:224A-230A. [DOI] [PubMed] [Google Scholar]
- 29.Sung, Y., K. E. Fletcher, K. M. Ritalahti, N. Ramos-Hernandez, R. A. Sanford, N. M. Mesbah, and F. E. Loeffler. 2006. Geobacter lovleyi strain SZ sp. nov., a novel metal-reducing and tetrachloroethene-dechlorinating bacterium. Appl. Environ. Microbiol. 72:2775-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Canstein, H., J. Ogawa, S. Shimizu, and J. R. Lloyd. 2008. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl. Environ. Microbiol. 74:615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]