Abstract
Anaerobic syntrophic associations of fermentative bacteria and methanogenic archaea operate at the thermodynamic limits of life. The interspecies transfer of electrons from formate or hydrogen as a substrate for the methanogens is key. Contrary requirements of syntrophs and methanogens for growth-sustaining product and substrate concentrations keep the formate and hydrogen concentrations low and within a narrow range. Since formate is a direct substrate for methanogens, a niche for microorganisms that grow by the conversion of formate to hydrogen plus bicarbonate—or vice versa—may seem unlikely. Here we report experimental evidence for growth on formate by syntrophic communities of (i) Moorella sp. strain AMP in coculture with a thermophilic hydrogen-consuming Methanothermobacter species and of (ii) Desulfovibrio sp. strain G11 in coculture with a mesophilic hydrogen consumer, Methanobrevibacter arboriphilus AZ. In pure culture, neither Moorella sp. strain AMP, nor Desulfovibrio sp. strain G11, nor the methanogens grow on formate alone. These results imply the existence of a previously unrecognized microbial niche in anoxic environments.
Much attention is paid to the environmental conditions that limit microbial growth and activity (24, 30, 31), such as high salt concentrations, high pressure, high and low pHs, high and low temperatures, and combinations thereof (3, 11, 23, 30, 32, 42). Less attention has been given to the thermodynamic limits of microbial life, although these are the most fundamental limits for any life form (19). These limits are approached in methanogenic environments, where syntrophic associations of anaerobic bacteria and methanogenic archaea obtain energy for growth from catalyzing pathways that operate close to thermodynamic equilibrium (ΔG, ∼0 kJ/mol) (20, 38). Methanogenic communities are generally schematized as four different functional groups (or guilds) of bacteria and archaea. Primary fermenters convert complex material into substrates for a group of secondary fermenters, also known as syntrophs. The syntrophs obligately depend on two groups of methanogens, one that uses hydrogen and formate and another that uses acetate (9, 38). For thermodynamic reasons, growth of the syntrophs is sustainable only through the removal of their waste products by the methanogens. Hydrogen is the main electron carrier in such syntrophic associations, but formate is important too, especially in associations where electron fluxes are high (5, 8, 41). It is assumed that formate and hydrogen are in thermodynamic equilibrium (26, 44) (Table 1), but this is not always the case. For instance, measurements in a shallow methanogenic aquifer in Denmark have indicated a potential energy gain of 5 to 10 kJ/mol electrons for the conversion of formate to H2 and bicarbonate (14). This implies a previously unrecognized niche for organisms that are able to catalyze this reaction.
TABLE 1.
Gibbs free-energy changes for the reactions involved in syntrophic conversion of formatea
| Reaction | ΔG0′b at 25°C | ΔG′b at 65°C |
|---|---|---|
| 4HCOO− + 4H2O → 4H2 + 4HCO3− | +5.2 | −6.3 |
| 4H2 + HCO3− + H+ → CH4 + 3H2O | −135.6 | −146.2 |
| 4HCOO− + H2O + H+ → CH4 + 3HCO3− | −130.4 | −152.5 |
Data were obtained or calculated from reference 1.
Gibbs free energy (kJ reaction−1) was calculated under standard conditions (solute concentration of 1 M, pH of 7, and partial pressure of gas of 105 Pa).
Hydrolytic cleavage of formate to H2 and bicarbonate has been described before (2, 7, 12, 29), but it has never been shown before that this can be coupled to growth (Table 1). Formate hydrogen lyase has been proposed to be coupled to energy conservation (15). Guyot and Brauman have reported formate-based coupling between a sulfate reducer and a non-formate-using methanogen, but growth was not demonstrated (12). Here we describe experiments that show that bacteria are able to grow by the conversion of formate to H2 and bicarbonate, provided that hydrogen is consumed by a methanogen.
MATERIALS AND METHODS
Strains and source of microorganisms.
Moorella sp. strain AMP (DSMZ 21394; GenBank accession number of the 16S rRNA gene sequence, AY884087) and Methanothermobacter sp. strain NJ1 were isolated from a methanol-degrading enrichment culture from a thermophilic upflow anaerobic sludge blanket reactor as described by Paulo et al. (35) and maintained routinely on methanol and H2-CO2, respectively. Desulfovibrio sp. strain G11 (DSM 7057) and Methanobrevibacter arboriphilus AZ (DSM 744) were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) and maintained routinely on formate plus sulfate and H2-CO2, respectively.
Media and growth conditions.
Media and growth conditions were as described previously (21, 35). All media used for the coculture studies were without addition of carbon and energy sources other than formate. For the thermophilic (65°C) coculture studies, pregrown pure cultures of Moorella sp. strain AMP on methanol and Methanothermobacter sp. strain NJ1 on H2-CO2 were inoculated (10%, inoculum size) into fresh mineral medium with 60 mM formate as the sole energy source. For the mesophilic (37°C) coculture studies, a pure culture of Desulfovibrio sp. strain G11 (10%, vol/vol) was inoculated into a dense culture of M. arboriphilus AZ that was pregrown on H2-CO2. Subcultures were made after 40 mM formate was consumed until stable cocultures were obtained. This repeated transfer was performed at least five times.
All inoculations and transfers were done aseptically with sterile needles and syringes, and all cultures were incubated statically in the dark.
Analytical and other methods.
Growth and growth yields were determined by measuring the increase in optical density at 600 nm, cell number counting with a Burker-Türk counting chamber, and dry weight content measurements by standard techniques.
Gas (H2 and CH4) samples were analyzed by gas chromatography with a Shimadzu GC-14B (Shimadzu, Kyoto, Japan) equipped with a packed column (Molsieve 13 × 60/80 mesh, 2-m length, 2.4-mm internal diameter; Varian, Middelburg, The Netherlands) and a thermal conductivity detector. The oven temperature was 100°C, and the injector and detector temperatures were 90 and 150°C, respectively. Argon was the carrier gas at a flow rate of 30 ml min−1. Formate was analyzed by high-pressure liquid chromatography from centrifuged (10,000 × g, 10 min) samples of the culture media. Formate was measured with a Polyspher OA HY column (300 by 6.5 mm; Merck, Darmstadt, Germany) and an RI SE-61 refractive index detector (Shodex, Tokyo, Japan). The mobile phase was 0.01 N H2SO4 at a flow rate of 0.6 ml min−1. The column temperature was 60°C.
Thermodynamic calculations were done as described by Amend and Shock (1). Temperature corrections for 65°C were made by linear interpolation from tabulated values for 55 and 70°C. This method yielded essentially the same results as the use of the Gibbs-Helmholtz equation for temperature correction as described by Hanselmann (13). Corrections for the actual concentrations of substrates were made with the Nernst equation (27, 40). Comparative analyses of genome sequences were performed with the integrated microbial genomes system (28), which is available from the U.S. Department of Energy Joint Genome Institute (www.jgi.doe.gov).
RESULTS AND DISCUSSION
Two different defined communities were studied, a thermophilic community consisting of Moorella sp. strain AMP in coculture with Methanothermobacter sp. strain NJ1 and a mesophilic community consisting of Desulfovibrio sp. strain G11 in coculture with M. arboriphilus AZ. Both methanogens can only use H2 as an electron donor.
Moorella sp. strain AMP and Methanothermobacter sp. strain NJ1 were isolated from a methanogenic bioreactor operated at 55°C (35). Based on 16S rRNA gene sequence analysis, strain AMP was closely related to Moorella thermoacetica and Moorella thermoautotrophica. The sequence was 98% identical to both Moorella strains. However, the new isolate had the special property of growth on CO, forming H2 rather than acetate as the end product. Strain NJ1 was a hydrogen-utilizing methanogen; its 16S rRNA sequence was 99.5% identical to that of Methanothermobacter thermoautotrophicus ΔH (21).
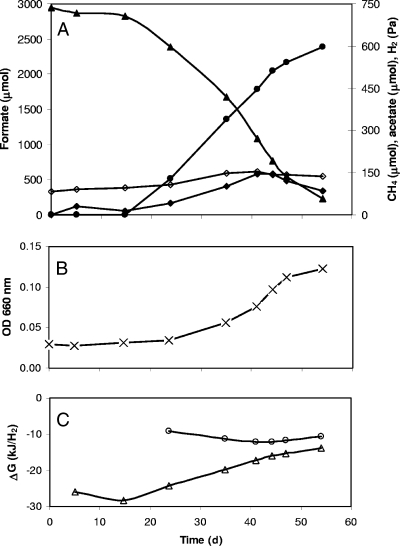
Moorella sp. strain AMP grew on formate when thiosulfate was added as an electron acceptor but did not grow when thiosulfate was replaced with sulfate, nitrate, or fumarate. In the absence of thiosulfate, H2 gradually accumulated to a partial pressure of 2,000 Pa. Removal of H2 from the headspace via flushing resulted again in accumulation of H2 (21). These observations indicate that accumulation of H2 was inhibitory to the conversion of formate. Therefore, we tested whether Methanothermobacter sp. strain NJ1 could serve as an alternative electron acceptor. A coculture of Moorella sp. strain AMP and Methanothermobacter sp. strain NJ1 grew at 65°C on formate as the sole carbon and energy substrate (Fig. 1), while the individual pure cultures did not grow in formate-containing media (data not shown). Consumption of 2.71 ± 0.13 mmol formate resulted in the formation of 0.60 ± 0.04 mmol CH4, which is consistent with the reaction 4HCOO− + H2O + H+ → CH4 + 3HCO3−, and gave rise to an increase in the total cell concentration from 9.0 × 106 ± 2.8 × 106 to 7.5 × 107 ± 1.4 × 107/ml. In the coculture, hydrogen levels were between 10 and 150 Pa. Under these conditions, Gibbs free-energy changes ranged between −16 and −29 kJ/mol H2 for the conversion of formate into H2 and bicarbonate and between −9 and −12 kJ/mol H2 for H2-driven methanogenesis. Taken together, these data indicate that interspecies hydrogen transfer is essential to sustain the growth of the coculture and that Moorella sp. strain AMP can grow by the conversion of formate to H2 and bicarbonate when the hydrogen concentration is kept low.
FIG. 1.
Syntrophic growth on formate by a coculture of Moorella sp. strain AMP and Methanothermobacter sp. strain NJ1. (A) Changes in formate (▴), methane (•), hydrogen (⧫), and acetate (⋄). (B) Growth. OD 600 nm, optical density at 600 nm. (C) Actual Gibbs free-energy changes for formate degradation to H2 and bicarbonate (▵) and methane formation from H2 and bicarbonate (○). Data are averages of duplicate incubations; the experiment was repeated once with essentially the same results.
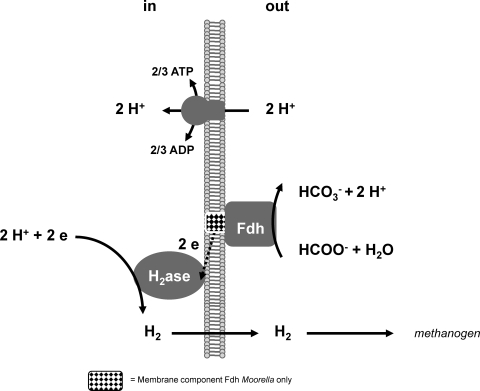
Moorella sp. strain AMP grew on carbon monoxide in pure culture. Carbon monoxide is converted into H2 and bicarbonate in a fashion similar to that described for Carboxydothermus hydrogenoformans and several other gram-positive bacteria (17). The energetics of CO conversion (CO + 2H2O → H2 + HCO3− + H+; ΔG0′ = −15.4 kJ at 25°C and ΔG′ = −12.4 kJ at 65°C) is more favorable than the energetics of formate conversion (HCOO− + H2O → H2 + HCO3−; ΔG0′ = 1.1 kJ at 25°C and ΔG′ = −1.6 kJ at 65°C). Thus, CO oxidation allows growth even if the partial pressure of H2 is high (17, 18). In Carboxydothermus hydrogenoformans, there is compelling evidence that the organism conserves energy via a novel CO-oxidizing, H2-evolving enzyme complex. The key for energy conservation is that the hydrogenase part of the proton-consuming-H2-generating enzyme complex is located at the cytoplasmic site of the cell membrane (15). It is possible that Moorella sp. strain AMP conserves energy from the formate-hydrogen-lyase reaction via an analogous enzyme complex with an energy-conserving hydrogenase located at the cytoplasmic side of the cell membrane. Support for this hypothesis was obtained via an analysis of the genome of Moorella thermoacetica, the closest relative of Moorella sp. strain AMP, with 98% 16S RNA sequence identity. In M. thermoacetica, the genes that encode the constituents of a putative energy-conserving formate hydrogen lyase complex are all located in one operon. M. thermoacetica also possesses a membrane-integrated formate dehydrogenase (Table 2) that can oxidize formate at the outside of the membrane, thus generating a proton gradient over the membrane (Fig. 2) (36). The electrons produced are transferred across the membrane to a hydrogenase. Energy from the resulting proton gradient is harnessed via a membrane-integrated ATPase.
TABLE 2.
Genes and gene clusters possibly involved in formate conversion and hydrogen production by M. thermoacetica and D. vulgarisa
| Organism and enzyme | Localizationb | Locus tag (GenBank accession no.) |
|---|---|---|
| M. thermoacetica | ||
| Formate dehydrogenase | Outside, membrane integrated | Moth_0450-0452 |
| Formate dehydrogenase | Cytoplasm | Moth_2312-2314 (U73807) |
| Formate hydrogen lyase | Cytoplasm, membrane integrated | Moth_2174-2193 |
| Fe-only hydrogenase | Cytoplasm | Moth_1717-1719 |
| D. vulgaris | ||
| Formate dehydrogenase | Periplasm | DVU2481-2484 |
| Formate dehydrogenase | Periplasm | DVU2809-2812 |
| Formate dehydrogenase | Cytoplasm | DVU0587-0588 |
| Hydrogenase (EchA, -B, -C, -D, -E, -F) | Cytoplasm, membrane integrated | DVU0429-0434 |
| Hydrogenase (Ech-CO dehydrogenase) | Cytoplasm, membrane integrated | DVU2286-2293 |
| Hydrogenase | Cytoplasm | DVU0325-0326 |
| Hydrogenase, Fe only | Periplasm | DVU1769-1770 |
| Hydrogenase NiFe isozyme 1 | Periplasm | DVU1921-1922 |
| Hydrogenase NiFeSe | Periplasm | DVU1917-1918 |
| Hydrogenase NiFe isozyme 2 | Periplasm | DVU2525-2526 |
| Hydrogenase Mvr/hdr type | Cytoplasm | DVU2399-2404 |
www.jgi.doe.gov and NCBI GenBank.
FIG. 2.
Schematic hypothetical representation of formate oxidation coupled to proton translocation and energy conservation in Desulfovibrio sp. strain G11 and Moorella sp. strain AMP based on genome annotations of related organisms. H2ase, hydrogenase; Fdh, formate dehydrogenase; e, electrons.
On the basis of these findings, it seems prudent to also test M. thermoacetica for the ability to grow syntrophically on formate. However, testing this experimentally is confounded by the fact that M. thermoacetica can grow on formate in pure culture via a different pathway whereby formate is converted to acetate (4HCOO− + H+ → CH3COO− + 2HCO3−; ΔG0′ = −99.7 kJ at 25°C and ΔG′ = −95.2 kJ at 65°C). Indeed M. thermoacetica is the model organism with which the pathway for the formation of acetate from hydrogen and formate was elucidated first (10). Moorella sp. strain AMP cannot grow homoacetogenically on H2-CO2 or formate, probably because it lacks cytochrome b (21).
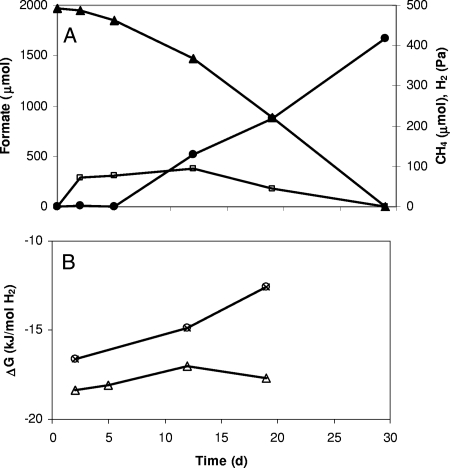
To further explore the occurrence of the ability to grow by the conversion of formate to H2 and bicarbonate, a mesophilic bacterium, Desulfovibrio sp. strain G11, was tested in coculture with a methanogen that could only use H2 as an electron donor (M. arboriphilus AZ). A coculture of Desulfovibrio sp. strain G11 and M. arboriphilus AZ grew well on formate as the sole carbon and energy substrate (Fig. 3), while the individual pure cultures did not grow in formate-containing media (data not shown). The growth yield of the coculture was 0.52 ± 0.12 g (dry weight)/mol of formate. Since floc formation was observed during syntrophic growth, growth could not be quantified by following the increase in turbidity. These flocs consisted of Desulfovibrio sp. strain G11 and the methanogen. The close proximity of the two microorganisms is beneficial for interspecies hydrogen transfer.
FIG. 3.
Syntrophic growth on formate by a coculture of Desulfovibrio strain sp. strain G11 and M. arboriphilus AZ. (A) Changes in formate (▴), hydrogen (□), and methane (•). (B) Actual Gibbs free-energy changes for formate degradation to H2 and bicarbonate (▵) and methane formation from H2 (○). Data shown are a typical example of six replicates.
In the coculture, hydrogen levels were between 40 and 100 Pa (Fig. 3). Formate degradation resulted in a nearly stoichiometric formation of methane (1 mol of CH4/4 mol of formate degraded) under transient accumulation of H2 to a level of ∼100 Pa. Under these conditions, Gibbs free-energy changes ranged between −17 and −19 kJ/mol H2 for the conversion of formate into H2 and bicarbonate and between −12 and −17 kJ/mol H2 for H2-driven methanogenesis. Formate was metabolized at a rate of ∼100 μmol/day.
Desulfovibrio sp. strain G11 is not able to grow in pure culture by the conversion of formate to H2 and bicarbonate. However, in pure culture it converts formate, resulting in a gradual accumulation of 100 Pa of hydrogen in the gas phase, after which the conversion of formate stopped. Removal of H2 from the headspace via flushing resulted again in accumulation of H2. A similar observation was done for Desulfovibrio sp. strain FOX1 (37). It is unclear if this bacterium is able to grow in pure culture from this conversion or growth was supported by the degradation of biomass, yeast extract, or other sources of organic carbon in the growth medium.
Desulfovibrio sp. strain G11 is a close relative of Desulfovibrio vulgaris strain Hildenborough, for which the genome sequence is available (16). We have also tested D. vulgaris for the ability to grow on formate in coculture with M. arboriphilus. D. vulgaris is indeed able to grow on formate with a syntrophic partner, but a stable consortium could only be obtained in the presence of low levels (0.01%) of yeast extract or acetate (data not shown). D. vulgaris Hildenborough is not capable of autotrophic growth, which is also apparent from its genome sequence (14). This is a clear difference from Desulfovibrio sp. strain G11, which is an autotroph. Nevertheless, the genome of D. vulgaris contains several genes that could be instrumental in growth on formate, including genes that encode periplasmic formate dehydrogenases, soluble hydrogenases, and an energy-conserving hydrogenase (Table 2). We propose that both Desulfovibrio sp. strain G11 and D. vulgaris can conserve energy via a periplasmic formate dehydrogenase coupled to an enzyme complex with an energy-conserving hydrogenase or a hydrogenase located at the cytoplasmic side of the cell membrane. The resulting proton gradient is the driving force of ATP synthesis by a membrane-integrated ATPase (Fig. 2).
This research describes the construction of syntrophic anaerobic microbial communities that grow by fermentation of formate, a compound that has thus far been disregarded by the scientific community as a substrate for syntrophic growth, although this is understandable from a biochemical and thermodynamic point of view. In nature, formate-converting syntrophs have to compete with methanogenic archaea that can directly convert formate to methane and have more energy available than the bacteria that convert formate to hydrogen and bicarbonate. This situation is analogous to that of syntrophic acetate oxidation, where acetate-oxidizing bacteria have to compete with aceticlastic methanogens (45). For some time, syntrophic acetate oxidation has been considered a slight metabolic and thermodynamic oddity, but it has now been shown to be feasible and occur in various situations, e.g., in Lake Kinneret sediments (34), in subsurface petroleum reservoirs (22), and in other environments with long solid retention times (39). Therefore, this type of metabolism, while seemingly paradoxical in the context of the existence of aceticlastic and formate-utilizing methanogens, may actually be a more fundamental component of methanogenic organic-carbon-mineralizing systems than previously recognized. On the other hand, since the discovery by Bryant et al. (6) that Methanobacillus omelianskii is not a pure culture but a syntrophic coculture, ethanol is a known substrate for syntrophic communities. Moreover, some methanogens are known to use ethanol or isopropanol directly as an electron donor for methanogenesis (25, 43). Further research is needed to get insight into the environmental conditions under which substrates are degraded by methanogens alone or by syntrophic communities.
Acknowledgments
We thank Ian M. Head for comments and suggestions.
This research was supported by the Research Councils for Earth and Life Sciences (ALW) and Chemical Sciences (CW) with financial aid from The Netherlands Organization for Scientific Research (NWO) and the Technology Foundation (STW), Applied Science Division of NWO, and by the European Commission, through ECOSERV, a Marie Curie Excellence Grant (EXT 023469).
Footnotes
Published ahead of print on 15 August 2008.
REFERENCES
- 1.Amend, J. P., and E. L. Shock. 2001. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 25:175-243. [DOI] [PubMed] [Google Scholar]
- 2.Bagramyan, K., and A. Trchounian. 2003. Structural and functional features of formate hydrogen lyase, an enzyme of mixed-acid fermentation from Escherichia coli. Biochemistry (Moscow) 68:1159-1170. [DOI] [PubMed] [Google Scholar]
- 3.Bakermans, C., A. I. Tsapin, V. Souza-Egipsy, D. A. Gilichinsky, and K. H. Nealson. 2003. Reproduction and metabolism at −10°C of bacteria isolated from Siberian permafrost. Environ. Microbiol. 5:321-326. [DOI] [PubMed] [Google Scholar]
- 4.Bendtsen, D. B., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 5.Boone, D. R., R. L. Johnson, and Y. Liu. 1989. Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 or formate uptake. Appl. Environ. Microbiol. 55:1735-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant, M. P., E. A. Wolin, M. J. Wolin, and R. S. Wolfe. 1967. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch. Microbiol. 59:20-31. [DOI] [PubMed] [Google Scholar]
- 7.Carroll, E. J., and R. E. Hungate. 1955. Formate dissimilation and methane production in bovine rumen contents. Arch. Biochem. Biophys. 56:525-536. [DOI] [PubMed] [Google Scholar]
- 8.de Bok, F. A. M., C. M. Plugge, and A. J. M. Stams. 2004. Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res. 38:1368-1375. [DOI] [PubMed] [Google Scholar]
- 9.Dolfing, J. 1988. Acetogenesis, p. 417-468. In A. J. B. Zehnder (ed.), Biology of anaerobic microorganisms. Wiley-Interscience, New York, NY.
- 10.Drake, H. L., and S. L. Daniel. 2004. Physiology of the thermophilic acetogen Moorella thermoacetica. Res. Microbiol. 155:869-883. [DOI] [PubMed] [Google Scholar]
- 11.Edwards, K. J., P. L. Bond, T. M. Gihring, and J. F. Banfield. 2000. An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 287:1796-1799. [DOI] [PubMed] [Google Scholar]
- 12.Guyot, J.-P., and A. Brauman. 1986. Methane production from formate by syntrophic association of Methanobacterium bryantii and Desulfovibrio vulgaris JJ. Appl. Environ. Microbiol. 52:1436-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanselmann, K. W. 1991. Microbial energetics applied to waste repositories. Experientia 47:645-687. [Google Scholar]
- 14.Hansen, L. K., R. Jakobsen, and D. Postma. 2001. Methanogenesis in a shallow sandy aquifer, Rømø, Denmark. Geochim. Cosmochim. Acta 65:2925-2935. [Google Scholar]
- 15.Hedderich, R. 2004. Energy-converting [NiFe] hydrogenases from Archaea and extremophiles: ancestors of complex I. J. Bioenerg. Biomembr. 36:65-75. [DOI] [PubMed] [Google Scholar]
- 16.Heidelberg, J. F., R. Seshadri, S. A. Haveman, C. L. Hemme, I. T. Paulsen, J. F. Kolonay, J. A. Eisen, N. Ward, B. Methe, L. M. Brinkac, S. C. Daugherty, R. T. Deboy, R. J. Dodson, A. S. Durkin, R. Madupu, W. C. Nelson, S. A. Sullivan, D. Fouts, D. H. Haft, J. Selengut, J. D. Peterson, T. M. Davidsen, N. Zafar, L. Zhou, D. Radune, G. Dimitrov, M. Hance, K. Tran, H. Khouri, J. Gill, T. R. Utterback, T. V. Feldblyum, J. D. Wall, G. Voordouw, and C. M. Fraser. 2004. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Nat. Biotechnol. 22:554-559. [DOI] [PubMed] [Google Scholar]
- 17.Henstra, A. M., and A. J. M. Stams. 2004. Novel physiological features of Carboxydothermus hydrogenoformans and Thermoterrabacterium ferrireducens. Appl. Environ. Microbiol. 70:7236-7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henstra, A. M. 2006. CO metabolism of Carboxydothermus hydrogenoformans and Archaeoglobus fulgidus. Ph.D. dissertation. Wageningen University, Wageningen, The Netherlands.
- 19.Hoehler, T. M., J. P. Amend, and E. L. Shock. 2007. A “follow the energy” approach for astrobiology. Astrobiology 7:819-823. [DOI] [PubMed] [Google Scholar]
- 20.Jackson, B. E., and M. J. McInerney. 2002. Anaerobic microbial metabolism can proceed close to thermodynamic limits. Nature 415:454-456. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, B. 2006. The effect of trace elements on the metabolism of methanogenic consortia. Ph.D. dissertation. Wageningen University, Wageningen, The Netherlands.
- 22.Jones, D. M., I. M. Head, N. D. Gray, J. J. Adams, A. K. Rowan, C. M. Aitken, B. Bennett, H. Huang, A. Brown, B. F. J. Bowler, T. Oldenburg, M. Erdmann, and S. R. Larter. 2008. Crude oil biodegradation in subsurface petroleum reservoirs proceeds via methanogenesis. Nature 451:176-180. [DOI] [PubMed] [Google Scholar]
- 23.Kashefi, K., and D. R. Lovley. 2003. Extending the upper temperature limit for life. Science 301:934. [DOI] [PubMed] [Google Scholar]
- 24.Kasting, J. F., and J. L. Siefert. 2002. Life and the evolution of earth's atmosphere. Science 296:1066-1068. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Y., and W. B. Whitman. 2008. Metabolic, phylogenetic, and ecological diversity of the methanogenic Archaea. Ann. N. Y. Acad. Sci. 1125:171-189. [DOI] [PubMed] [Google Scholar]
- 26.Lueders, T., and M. W. Friedrich. 2000. Archaeal population dynamics during sequential reduction processes in rice field soil. Appl. Environ. Microbiol. 66:2732-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madsen, E. L. 2008. Environmental microbiology: from genes to biogeochemistry. Blackwell, Wiley-Interscience, New York, NY.
- 28.Markowitz, V. M., E. Szeto, K. Palaniappan, Y. Grechkin, K. Chu, I.-M. A. Chen, I. Dubchak, I. Anderson, A. Lykidis, K. Mavromatis, N. Ivanova, and N. C. Kyrpides. 2008. The integrated microbial genomes (IMG) system in 2007: data content and analysis tool extensions. Nucleic Acids Res. 36(database issue):D528-D533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meshulam-Simon, G., S. Behrens, A. D. Choo, and A. M. Spormann. 2007. Hydrogen metabolism in Shewanella oneidensis MR-1. Appl. Environ. Microbiol. 73:1153-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro-González, R., F. A. Rainey, P. Molina, D. R. Bagaley, B. J. Hollen, J. de la Rosa, A. M. Small, R. C. Quinn, F. J. Grunthaner, L. Cáceres, B. Gomez-Silva, and C. P. McKay. 2003. Mars-like soils in the Atacama Desert, Chile, and the dry limit of microbial life. Science 302:1018-1021. [DOI] [PubMed] [Google Scholar]
- 31.Nealson, K. H. 1997. The limits of life on earth and searching for life on Mars. J. Geophys. Res. Planets 102:23675-23686. [PubMed] [Google Scholar]
- 32.Newman, D. K., and J. F. Banfield. 2002. Geomicrobiology: how molecular-scale interactions underpin biogeochemical systems. Science 296:1071-1077. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 34.Nüsslein, B., K.-J. Chin, W. Eckert, and R. Conrad. 2001. Evidence for anaerobic syntrophic acetate oxidation during methane production in the profundal sediment of subtropical Lake Kinneret (Israel). Environ. Microbiol. 3:460-470. [DOI] [PubMed] [Google Scholar]
- 35.Paulo, P. L., B. Jiang, D. Cysneiros, A. J. M. Stams, and G. Lettinga. 2004. Effect of cobalt on the anaerobic thermophilic conversion of methanol. Biotechnol. Bioeng. 85:434-441. [DOI] [PubMed] [Google Scholar]
- 36.Pierce, E., G. Xie, R. D. Barabote, E. Saunders, C. S. Han, J. C. Detter, P. Richardson, T. S. Brettin, A. Das, L. G. Ljungdahl, and S. W. Ragsdale. 2008. The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum). Environ. Microbiol. [Epub ahead of print.] doi: 10.1111/j.1462-2920.2008.01679.x. [DOI] [PMC free article] [PubMed]
- 37.Sanford, R. A., J. W. Urbance, and J. M. Tiedje. 1996. Anaerobic oxidation of formate to H2 supports growth in strain FOX1, a novel sulfate reducer, abstr. O-66. In Abstr. 96th Annu. Meet. Am. Soc. Microbiol. 1996. American Society for Microbiology, Washington, DC.
- 38.Schink, B., and A. J. M. Stams. 2006. Syntrophism among prokaryotes. Prokaryotes 2:309-335. [Google Scholar]
- 39.Shigematsu, T., Y. Tang, T. Kobayashi, H. Kawaguchi, S. Morimura, and K. Kida. 2004. Effect of dilution rate on metabolic pathway shift between aceticlastic and nonaceticlastic methanogenesis in chemostat cultivation. Appl. Environ. Microbiol. 70:4048-4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thauer, R. K., K. Jungermann, and K. Decker. 1977. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41:100-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiele, J. H., and J. G. Zeikus. 1988. Control of interspecies electron flow during anaerobic digestion: significance of formate transfer during syntrophic methanogenesis in flocs. Appl. Environ. Microbiol. 54:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker, J. J., J. R. Spear, and N. R. Pace. 2005. Geobiology of a microbial endolithic community in the Yellowstone geothermal environment. Nature 434:1011-1014. [DOI] [PubMed] [Google Scholar]
- 43.Widdel, F. 1986. Growth of methanogenic bacteria in pure culture with 2-propanol and other alcohols as hydrogen donors. Appl. Environ. Microbiol. 51:1056-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu, W.-M., R. F. Hickey, and J. G. Zeikus. 1991. Characterization of metabolic performance of methanogenic granules treating brewery wastewater: role of sulfate-reducing bacteria. Appl. Environ. Microbiol. 57:3438-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zinder, S. H., and M. Koch. 1984. Nonaceticlastic methanogenesis from acetate: acetate oxidation by a thermophilic syntrophic coculture. Arch. Microbiol. 138:263-272. [Google Scholar]