Abstract
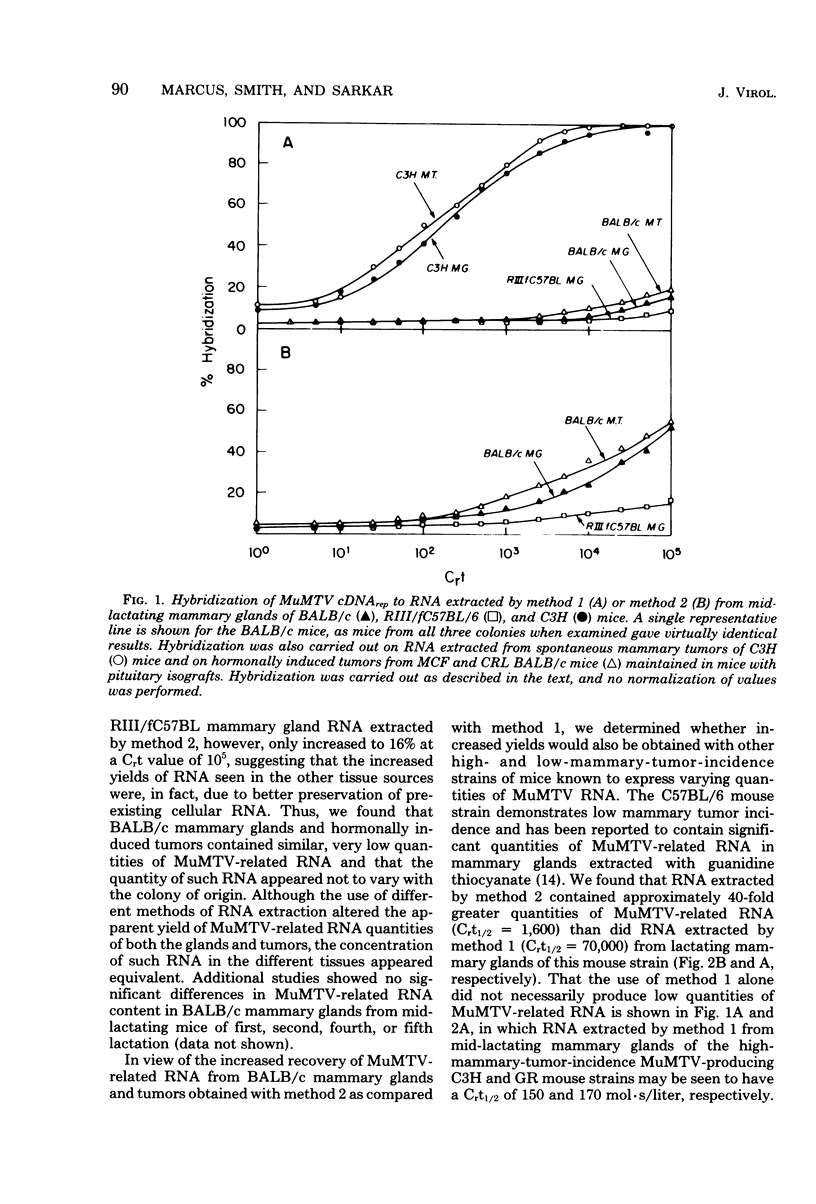
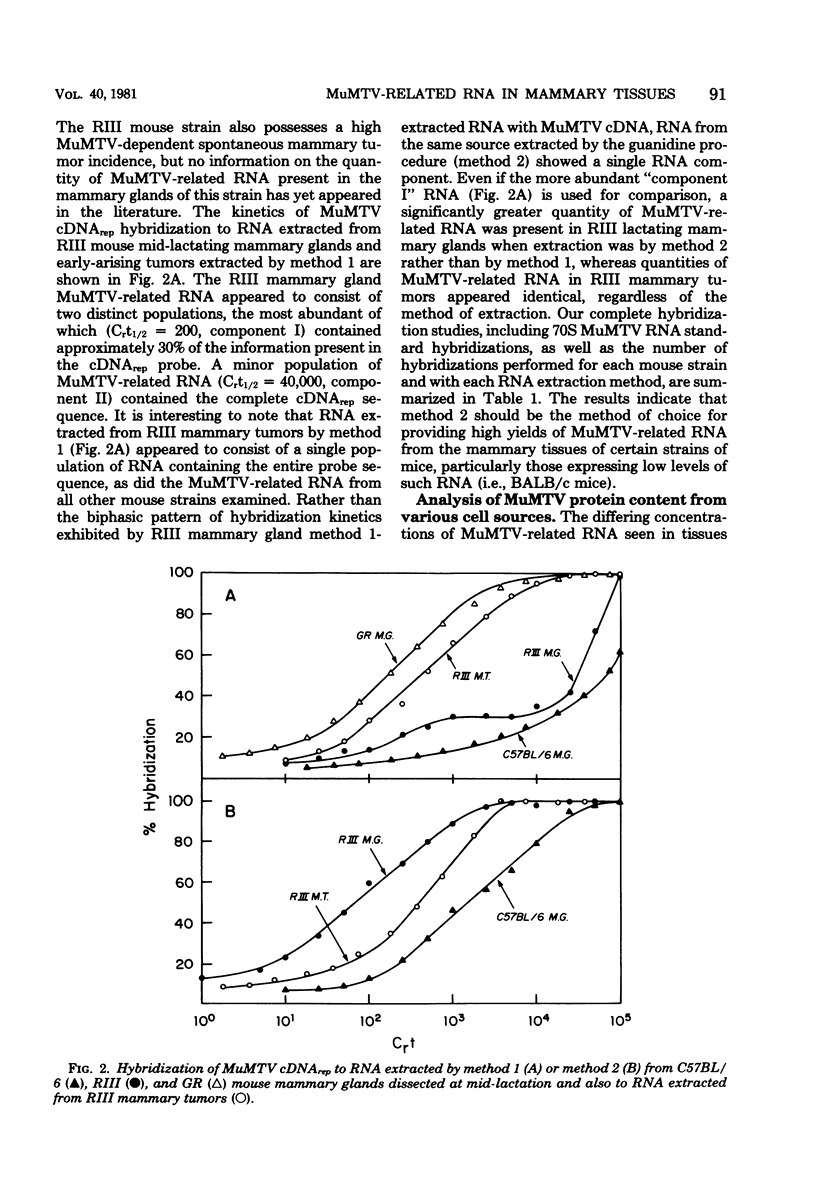
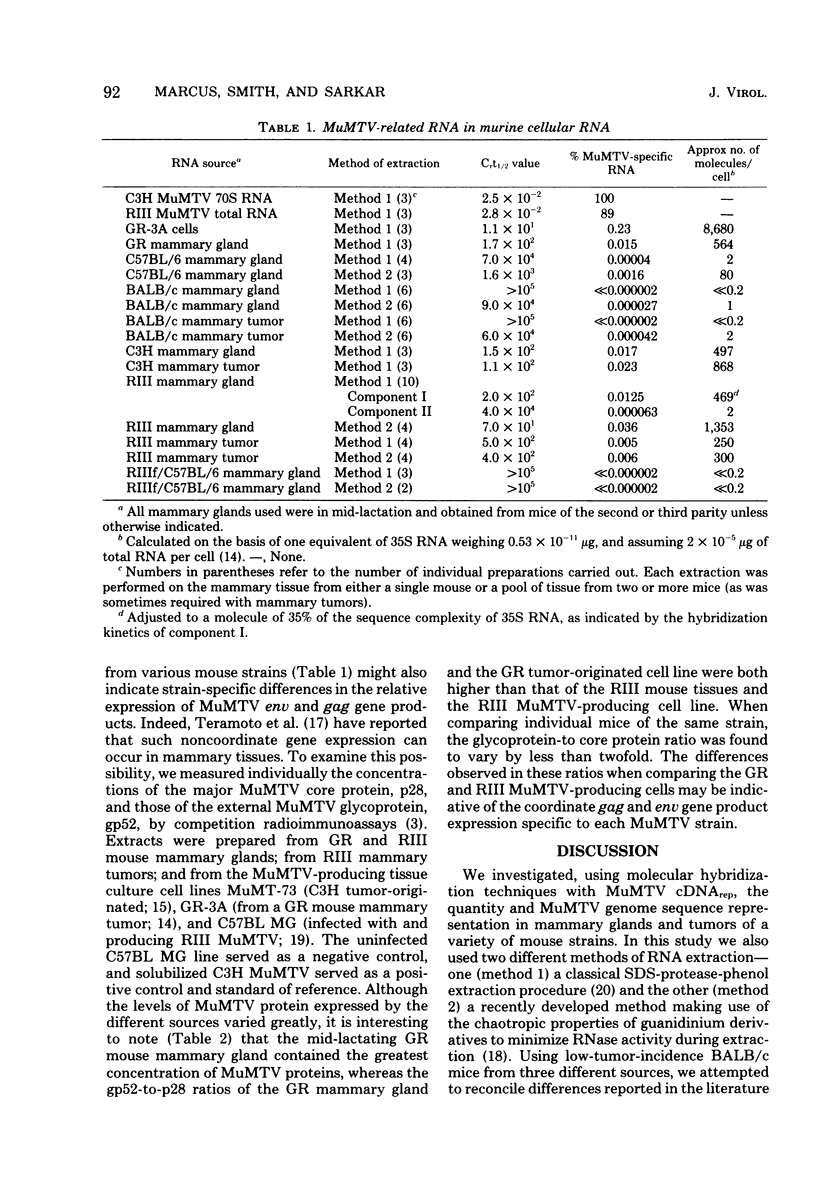
Lactating mammary glands and hormonally induced mammary tumors of BALB/c mice from three geographically separated breeding colonies were examined by molecular hybridization, using murine mammary tumor virus (MuMTV) cDNA representing the entire viral genome to determine the amount of MuMTV-related RNA expressed in these tissues. The RNA extracted from these tissues by the classical sodium dodecyl sulfate-pronase, phenol-chloroform procedure (method 1) contained barely detectable levels of MuMTV-related sequences. In contrast, both normal lactating mammary glands and hormonally induced mammary tumors of these mice were found to contain approximately one to two copies of the MuMTV genome per cell by using a new procedure in which the RNA was extracted with guanidine derivatives (method 2). No significant differences in the MuMTV-related RNA content of the BALB/c mammary tissues were observed regardless of their colony of origin. Our results suggest that expression of MuMTV RNA does not change in BALB/c mammary glands during transformation to a malignant state and that MuMTV expression does not play a role in tumorigenesis in these mice. In view of the increased recovery of MuMTV-related RNA from BALB/c mice with method 2, we compared the level of MuMTV RNA expression in lactating mammary glands and mammary tumors of other mouse strains, including C57BL/6 and RIII, using both extraction methods. Yields of MuMTV-related RNA from mammary tissues increased by as much as 35- to 40-fold, using method 2 as compared with method 1. Therefore method 2, involving guanidine derivatives, appears to be method of choice for MuMTV-related RNA extraction from the mammary tissues of certain strains of mice, particularly those expressing relatively low levels of MuMTV RNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dudley J. P., Rosen J. M., Butel J. S. Differential expression of poly(A)-adjacent sequences of mammary tumor virus RNA in murine mammary cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5797–5801. doi: 10.1073/pnas.75.12.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusing-Swartz S., Medina D., Butel J. S., Socher S. H. Mouse mammary tumor virus genome expression in chemical carcinogen-induced mammary tumors in low- and high-tumor-incidence mouse strains. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5360–5364. doi: 10.1073/pnas.76.10.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S. L., Kopelman R., Sarkar N. H. Simultaneous purification of murine mammary tumor virus structural proteins: analysis of antigenic reactivities of native gp34 by radioimmunocompetition assays. J Virol. 1979 Aug;31(2):341–349. doi: 10.1128/jvi.31.2.341-349.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath C. M., Jones R. F. Hormonal induction of mammary tumor viruses and its implications for carcinogenesis. Cancer Res. 1978 Nov;38(11 Pt 2):4112–4125. [PubMed] [Google Scholar]
- McGrath C. M., Marineau E. J., Voyles B. A. Changes in MuMTV DNA and RNA levels in Balb/c mammary epithelial cells during malignant transformation by exogenous MuTV and by hormones. Virology. 1978 Jun 15;87(2):339–353. doi: 10.1016/0042-6822(78)90139-3. [DOI] [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nusse R., Hageman P. Induction of mouse mammary tumor virus RNA in mammary tumors of BALB/c mice treated with urethane, X-irradiation, and hormones. J Virol. 1979 Jul;31(1):63–72. doi: 10.1128/jvi.31.1.63-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nusse R., Röpcke G., Boot L. Involvement of mouse mammary tumor virus in spontaneous and hormone-induced mammary tumors in low-mammary-tumor mouse strains. J Virol. 1978 Sep;27(3):551–559. doi: 10.1128/jvi.27.3.551-559.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. H., Sarkar N. H., Holben J. A., Sheffield J. B. Idiopathic mammary tumors in BALB/c mice. Int J Cancer. 1979 May 15;23(5):713–717. doi: 10.1002/ijc.2910230519. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Vlasschaert J. E., Beard C. L., Milazzo M. F., Bradbury W. C. Mammary tumors from BALB/c mice with a reported high mammary tumor incidence have acquired new mammary tumor virus DNA sequences. Virology. 1980 Jan 15;100(1):101–109. doi: 10.1016/0042-6822(80)90555-3. [DOI] [PubMed] [Google Scholar]
- Pauley R. J., Medina D., Socher S. H. Hormonal regulation of murine mammary tumor virus RNA expression during mammary tumorigenesis in BALB/c mice. J Virol. 1979 Nov;32(2):557–566. doi: 10.1128/jvi.32.2.557-566.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley R. J., Medina D., Socher S. H. Murine mammary tumor virus expression during mammary tumorigenesis in BALB/c mice. J Virol. 1979 Feb;29(2):483–493. doi: 10.1128/jvi.29.2.483-493.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. L., Varmus H. E. Structural analysis of the intracellular RNAs of murine mammary tumor virus. J Virol. 1979 May;30(2):576–589. doi: 10.1128/jvi.30.2.576-589.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. H., Pomenti A. A., Dion A. S. Replication of mouse mammary tumor virus in tissue culture. 1. Establishment of a mouse mammary tumor cell line, virus characterization, and quantitation of virus production. Virology. 1977 Mar;77(1):12–30. doi: 10.1016/0042-6822(77)90402-0. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Smith S. W., Marcus S. L., Sarkar N. H. Identification of the messenger RNAs coding for the gag and env gene products of the murine mammary tumor virus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1736–1740. doi: 10.1073/pnas.76.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto Y. A., Medina D., McGrath C., Schlom J. Noncoordinate expression of murine mammary tumor virus gene products. Virology. 1980 Dec;107(2):345–353. doi: 10.1016/0042-6822(80)90302-5. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Lasfargues E. Y., Sheffield J. B., Coutinho W. G. Murine mammary tumor virus (MuMTV) infection of an epithelial cell line established from C57BL/6 mouse mammary glands. Virology. 1978 Oct 1;90(1):12–22. doi: 10.1016/0042-6822(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]



