Abstract
Microbial succession during Parmigiano-Reggiano cheesemaking was monitored by length heterogeneity PCR (LH-PCR), considering the intact and lysed cells at different stages of cheese production and ripening. When starter species underwent autolysis, species coming from milk were able to grow. For the first time, the LH-PCR technique was applied to study a fermented food.
Parmigiano-Reggiano (PR) is a protected-designation-of-origin cheese, produced in specific areas of Northern Italy. It is a hard-textured, cooked, and long-ripened cheese made from raw cow's milk supplemented with natural whey starter rich in thermophilic lactic acid bacteria (LAB). Microbiological features of PR have been studied, isolating on traditional growth media a large number of strains from a large amount of samples representative of production and of the earlier and advanced stages of ripening (2, 3). Other studies have focused on the biodiversity of different strains of Lactobacillus helveticus isolated from natural whey cultures (6, 7). These previous studies showed the microbial biodiversity of PR but not its real microbial succession during production and ripening, and the use of culture-dependent methods could have underestimated the less-abundant components of microflora, which are equally important for cheese ripening and flavor development (15). Thus, the microbial ecology of PR still appears not to be completely understood. From this perspective, a detailed knowledge of LAB dynamics during the manufacturing and ripening stages is necessary for a deeper insight into the complex processes which contribute to the development of this appreciated cheese.
In the present study, length heterogeneity PCR (LH-PCR) was used to monitor the microbial dynamics during 24 months of PR ripening for both the whole and lysed cells. The availability of PR twin wheels allowed us to have samples representative of the subsequent stages of the same cheesemaking process.
In our opinion, the new findings in this article contribute to a better understanding of microbial dynamics in a complex fermented ecosystem.
Cheesemaking.
A unique cheese factory was selected for its equipment and technological conditions in order to obtain a suitable number of PR twin wheels. The same milk was distributed in equal volumes in eight vats and was worked according to PR production standards.
Thirty-four liters of natural whey starter was added in each vat, containing 1,070 liters of milk (525 liters of partially skimmed evening milk and 545 liters of whole morning milk), bringing the pH of the mixture to 6.20. After cheesemaking and molding, the wheels, two from each vat, were held for 3 days and frequently turned to enable complete whey drainage. They were then salted by immersion in brine at 300 g NaCl·liter−1 for 23 days. Ripening was carried out in aging rooms with 85% relative humidity and a temperature of about 18°C for 24 months.
Cheese sampling and bacterial recovery.
Samples were collected from milk to cheese ripened for 24 months. Aliquots of the total mass of raw milk, whey starter, milk plus whey starter in the vat, and curd at the vat extraction stage were sampled. For each of the following 14 samples, the whole wheel was sacrificed: curd after 6, 12, and 48 h from extraction, salted cheese, and cheese at different stages of ripening (2, 3, 4, 6, 8, 10, 12, 16, 20, and 24 months). The remaining two wheels will be the objects of further research. The wheels were cut lengthwise along the vertical axis, and a central 10-cm-thickness cheese section was obtained. Representative portions of internal (i.e., central) and external (i.e., ∼0.5 cm from the cheese crust) zones were obtained from these cheese sections. In order to have a sample representative of the whole wheel, the two portions were mixed at equal weights and grated before the analysis. Raw milk, whey starter, and milk plus whey starter in the vat were 10-fold serially diluted in 50 mM sodium citrate buffer (pH 7.0), while 10 g of curd and cheese samples were first homogenized with 90 ml of sodium citrate buffer for 3 min in a blender (Stomacher 400; Seward, United Kingdom).
In order to recover a heterogeneous bacterial population, different types of nutritional media and the following time/temperature conditions of incubation were used: MRS agar (pH 5.4) (Oxoid Spa, Italy) at 42°C for 48 h and 25°C for 72 h; M17-SSW (5) at 42°C for 42 h and 25°C for 72 h; whey agar medium (6) at 42°C for 48 h; curd agar medium (10); and cheese agar medium (4, 10) at 42°C for 72 h, all under anaerobic conditions.
The level of LAB originating from raw milk was lower than 4 log CFU ml−1. The natural whey starter was characterized by a high number of thermophilic lactobacilli (Table 1). The total cultivable microbial population was high and not variable during the first 6 months. After 9/10 months of ripening, a substantial decrease in cultivable bacteria was observed. Similar growth trends were observed in previous studies (2, 3), though the results are not easily comparable because different media were used.
TABLE 1.
Bacterial counts in different media of cultivable lactic acid thermophilic and mesophilic bacteria during PR cheese production and ripening
| Sample | Bacterial count with indicated medium and temperaturea
|
||||||
|---|---|---|---|---|---|---|---|
| M17-SSW
|
MRS (pH 5.4)
|
WAM, 42°C | CURDAM, 42°C | CAM, 42°C | |||
| 25°C | 42°C | 25°C | 42°C | ||||
| Raw milk | 3.61 ± 2.15 | 3.89 ± 2.08 | 2.13 ± 0.93 | 1.95 ± 0.63 | 2.43 ± 1.26 | 3.17 ± 1.96 | 3.23 ± 2.13 |
| Whey starter | NDb | 4.89 ± 3.33 | ND | 7.00 ± 5.45 | 8.53 ± 7.13 | 7.00 ± 5.66 | 1.00 ± 0.15 |
| Milk enriched with whey starter in vat | 3.61 ± 2.15 | 4.00 ± 2.75 | 2.13 ± 0.93 | 5.00 ± 3.55 | 7.00 ± 5.69 | 5.00 ± 3.66 | 3.23 ± 1.88 |
| Curd at vat extraction | 3.52 ± 2.28 | 5.05 ± 3.20 | 3.03 ± 1.77 | 6.36 ± 5.19 | 7.00 ± 5.80 | 4.00 ± 2.53 | 2.96 ± 1.60 |
| Curd, 6 h | 3.66 ± 2.42 | 5.55 ± 4.19 | 2.35 ± 0.85 | 7.25 ± 6.19 | 8.17 ± 6.89 | 7.53 ± 6.14 | 3.31 ± 1.93 |
| Curd, 12 h | 4.76 ± 3.24 | 4.87 ± 3.24 | 3.05 ± 1.83 | 6.97 ± 5.32 | 7.89 ± 6.19 | 6.93 ± 5.47 | 3.70 ± 2.10 |
| Curd, 48 h | 4.85 ± 3.39 | 4.34 ± 3.17 | 2.26 ± 1.10 | 5.70 ± 4.04 | 7.67 ± 6.14 | 7.19 ± 5.93 | 3.11 ± 1.95 |
| Salted cheese | 5.65 ± 4.47 | 5.94 ± 4.55 | 5.64 ± 4.09 | 5.63 ± 3.99 | 6.48 ± 5.08 | 4.15 ± 2.88 | 5.06 ± 3.83 |
| Cheese, 2 mo | 6.40 ± 5.14 | 6.21 ± 5.10 | 6.18 ± 4.81 | 6.77 ± 5.35 | 7.21 ± 6.15 | 7.07 ± 5.71 | 7.18 ± 5.55 |
| Cheese, 3 mo | 4.86 ± 3.30 | 4.18 ± 3.05 | 6.75 ± 4.88 | 7.29 ± 6.29 | 6.54 ± 5.03 | 7.13 ± 5.84 | 7.08 ± 5.82 |
| Cheese, 4 mo | 5.00 ± 3.94 | 4.78 ± 3.14 | 7.08 ± 5.68 | 7.08 ± 5.66 | 6.91 ± 5.51 | 6.15 ± 4.66 | 6.88 ± 5.55 |
| Cheese, 6 mo | 4.15 ± 2.98 | 3.74 ± 2.24 | 7.10 ± 5.96 | 6.85 ± 5.26 | 7.02 ± 5.95 | 6.35 ± 4.96 | 7.19 ± 7.19 |
| Cheese, 8 mo | 4.91 ± 3.41 | 4.90 ± 3.56 | 7.56 ± 6.30 | 6.65 ± 5.19 | 6.84 ± 5.18 | 5.17 ± 3.87 | 6.91 ± 5.66 |
| Cheese, 10 mo | 4.57 ± 3.12 | 4.17 ± 3.05 | 6.17 ± 5.09 | 6.28 ± 4.96 | 5.94 ± 4.45 | 5.10 ± 3.95 | 6.42 ± 5.16 |
| Cheese, 12 mo | 4.77 ± 3.15 | 4.54 ± 3.15 | 5.83 ± 3.85 | 5.93 ± 4.66 | 5.51 ± 4.15 | 5.39 ± 4.24 | 6.06 ± 4.98 |
| Cheese, 16 mo | 4.30 ± 3.08 | 4.00 ± 2.87 | 5.13 ± 3.69 | 5.30 ± 4.15 | 5.40 ± 4.09 | 4.60 ± 3.15 | 5.11 ± 3.51 |
| Cheese, 20 mo | 3.77 ± 2.23 | 3.00 ± 1.68 | 3.95 ± 2.54 | 4.34 ± 3.05 | 4.00 ± 2.95 | 3.18 ± 1.91 | 3.65 ± 2.56 |
| Cheese, 24 mo | 2.60 ± 1.33 | 3.00 ± 1.88 | 2.30 ± 1.19 | 3.10 ± 1.88 | 2.70 ± 1.60 | 2.34 ± 0.85 | 2.25 ± 1.10 |
Logarithmic bacterial counts (± standard deviations) are expressed in log CFU ml−1 for the first three samples and log CFU g−1 for the others. WAM, whey agar medium; CURDAM, curd agar medium; CAM, cheese agar medium.
ND, not determined.
Strain isolation and clustering and setup of an LH-PCR database.
To set up an appropriate LH-PCR database, a total of 187 strains were isolated from the five media used for all 18 stages of PR manufacturing and ripening. Genomic DNA of the isolated strains was extracted from overnight cultures by a Chelex-based procedure according to the method of Rossetti and Giraffa (13). DNA amplification and sequencing were performed as previously described (8), and each sequence obtained was checked manually and searched for sequence homology using the basic local alignment search tool (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi).
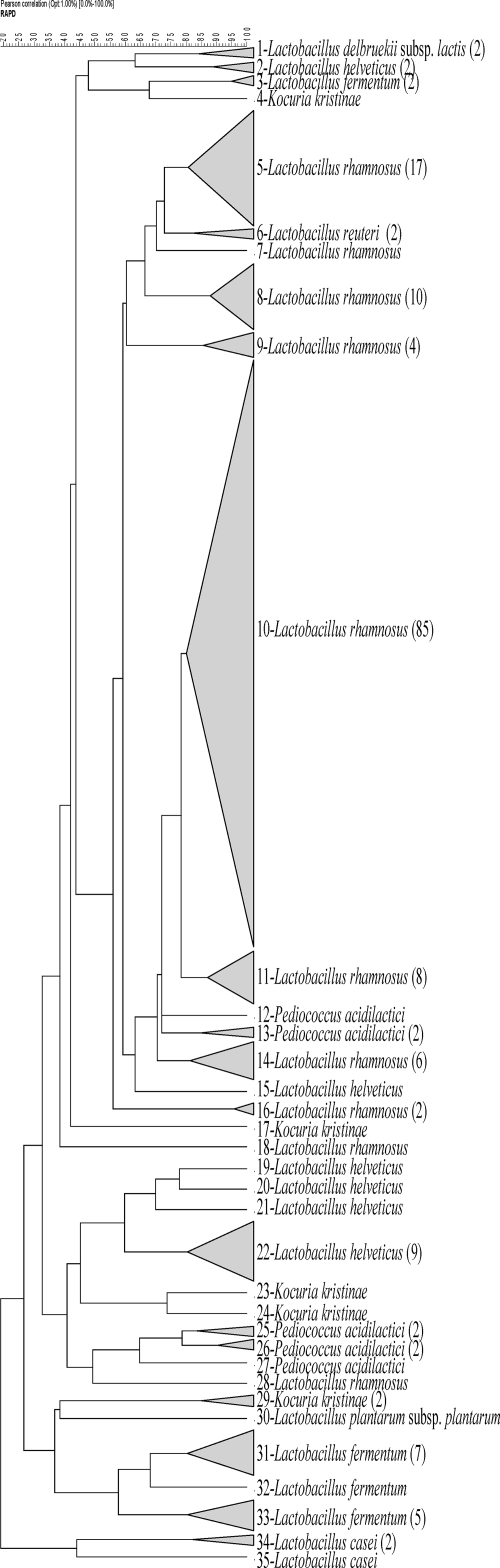
With the aim of clustering strains with genotypic relatedness, all species identified by 16S rRNA gene sequencing were fingerprinted by randomly amplified polymorphic DNA (RAPD)-PCR as previously described (13). Clustering of the patterns was achieved through the unweighted pair group method and employing arithmetic averages using the BioNumerics software program (package version 3.0; Applied Maths BVBA, Belgium). Calculation of the similarities of the PCR fingerprinting profiles was based on the Pearson product-moment correlation coefficient. Strains with a similarity coefficient higher than 80% in the dendrogram were considered to belong to the same biotype.
The dendrogram revealed 35 clusters, showing a high biodiversity among strains and species present in a complex system like PR cheese (Fig. 1).
FIG. 1.
Cluster analysis of RAPD-PCR patterns obtained with a primer, M13, from 187 strains isolated from samples. Clustering was performed using an unweighted pair group method and employing arithmetic averages of Pearson's product moment correlation coefficient (expressed as a percentage). Numbers from 1 to 35 report the RAPD-PCR clusters or branches. The number of strains isolated for each cluster is indicated in parentheses.
Thirteen strains, belonging to eight different species or belonging to the same species but with a different RAPD-PCR profile and coming from different samples, were chosen to generate the database. Moreover, six type strains of other species isolated from PR cheese but not found in this study were included (Table 2).
TABLE 2.
LH-PCR fragment length database of 13 strains isolated from cheese samples belonging to different RAPD-PCR clusters and 6 type strains
| Strain | RAPD-PCR cluster or branch | No. of isolatesa | Origin | Closest relative or type strainb | Accession no.c | % Match | Fragment size (bp)d |
|---|---|---|---|---|---|---|---|
| 750 | 23 | 1 | Curd, 12 h | Kocuria kristinae | AF375912 | 100 | 310 |
| 714 | 1 | 2 | Milk | Lactobacillus delbrueckii subsp. lactis | AB289095 | 99 | 330 (284) |
| 779 | 2 | 2 | Curd, 48 h | Lactobacillus helveticus | AB008210 | 100 | 334 |
| 776 | 22 | 9 | Curd, 6 h | Lactobacillus helveticus | AB008210 | 98 | 334 |
| 1056 | 34 | 2 | Cheese, 6 mo | Lactobacillus casei | AJ558112 | 100 | 335 |
| 1247 | 35 | 1 | Curd, 12 h | Lactobacillus casei | AB008205 | 100 | 335 |
| 830 | 10 | 85 | Salted cheese | Lactobacillus rhamnosus | EU184020 | 100 | 336 (290) |
| 1489 | 28 | 1 | Cheese, 20 mo | Lactobacillus rhamnosus | EF533991 | 99 | 336 (290) |
| 710 | 30 | 1 | Curd, 48 h | Lactobacillus plantarum subsp. plantarum | EF577047 | 99 | 337 (290, 308) |
| 1026 | 3 | 2 | Cheese, 6 mo | Lactobacillus fermentum | AF477498 | 99 | 342, 344 |
| 730 | 31 | 7 | Curd, 6 h | Lactobacillus fermentum | EU221276 | 99 | 342, 344 |
| 1466 | 12 | 1 | Cheese, 20 mo | Pediococcus acidilactici | EU147316 | 99 | 345 |
| 805 | 25 | 2 | Whey starter | Pediococcus acidilactici | EU147316 | 100 | 345 |
| Type LMG 6897 | Lactococcus lactis subsp. cremoris | 318 (272) | |||||
| Type LMG 6896 | Streptococcus thermophilus | 319 (273) | |||||
| Type LMG 6890 | Lactococcus lactis subsp. lactis | 318 (272, 291) | |||||
| Type LMG 11423 | Enterococcus faecium | 328, 331 (284, 301, 303) | |||||
| Type LMG 7937 | Enterococcus faecalis | 329 (283, 299) | |||||
| Type LMG 6901 | Lactobacillus delbrueckii subsp. bulgaricus | 330 | |||||
| Type LMG 11457 | Lactobacillus parabuchneri | 345 (255, 299) |
Quantity of strains isolated for this RAPD-PCR cluster or branch.
Determined by 16S-rRNA BLAST.
GenBank.
The fragment length is reported with an approximation of ±1 bp. The lengths of the secondary peaks are reported in parentheses.
The V1, V2, and V3 variable regions of the 16S rRNA genes of the 20 strains were analyzed by LH-PCR as previously described (9). Amplicon sizes were determined using the GeneMapper software program (version 4.0; Applied Biosystems, Foster City, CA) with a threshold of 150 fluorescence units.
The database reports fragment lengths from 310 to 346 bp. LH-PCR fragment sizes of the LAB agree with the findings of Lazzi et al. (9). In a difference from the work of these authors, other secondary peaks were found. As expected, strains belonging to different clusters but to the same species gave the same fragment sizes in base pairs (Table 2).
LH-PCR analysis of cheese samples.
To investigate evolution of the microbial community in PR cheese, grated cheese (and curd) samples were diluted 1:10 in 50 mM sodium citrate buffer (pH 7.0) and homogenized in a blender (Stomacher 400) for 3 min, while raw milk, whey starter, and milk plus whey starter in vat samples were directly subjected to the following steps. In order to separate whole bacterial cells from the DNA coming from lysed cells, 1 ml of each sample was filtered on a 0.2-μm filter (Whatman GmbH, Dassel, Germany) to obtain a free-cell fraction and another milliliter was left unfiltered and was directly placed in a 1.5-ml microtube. In order to digest free DNA arising from lysed cells, the nonfiltered fraction was treated with 0.14 U μl−1 of amplification-grade DNase I (Sigma-Aldrich Co., St. Louis, MO) under conditions given by the supplier. The samples were centrifuged at 7,500 rpm for 5 min, and the pellets were suspended in 100 μl of pure water with the addition of 20 μl 10× reaction buffer (Sigma-Aldrich Co., St. Louis, MO) and 20 μl amplification-grade DNase. This mixture was incubated for 1 h at room temperature. DNase was inactivated by adding 20 μl of stop solution (Sigma-Aldrich Co., St. Louis, MO) and heating at 70°C for 10 min.
DNA was extracted both from the filtered untreated fractions (lysed cells) and from the nonfiltered treated ones (whole cells) by means of a Qiagen-DNeasy blood and tissue kit (Qiagen GmbH, Hilden, Germany) as described by the manufacturer. DNA was quantified by measuring the absorbances at 260 nm and 280 nm (using a Jasco spectrophotometer), diluted up to 20 ng μl−1, and stored at −20°C until use. LH-PCR was performed as described for the database setup.
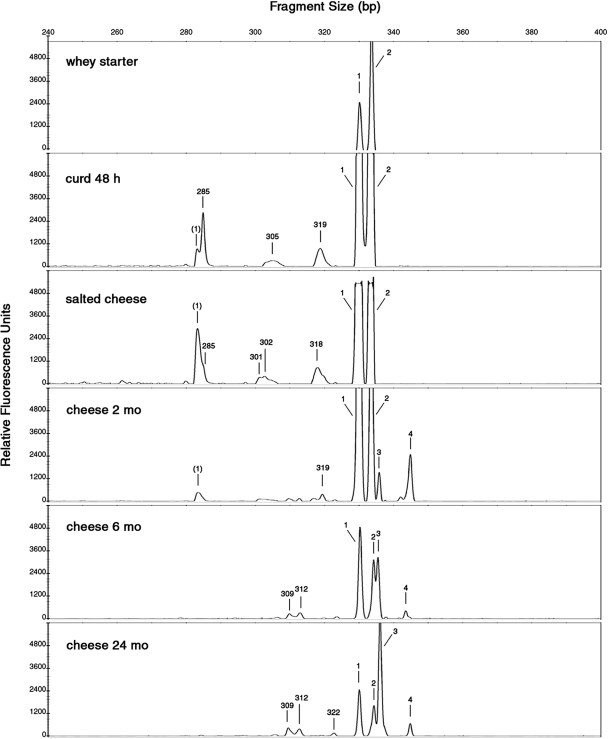
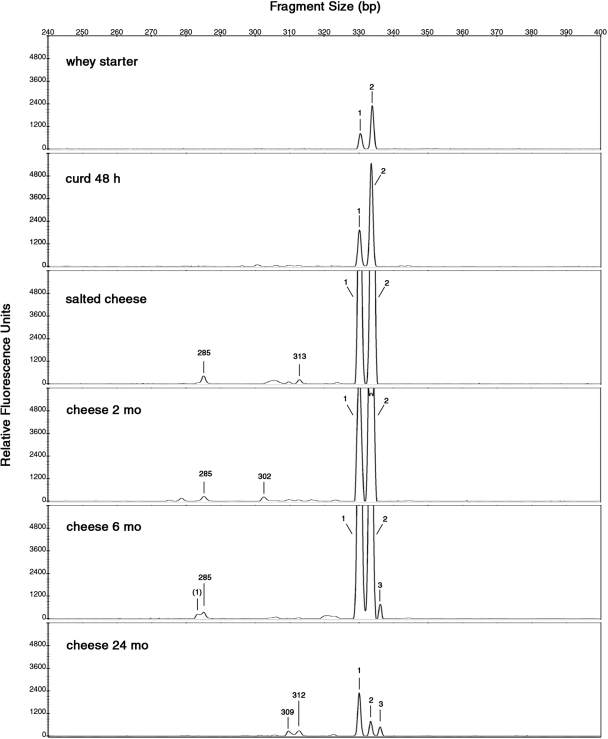
Figures 2 and 3 show the LH-PCR profiles obtained from LAB whole and lysed cells during PR cheese manufacturing. Only the electropherograms referring to the most representative samples (whey starter, curd at 48 h, salted cheese, and 2-, 6-, and 24-month cheeses) are shown. Electropherograms for 6-, 12-, and 48-h curds were similar. Two-, 3-, and 4-month cheeses gave similar patterns, and any evident differences were found in 6-, 8-, 10-, 12-, 16-, and 20-month cheese samples.
FIG. 2.
Length heterogeneity LH-PCR electropherograms of whole cells of LAB communities present in the samples studied at different stages of manufacturing and ripening of PR cheese. The x axis shows the peak size in base pairs, and the y axis shows the peak intensity in relative fluorescence units. The peak sizes were attributed to bacterial species according to the LH-PCR database (Table 2) as follows: 1, L. delbrueckii subsp. lactis or subsp. bulgaricus; (1), secondary peak of L. delbrueckii subsp. lactis; 2, L. helveticus; 3, L. rhamnosus, L. casei, or L. plantarum; 4, L. parabuchneri or P. acidilactici. Unattributed peaks higher than 150 fluorescence units are indicated with the respective base pair sizes.
FIG. 3.
Length heterogeneity LH-PCR electropherograms of lysed cells of LAB communities present in the samples studied at different stages of manufacturing and ripening of PR cheese. The x axis shows the peak size in base pairs, and the y axis shows the peak intensity in relative fluorescence units. The peak sizes were attributed to bacterial species according to the LH-PCR database (Table 2) as follows: 1, L. delbrueckii subsp. lactis or subsp. bulgaricus; (1), secondary peak of L. delbrueckii subsp. lactis; 2, L. helveticus; 3, L. rhamnosus, L. casei, or L. plantarum. Unattributed peaks higher than 150 fluorescence units are indicated with the respective base pair sizes.
The different fragment sizes in the LH-PCR profiles were attributed to bacterial species according to the LH-PCR database (Table 2).
The peaks detected in the raw milk whole-cell electropherogram were as follows: 330 bp (attributed to Lactobacillus delbrueckii subsp. lactis or subsp. bulgaricus or to Enterococcus faecium or Enterococcus faecalis), 334 bp (L. helveticus), 336 bp (Lactobacillus rhamnosus, Lactobacillus plantarum, or Lactobacillus casei), and 339 bp (not attributed). The fluorescence intensity was more than 10-fold lower than the average found in the other samples (data not shown). As expected, the amount of DNA from filtered raw milk, released from lysed cells, was too small to be amplified (data not shown).
The LH-PCR profile referring to whole LAB cells from natural whey starter showed two peaks (Fig. 2), corresponding to L. delbrueckii subsp. lactis or subsp. bulgaricus and L. helveticus species. In a difference from previous studies on natural whey starter for a similar cheese, Grana Padano (5, 9, 14), a Streptococcus thermophilus peak (320 ± 1 bp) was not found. Contrary to findings of Coppola et al. (3), we never in our samples isolated a L. delbrueckii subsp. bulgaricus species which, on the other hand, could have been viable but hardly cultivable. Whey starter is expected to have a high percentage of metabolically active cells; however, the LH-PCR profile from lysis-released DNA is similar to, even if three- to fourfold lower than, the whole-cell profile, showing an autolysis phenomenon.
In the 48-h curd whole-cell electropherogram, two major peaks, attributable to L. helveticus and L. delbrueckii subsp. lactis or subsp. bulgaricus, were found. Three other, unattributed peaks of minor fluorescence intensity were detected. In the LH-PCR profile from lysis-released DNA of 48-h curd, only the two attributable peaks were found.
After 1 month of brining, the salted cheese sample was characterized by an attributable peak pattern of whole cells similar to the previous one. In the lysis-released DNA profile, the attributed peaks were higher than those in the 48-h curd and two unknown peaks appeared. This result could highlight an increase in cell autolysis after the brining.
After 2 months of ripening, whole cells of L. helveticus and L. delbrueckii subsp. lactis or subsp. bulgaricus were found in great amounts but none of these species was isolated from agar plates (Table 2). These cells could be quiescent, might be viable but not cultivable, and might still not be lysed. They also could be dead, not-yet-lysed cells, as suggested by fluorescent dyes in the live-and-dead cell assay (data not shown). In addition to L. helveticus and L. delbrueckii subsp. lactis or subsp. bulgaricus, two other peaks, attributable to L. rhamnosus, L. casei, or L. plantarum and to Lactobacillus parabuchneri or Pediococcus acidilactici, were able to grow and appear only in the whole-cell electropherogram.
The electropherogram of whole cells from 6-month cheese showed the same peaks as previous samples but with reduced peak intensities. Instead, in the lysis-released DNA electropherogram, a peak attributable to L. rhamnosus, L. casei, or L. plantarum appeared. This trend persisted without appreciable variation in the subsequent samples until 24 months of ripening (data not shown).
In the 24-month cheese whole-cell electropherogram, the major peak was attributable to L. rhamnosus, L. casei, or L. plantarum. Notably, in the lysis-released DNA electropherogram, an important decrease in fluorescence intensity was observed. In agreement with Thomas et al. (16) and Nielsen et al. (12), we can suppose that DNA degradation of some microorganisms could be a source of carbon, nitrogen, phosphorus, and nucleic acid precursor for less nutritionally demanding bacterial cells in the particularly hostile nutritional environment occurring during the last months of PR ripening. Thomas et al. (16) and Williams et al. (17) have hypothesized that nonstarter LAB could use nucleic acids derived from the autolysis of starter LAB as an alternative potential energy source. Even though pure nucleic acids are generally not sufficient as a sole carbon source for bacteria, it has been demonstrated that Serratia marcescens and Escherichia coli are capable of utilizing DNA exclusively for carbon (12).
LH-PCR and reverse transcription-LH-PCR have already been successfully used for the analysis of fresh dairy products (9, 14) and to monitor LAB dynamics during maize ensiling (1). As far as we know, this technique has never been used to study a ripened cheese where viable, not viable, and lysed microbial cells are contemporaneously present. Analysis of intact DNA extracted from degraded specimens and tissue samples has become a useful tool for criminal and conservation forensics. In an intriguing review, Nielsen et al. (12) studied the release, breakdown, and persistence of bacterial and plant DNA in soil, sediment, and water. The recovery of DNA from processed food has already been used for detection and quantification of genetically modified ingredients (11). However, to our knowledge this approach has never been used to evaluate the presence of DNA from lysed cells in fermented food.
Due to its low sensitivity, LH-PCR is not meant to provide a quantitative analysis. However, it gave us a way to follow and be aware of the dynamics of whole and lysed bacterial cells during PR cheese production and ripening, letting us make important new findings for knowledge of this appreciated cheese.
Acknowledgments
This work was partially supported by Emilia Romagna Region (LR28/98), Bologna, Italy, project SMEPR Studio e modelazione degli aspetti enzimatici legati alla stagionatura del formaggio Parmigiano-Reggiano.
Footnotes
Published ahead of print on 8 August 2008.
REFERENCES
- 1.Brusetti, L., S. Borin, D. Mora, A. Rizzi, N. Raddadi, C. Sorlini, and D. Daffonchio. 2006. Usefulness of length heterogeneity-PCR for monitoring lactic acid bacteria succession during maize ensiling. FEMS Microbiol. Ecol. 56:154-164. [DOI] [PubMed] [Google Scholar]
- 2.Coppola, R., M. Nanni, M. Iorizzo, A. Sorrentino, E. Sorrentino, and L. Grazia. 1997. Survey of lactic acid bacteria isolated during the advance stages of the ripening of Parmigiano Reggiano cheese. J. Dairy Res. 64:305-310. [Google Scholar]
- 3.Coppola, R., M. Nanni, M. Iorizzo, A. Sorrentino, E. Sorrentino, C. Chiavari, and L. Grazia. 2000. Microbiological characteristics of Parmigiano Reggiano cheese during the cheesemaking and the first months of the ripening. Lait 80:479-490. [Google Scholar]
- 4.De Dea Lindner, J. 2008. Traditional and innovative approaches to evaluate microbial contribution in long ripened fermented foods: the case of Parmigiano Reggiano cheese. Ph.D. thesis. University of Parma, Parma, Italy.
- 5.Fornasari, M. E., L. Rossetti, D. Carminati, and G. Giraffa. 2006. Cultivability of Streptococcus thermophilus in Grana Padano cheese whey starters. FEMS Microbiol. Lett. 257:139-144. [DOI] [PubMed] [Google Scholar]
- 6.Gatti, M., C. Lazzi, L. Rossetti, G. Mucchetti, and E. Neviani. 2003. Biodiversity in Lactobacillus helveticus strains present in natural whey starter used for Parmigiano Reggiano cheese. J. Appl. Microbiol. 95:463-470. [DOI] [PubMed] [Google Scholar]
- 7.Gatti, M., C. Trevisano, E. Fabrizi, E. Neviani, and F. Gardini. 2004. Biodiversity within Lactobacillus helveticus isolated from different natural whey starter cultures as revealed by classification trees. Appl. Environ. Microbiol. 70:182-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giraffa, G., C. Lazzi, M. Gatti, L. Rossetti, D. Mora, and E. Neviani. 2003. Molecular typing of Lactobacillus delbrueckii of dairy origin by PCR-RFLP of protein coding genes. Int. J. Food Microbiol. 82:163-172. [DOI] [PubMed] [Google Scholar]
- 9.Lazzi, C., L. Rossetti, M. Zago, E. Neviani, and G. Giraffa. 2004. Evaluation of bacterial communities belonging to natural whey starters for Grana Padano cheese by length heterogeneity-PCR. J. Appl. Microbiol. 96:481-490. [DOI] [PubMed] [Google Scholar]
- 10.Lazzi, C., M. Gatti, V. Bernini, J. De Dea Lindner, and E. Neviani. 2007. Impiego di nuovi terreni colturali a base di cagliata e di formaggio per il recupero e la differenziazione della microflora caratteristica di formaggi a lunga stagionatura. Sci. Tec. Lattiero-Casearia 58:55-69. [Google Scholar]
- 11.Miraglia, M., K. G. Berdal, C. Brera, P. Corbisier, A. Holst-Jensen, E. J. Kok, H. J. Martin, H. Schimmel, J. Rentsch, J. P. van Rie, and J. Zagon. 2004. Detection and traceability of genetically modified organisms in the food production chain. Food Chem. Toxicol. 42:1157-1180. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen, K. M., P. J. Johnsen, D. Bensasson, and D. Daffonchio. 2007. Release and persistence of extracellular DNA in the environment. Environ. Biosafety Res. 6:37-53. [DOI] [PubMed] [Google Scholar]
- 13.Rossetti, L., and G. Giraffa. 2005. Rapid identification of dairy lactic acid bacteria by M13-generated, RAPD-PCR fingerprint databases. J. Microbiol. Methods 63:135-144. [DOI] [PubMed] [Google Scholar]
- 14.Santarelli, M., M. Gatti, C. Lazzi, V. Bernini, G. A. Zapparoli, and E. Neviani. 2008. Whey starter for Grana Padano cheese: effect of technological parameters on viability and composition of the microbial community. J. Dairy Sci. 91:883-891. [DOI] [PubMed] [Google Scholar]
- 15.Steele, J. L., M. F. Budinich, H. Cai, S. C. Curtis, and J. R. Broadbent. 2006. Diversity and metabolic activity of Lactobacillus casei in ripening Cheddar cheese. Aust. J. Dairy Technol. 61:53-60. [Google Scholar]
- 16.Thomas, T. D. 1986. Oxidative activity of bacteria from Cheddar cheese. N. Z. J. Dairy Sci. Technol. 21:37-47. [Google Scholar]
- 17.Williams, A. G., S. E. Withers, and J. M. Banks. 2000. Energy sources of non-starter lactic acid bacteria isolated from Cheddar cheese. Int. Dairy J. 10:17-23. [Google Scholar]