Abstract
The CorA Mg2+ channel is the primary source of intracellular Mg2+ in Salmonella enterica serovar Typhimurium. In another study, we found that a strain lacking corA was attenuated in mice and also defective for invasion and replication within Caco-2 epithelial cells (K. M. Papp-Wallace, M. Nartea, D. G. Kehres, S. Porwollik, M. McClelland, S. J. Libby, F. C. Fang, and M. E. Maguire, J. Bacteriol. 190:6517-6523, 2008). Therefore, we further examined Salmonella interaction with Caco-2 epithelial cells. Inhibiting CorA acutely or chronically with a high concentration of a selective inhibitor, Co(III) hexaammine, had no effect on S. enterica serovar Typhimurium invasion of Caco-2 epithelial cells. Complementing the corA mutation with corA from various species rescued the invasion defect only if the complementing allele was functional and if it was evolutionarily similar to S. enterica serovar Typhimurium CorA. One explanation for these results could be that regulation of CorA function is needed for optimal virulence. Further experiments examining corA transcription, CorA protein content, CorA transport, and cell Mg2+ content indicated that both CorA expression and CorA function are differentially regulated. Moreover, the rates of Mg2+ influx via CorA are not closely correlated with either protein levels or Mg2+ content. We conclude that loss of the CorA protein disrupts a regulatory network(s) with the ultimate phenotype of decreased virulence. This conclusion is compatible with the microarray results in our other study, which showed that loss of corA resulted in changes in transcription (and protein expression) in multiple metabolic pathways (Papp-Wallace et al., J. Bacteriol. 190:6517-6523, 2008). Further study of the regulation of CorA expression and function provides an opportunity to dissect the complexity of Mg2+ homeostasis and its ties to virulence within the bacterium.
Mg2+ is implicated in several stages of Salmonella enterica serovar Typhimurium infection. For example, exposure to low extracellular Mg2+ concentrations results in activation of the PhoP/PhoQ two-component system and consequently markedly alters the expression of many genes necessary for virulence, including but not limited to those that encode Salmonella pathogenicity island 1, Salmonella pathogenicity island 2, host antimicrobial peptide resistance, bile resistance, and biofilm formation (1, 5, 18). mgtA and mgtB, which encode two inducible Mg2+ transport systems, are also PhoP/PhoQ regulated (5, 32, 34).
The primary source of intracellular Mg2+ in S. enterica serovar Typhimurium, about 50% of other bacteria, and about 20% of archaea is the CorA Mg2+ channel (12-14, 21). A strain lacking corA shows no Mg2+ growth phenotype or other growth deficit. Despite this lack of an overt growth phenotype, in another study we found that a strain lacking corA (MM2242) was attenuated in mice after infection by both the oral and intraperitoneal routes and was defective for invasion and replication within Caco-2 epithelial cells (22). Microarray data indicated that multiple metabolic pathways are affected by corA mutation. The simplest explanation for these defects is that, in the absence of CorA, the organism cannot obtain sufficient intracellular Mg2+ for optimal virulence despite having two other Mg2+ transporters. However, here we show that intracellular Mg2+ is relatively unaffected by loss of corA. The virulence phenotype and changes in gene expression are related to the presence of a functional CorA protein and most likely regulation of CorA function rather than the actual transport process.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains used in this study are listed in Table 1. Unless otherwise indicated, all are derived from S. enterica serovar Typhimurium SL1344, referred to as our wild-type strain. Cloning of Methanococcus jannaschii corA into the pUC18 plasmid (pMJcorA) has been previously reported (27). This plasmid carrying M. jannaschii corA was isolated from MM1556 and transformed into the corA mutant strain (MM2242), creating MM3203. The Escherichia coli corA and Bacillus subtilis mgtE genes were cloned from E. coli DH5α cells (MM3097) and B. subtilis genomic DNA (ATCC 23857D), respectively, into a pBADMycHis(a) vector (Invitrogen), creating pBADECcorA and pBADBSmgtE. The restriction sites used for insertion into this vector were XhoI and EcoRI. An additional stop codon was added to prevent the MycHis tag from being added. Expression from the pBAD vectors was dependent on the arabinose-inducible araBAD promoter. These plasmids were shuttled into the restrictionless MM1242 strain and then into MM2242, creating strains MM3218 and MM3217, respectively. MM3217 and MM3218 were grown in Luria-Bertani (LB) broth with 1 mM arabinose overnight for induction prior to experiments. pCorAF266A and pCorAP269A are low-copy pAlter plasmids carrying the corA gene with site-directed substitutions at the indicated amino acid positions and have been previously described (33). The pCorAF266A and pCorAP269A plasmids were isolated from MM2034 and MM2036 and transformed into the corA mutant strain (MM2242), generating MM3227 and MM3228, respectively. To create the corB, corC, and corD strains, the genes were deleted by the technique of Datsenko and Wanner (4). Deletions were confirmed by PCR. The high-frequency generalized transducing bacteriophage P22 HT 105/1 int-201 was used to transduce the corB, corC, and corD deletions into MM2089, thus creating strains MM3238, MM3239, and MM3240; phage-free, phage-sensitive transductants were purified by successive rounds of purification on EBU agar (17). The pTTCAlux plasmid carries the corA promoter to drive the expression of luxAB to produce luciferase and has been previously described (35). The pTTCAlux plasmid was isolated from MM1103 and transformed into wild-type S. enterica serovar Typhimurium SL1344 (MM2089) and the corA mutant strain (MM2242), creating MM3252 and MM3253, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain | Genotype | Source(s) |
|---|---|---|
| MM281 | S. enterica serovar Typhimurium LT2 DEL485(leuBCD) corA45::MudJ mgtA21::MudJ mgtB10::MudJ zjh-1628::Tn10Δ16Δ17(Cam) | 9 |
| MM387 | S. enterica serovar Typhimurium LT2 DEL485(leuBCD) corA185::Tn10Δ16Δ17(Tet) | 26 |
| MM1103 | MM387 with pTTCAlux | 35 |
| MM1242 (JR501) | S. enterica serovar Typhimurium LT2 hsdSA29 hsdSB121 hsdL6 metA22 metE551 trpC2 ilv-452 rpsL120 xyl-404 galE719 H1-b H2-en,n,x (Fels2) fla-66 nml | 2 |
| MM1556 | MM281 with pMJcorA | 27 |
| MM2034 | MM281 with pCorAF266A | 33 |
| MM2036 | MM281 with pCorAP269A | 33 |
| MM2089 | S. enterica serovar Typhimurium SL1344 hisG rpsL xyl (wild type) | 10; B. B. Finlaya |
| MM2242 | SL1344 corA52::Tn10Δ16Δ17 | J. Linb |
| MM2320 | MM2242 with pCorA (pJL10) | 22; J. Linb |
| MM3097 | Escherichia coli DH5α | |
| MM3203 | MM2242 with pMJcorA | This study |
| MM3217 | MM2242 with pBADBSmgtE | This study |
| MM3218 | MM2242 with pBADECcorA | This study |
| MM3227 | MM2242 with pCorAF266A | This study |
| MM3228 | MM2242 with pCorAP269A | This study |
| MM3238 | MM2089 with corB::Kan | This study |
| MM3239 | MM2089 with corC::Kan | This study |
| MM3240 | MM2089 with corD::Kan | This study |
| MM3252 | MM2089 with pTTCAlux | This study |
| MM3253 | MM2242 with pTTCAlux | This study |
Michael Smith Laboratories, University of British Columbia, Vancouver, British Columbia, Canada.
Case Western Reserve University, Cleveland, OH.
Luciferase assays.
Cells were grown in LB broth with antibiotics overnight at 37°C with shaking, spun down in the cold (4°C), and washed three times in cold N-minimal medium (19). Cells were subcultured into either LB or N-minimal medium with 0.1% Casamino Acids and 0.4% glucose. Either 10 μM or 10 mM MgSO4 was added to the N-minimal medium cultures, and all cultures were grown at 37°C with shaking. The measurement time points used were approximately 2, 4, 6, and 20 h, roughly corresponding to the early, mid, and late exponential and stationary phases, respectively. Wild-type and corA mutant strains have identical time courses of growth in each of the growth media used (data not shown). At each time point, cells were diluted 1:100 in N-minimal medium and vortexed. Then, 50 μl of cell suspension was immediately added to 500 μl luciferase reaction buffer (0.01% dodecyl aldehyde in 50 mM sodium phosphate, pH 7.5). Reaction mixtures were vortexed for 10 s and immediately read on a liquid scintillation counter for 30 s. Relative light units obtained were corrected for optical density at 600 nm (OD600).
Epithelial cell invasion experiments.
Epithelial cell invasion experiments were conducted as described elsewhere (22). Briefly, bacteria were grown overnight at 37°C without shaking in LB broth without antibiotics, washed once with phosphate-buffered saline (PBS), and suspended in complete cell culture growth medium without fetal bovine serum and antibiotics. Medium was removed from the Caco-2 epithelial cells, and the bacterial suspension was added at a multiplicity of infection of 10:1. Plates were centrifuged at 1,000 rpm for 10 min at room temperature and incubated at 37°C with 5.0% CO2. After 1 h, the cells were washed three times with PBS and treated with gentamicin (100 μg/ml) in complete cell culture growth medium without fetal bovine serum and antibiotics for 2 h to kill extracellular bacteria. At each time point, the cultured cells were washed three times with PBS, lysed with 0.1% Triton X-100 detergent for 5 min, collected, diluted, plated onto LB plates, and grown overnight at 37°C for CFU determination. The number of bacteria obtained from within the cultured cells was divided by the total number of bacteria added to the cultured cells to obtain the percentage of gentamicin-protected bacteria.
Atomic absorption.
Bacteria were grown overnight in LB broth with antibiotics at 37°C with shaking. Cells were then washed in the cold three times with cold N-minimal medium (20) and then subcultured into LB or N-minimal medium containing 0.4% glucose and 0.1% Casamino Acids, all without antibiotics. N-minimal medium cultures had 10 μM, 10.0 mM, or 100 mM MgSO4 added before incubation at 37°C with shaking. During the log and stationary phases, 1.0 ml of each culture was collected and pelleted through a 2:1 dibutyl-dioctyl phthalate solution to strip extracellular water. The phthalate solution was removed, the sides of the tube were dried carefully with a cotton swab to remove residual phthalate and residual water, and 1.0 N nitric acid was added to digest the pellet. Samples were sonicated for 30 s in a water bath sonicator and read by atomic absorption spectrometry. A standard curve of known Mg2+ concentrations was used to obtain the mass content within the cells after correction for OD600.
Western blot assays.
Triplicate aliquots were grown as described for luciferase assays to log or stationary phase in the various media. Cells were pelleted and resuspended in 1.0 ml of N-minimal medium. Resuspended cells were lysed by sonication for 30 s, and protein concentrations were determined with the Bradford protein assay (Bio-Rad). Ten micrograms of total protein was loaded onto 12% sodium dodecyl sulfate-polyacrylamide gels. Gels were electrophoresed and transferred onto nitrocellulose (Schleicher & Schuell, Keene, NH). The CorA antibody (29) was used at a dilution of 1:10,000, and a DnaK antibody (Bio-Rad) was used at a dilution of 1:5,000. Secondary horseradish peroxidase-linked anti-rabbit and anti-mouse antibodies (Amersham) were each used at a dilution of 1:10,000. Proteins were visualized by enhanced chemiluminescence as recommended by the manufacturer (Amersham). Western blot assays were scanned and quantitated by densitometry with DnaK as a loading control.
Transport assays.
Bacteria were grown as described above for luciferase assays. The uptake of 63Ni2+ (GE Healthcare) and 57Co2+ (MP Biomedicals) was assayed instead of Mg2+ uptake, as 28Mg2+ is very difficult to obtain. Methods for transport have been described in detail previously (7, 15). Briefly, cells were centrifuged at 4°C, washed once in cold cell wash buffer (N-minimal medium with 0.1% Casamino Acids and 0.4% glucose), resuspended in cell wash buffer to an OD600 between 1.0 and 2.0, and kept on ice until assayed. Cell aliquots of 100 μl were added to tubes containing various concentrations of inhibitor cation plus 200 μM NiCl2 and 0.3 to 1 μCi 63Ni2+ in a final volume of 1 ml. The reaction mixtures were incubated for 5 min at 37°C, stopped by the addition of 5 ml of ice-cold transport wash buffer (N-minimal medium with 10 mM MgSO4 and 0.5 mM EDTA), and filtered immediately on nitrocellulose filters (Schleicher & Schuell, Keene, NH). Filters were washed once with 5 ml of transport wash buffer and placed in 3 ml of Biosafe II scintillation cocktail (Research Products International Corp., Mount Prospect, IL) for measurement of radioactivity by scintillation counting.
RESULTS
A functional and evolutionarily related CorA protein is required for replication of S. enterica serovar Typhimurium within Caco-2 epithelial cells.
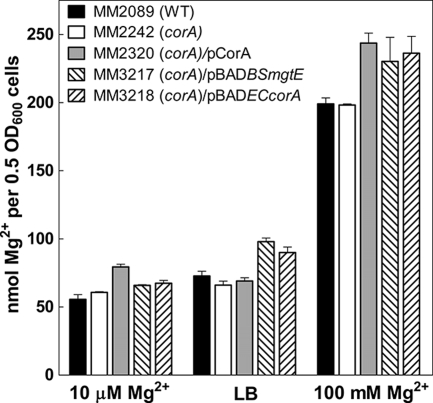
Since CorA is the primary Mg2+ channel in Salmonella, one reasonable hypothesis for a virulence defect is that a corA mutant strain has insufficient intracellular levels of Mg2+ even though a corA mutant strain does not exhibit any Mg2+-dependent or other growth defect. To address this question, we measured total intracellular Mg2+ content by atomic absorption. Mg2+ content was measured in N-minimal medium with 10 μM or 100 mM Mg2+ and in LB broth. Total intracellular Mg2+ levels were similar in N-minimal medium with 10 μM Mg2+ and LB medium but were about two- to fourfold increased in cells grown in N-minimal medium with 100 mM Mg2. Regardless of the absolute level, however, total intracellular Mg2+ did not differ between wild-type (MM2089) and corA mutant (MM2242) strains under any growth condition (Fig. 1). Thus, a corA mutant strain does not appear to lack Mg2+. A caveat to this conclusion is that only “total” intracellular Mg2+ is being measured by atomic absorption. It is possible that the level of “free” Mg2+ is altered in a corA mutant strain, but there is currently no known method to accurately measure intracellular “free” Mg2+ in actively growing cells.
FIG. 1.
Intracellular Mg2+ content. Total intracellular Mg2+ content was measured by atomic absorption after growing the wild-type (WT) strain (MM2089), a corA mutant strain (MM2242), a corA mutant strain with pCorA (MM2320), a corA mutant strain with pBADBSmgtE (MM3217), and a corA mutant strain with pBADECcorA (MM3218) either in LB medium or in N-minimal medium with 10 μM or 100 mM MgSO4. Data represent the average of three samples per strain.
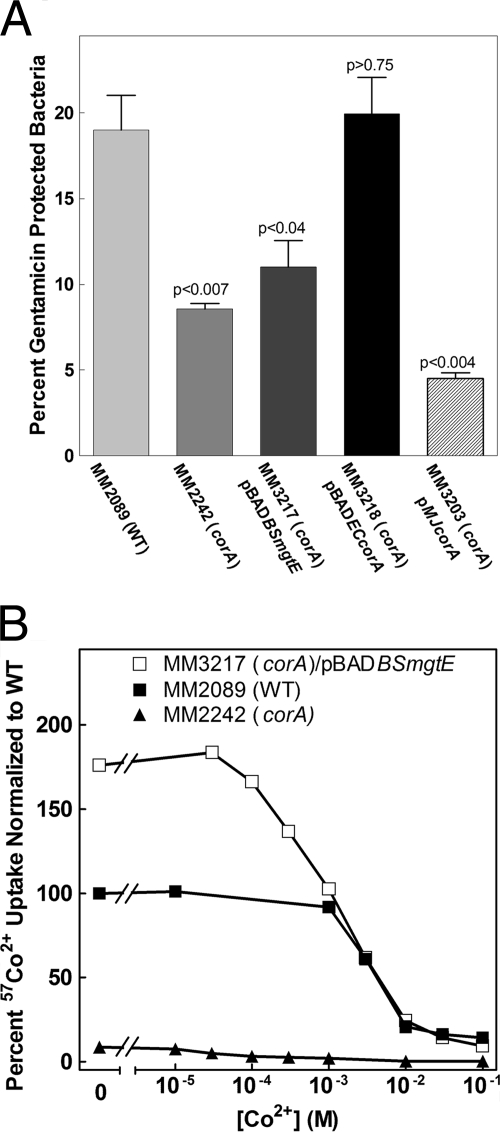
In another study, we found that a corA mutant strain (MM2242) has decreased invasion and replication within epithelial cells (22). Thus, we wanted to determine whether Mg2+ transport could rescue this corA epithelial cell phenotype. CorA from E. coli (MM3218) rescues the invasion defect (Fig. 2A), as does S. enterica serovar Typhimurium CorA (MM2320) (22). However, E. coli CorA and S. enterica serovar Typhimurium CorA differ by only eight amino acids, all conservative substitutions. We then determined whether a more phylogenetically distant CorA protein could rescue the corA mutant phenotype. The corA gene from the archaeal organism M. jannaschii can be stably expressed in S. enterica serovar Typhimurium (MM3203). M. jannaschii CorA exhibits a rate of transport that is about 15% of that of wild-type S. enterica serovar Typhimurium CorA. This rate of uptake is about threefold greater than the rate of uptake via MgtA and MgtB combined under these growth conditions (9, 31). Mg2+ affinity for influx and the Ki of Co(III) hexaammine for M. jannaschii CorA are identical to those for S. enterica serovar Typhimurium CorA (27). Despite this level of Mg2+ uptake, M. jannaschii CorA cannot rescue the invasion defect (Fig. 2A). MgtE is another class of Mg2+ transporter in prokaryotes that is structurally and mechanistically unrelated to CorA (8, 30, 36). MgtE from B. subtilis is functional in S. enterica serovar Typhimurium (MM3217) (Fig. 2B); however, MgtE also cannot rescue the corA defect (Fig. 2A). Thus, one interpretation of the above results is that only an evolutionarily similar CorA protein can rescue the invasion/replication defect. Moreover, simply supplying Mg2+ via another transporter also cannot rescue the invasion/replication phenotype. These results imply that the CorA protein itself is what is important for virulence.
FIG. 2.
Effect of complementation with foreign CorA proteins on invasion of Caco-2 epithelial cells. (A) The ability of E. coli corA (MM3218), M. jannaschii corA (MM3203), or B. subtilis MgtE (MM3217) to complement the invasion defect of a corA mutant strain was determined. S. enterica serovar Typhimurium strains were allowed to invade Caco-2 epithelial cells for 1 h, and intracellular bacteria were left to replicate for 6 h in the presence of gentamicin, except with the M. jannaschii corA mutant, where replication was for 4 h. P values indicate t tests comparing the wild type (MM2089) to the other strains. Data represent the average of three independent experiments (two experiments for the M. jannaschii corA mutant). (B) Transport via the B. subtilis MgtE transporter expressed in S. enterica serovar Typhimurium. 57Co2+ uptake was measured in the wild type (MM2089), a corA mutant strain (MM2242), and a corA mutant strain with a plasmid carrying a functional B. subtilis mgtE gene (MM3217). The data were normalized to the amount of 57Co2+ uptake in wild-type cells in the absence of additional CoCl2. The data shown are from a single experiment representative of two independent experiments. WT, wild type.
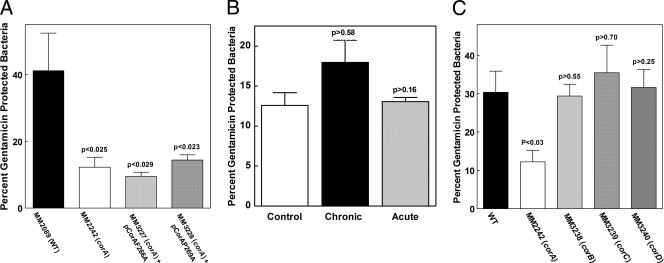
We next tested whether the S. enterica serovar Typhimurium CorA protein needed to be functional to rescue the invasion/replication defect. We compared our collection of S. enterica serovar Typhimurium CorA mutants (29, 33) to the recent crystal structure of Thermotoga maritima CorA (12) and chose two S. enterica serovar Typhimurium CorA mutants with alanine substitutions at positions F266 and P269 (33). Residues F266 and P269 are approximately one helical turn apart on transmembrane segment one, which forms the pore of the channel in its closed form. Previous Western blot assay and transport experiments with these mutants indicated that CorA is expressed at approximately wild-type levels and that each mutant mediates influx at 40 to 50% of the wild-type rate of transport (33). However, when expressed in a strain (MM281) lacking all three Mg2+ transporters, the strain expressing either of these mutant CorA proteins requires 0.25 mM Mg2+ for growth in N-minimal medium. A strain expressing wild-type CorA requires only 0.01 mM Mg2+. This result suggests that the F266A CorA and P269A CorA channels cannot close properly and are partially open or “leaky,” thus making it harder for the strain to acquire and retain sufficient Mg2+. We expressed these two mutant corA alleles (MM3227 and MM3228) on a low-copy plasmid (pAlter) in the corA mutant strain (which still retains functional alleles of mgtA and mgtB). Neither of these mutant CorA proteins could rescue the invasion/replication defects in Caco-2 cells (Fig. 3A). Thus, CorA must be functional for S. enterica serovar Typhimurium to be fully virulent. In addition, the failure of the two mutant CorA channels to rescue the invasion defect suggests that the channel must be able to close normally to obtain wild-type levels of invasion/replication.
FIG. 3.
Inhibition of CorA and the role of Mg2+ efflux in Caco-2 epithelial cell invasion. (A) S. enterica serovar Typhimurium cells were allowed to invade Caco-2 epithelial cells for 1 h, and intracellular bacteria were left to replicate for 6 h in the presence of gentamicin. The effects of mutations of CorA (F266A and P269A) putatively affecting Mg2+ transport on the invasion of Caco-2 epithelial cells were measured. P values indicate t tests comparing the wild type (MM2089) to the F266A and P269A corA mutant strains (MM3227 and MM3228). Data represent the average of two independent experiments. (B) Wild-type S. enterica serovar Typhimurium cells (MM2089) were allowed to invade Caco-2 epithelial cells for 1 h, which were then treated with gentamicin for 1.5 h to kill extracellular bacteria. The effect of chronic (overnight during growth) or acute (immediately prior to invasion) inhibition of CorA with Co(III) hexaammine on Caco-2 cell invasion was measured. The control represents wild-type invasion in the absence of Co(III) hexaammine. In both cases, Co(III) hexaammine was maintained in the medium during invasion. Chronic exposure of the Caco-2 cells to the inhibitor did not affect their viability (data not shown). (C) S. enterica serovar Typhimurium cells were allowed to invade Caco-2 epithelial cells for 1 h, and intracellular bacteria were left to replicate for 6 h in the presence of gentamicin. The effects of mutations of the corB, corC, and corD genes affecting Mg2+ efflux via CorA were determined. P values indicate t tests comparing the wild type (MM2089) to the corB, corC, or corD mutant strain (MM3238, MM3239, or MM3240, respectively). A single experiment representative of three additional experiments is shown.
Next, we took advantage of Co(III) hexaammine, a selective competitive inhibitor of CorA influx (11). In a strain lacking mgtA and mgtB, chronic inhibition with Co(III) hexaammine is bacteriostatic, indicating that substantial Mg2+ influx does not occur in the presence of a high concentration of Co(III) hexaammine. We reasoned that giving wild-type cells (MM2089) a maximum inhibitory dose of Co(III) hexaammine would mimic a closed channel but one which has the potential to function normally. Chronic (overnight) or acute (immediately before infection) exposure of wild-type S. enterica serovar Typhimurium (MM2089) to Co(III) hexaammine to inhibit Mg2+ uptake had no significant effect on the invasion of Caco-2 epithelial cells (Fig. 3B). We have previously shown that the inhibitor does not enter the bacterial cells (11) and by the same principal should not enter the host cells. Thus, these experiments only measure effects on invasion with the inhibitor. The results suggest that significant flux of Mg2+ through CorA is not essential for invasion either at the time of invasion or during growth before invasion.
We next examined whether Mg2+ efflux via CorA was relevant. When the extracellular [Mg2+] is below 1 mM, no Mg2+ efflux via CorA or any other system can be detected in S. enterica serovar Typhimurium with 28Mg2+ as a tracer (6). However, when cells are exposed to high (>5 mM) extracellular Mg2+ concentrations, CorA mediates 28Mg2+ efflux. We have previously described three additional loci, corB, corC, and corD, that markedly increase the extracellular Mg2+ concentration required to elicit CorA-mediated efflux (6). S. enterica serovar Typhimurium invasion of and replication within Caco-2 epithelial cells were unaffected by the mutation of any of these loci (MM3238, MM3239, and MM3240) (Fig. 3C). Thus, the corB, corC, or corD loci do not appear important for the invasion/replication phenotype. Further, since free Mg2+ within most mammalian cells, and therefore presumably Caco-2 epithelial cells, is less than 1 mM, efflux via CorA would not be activated (16, 23). Therefore, we conclude that Mg2+ efflux via CorA is unlikely to be important for invasion.
CorA-mediated influx is regulated.
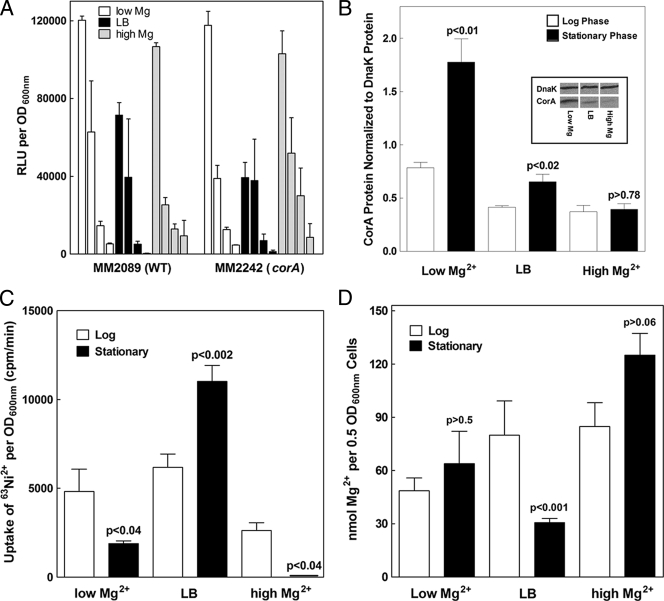
A previous study by Chamnongpol and Groisman indicated that CorA-mediated influx was increased in a phoP mutant strain compared to wild-type cells but that the CorA protein level was not altered (3). This result implies that Mg2+ influx via CorA is being regulated in some manner in a phoP mutant strain. However, since PhoP is crucial for multiple signaling cascades in Salmonella, a phoP mutant strain is not an optimal background in which to assess CorA regulation in more detail. Therefore, we investigated the possible regulation of corA in wild-type (MM2089) and corA mutant (MM2242) bacteria. Cells were grown in either N-minimal medium with 10 μM MgSO4 (low Mg) or 10 mM MgSO4 (high Mg) or in LB broth, which contains 30 to 50 μM Mg2+ (31). Wild-type and corA mutant strains were grown in these different media through log phase and into stationary phase. corA transcription was measured with a low-copy-plasmid-borne luciferase reporter driven by the endogenous corA promoter. Total CorA protein was determined by anti-CorA Western blot assays. Mg2+ influx was quantified by determining the initial rate of uptake of 63Ni2+ via CorA. Finally, total intracellular Mg2+ content was measured by atomic absorption.
The general pattern of corA transcription versus time was essentially the same in all three growth media (Fig. 4A); transcription was highest during early log phase and decreased markedly by stationary phase. Moreover, neither the pattern nor the relative amount of transcription from the corA promoter was altered in the absence of a functional corA allele. While these results suggest that transcription of corA is sensitive to growth phase, they also confirm previous data that corA transcription is independent of the extracellular Mg2+ concentration (28, 34). Additionally, the levels of corA transcription do not correlate with the amounts of CorA protein measured at similar growth phases in all three growth media (Fig. 4A and B). For example, despite a difference in the transcription of corA over time during growth in high Mg2+ concentrations, the corresponding CorA protein levels do not differ between the log and stationary phases.
FIG. 4.
Regulation of corA expression during growth. CorA expression and function during growth were measured for cells grown either in LB medium or in N-minimal medium with 10 μM Mg2+ (low Mg) or 10 mM Mg2+ (high Mg). (A) corA transcription was determined with a luciferase reporter at 2, 4, 6, and 20 h expressed in the wild type (MM3252) and a corA mutant strain (MM3253). Data represent an average of four independent experiments. RLU, relative light units; WT, wild type. (B) CorA protein levels in wild-type bacteria (MM2089) were measured by anti-CorA Western blot assays. CorA protein levels were normalized to DnaK protein, a loading control. Data represent an average of four independent experiments. The Western blot assay from one representative experiment with stationary-phase cells grown in low Mg, high Mg, and LB is shown in the insert. (C) The initial rate of CorA-mediated influx was determined with 63Ni2+ with wild-type cells (MM2089). The data represent an average of three independent experiments. (D) Mg2+ content was determined by atomic absorption with wild-type bacteria (MM2089). The data represent an average of three independent experiments. Panels B, C, and D show measurements of cells sampled at mid-log phase and after entry into stationary phase. P values indicate t tests comparing log- to stationary-phase cells in the same medium.
In low Mg, stationary-phase cells contain more CorA protein than log-phase cells (Fig. 4B). However, transport of 63Ni2+ is decreased in stationary phase (Fig. 4C). Thus, protein content does not correlate with transport. Furthermore, the total intracellular Mg2+ content is the same between log- and stationary-phase cells grown in low Mg (Fig. 4D).
After growth in LB broth, CorA protein content (Fig. 4B) is increased in stationary phase, as is transport of 63Ni2+ (Fig. 4C). However, Mg2+ content is significantly lower in stationary phase, despite increased levels of both CorA protein and transport (Fig. 4D).
Finally, in high Mg, similar amounts of CorA protein are found in stationary-phase versus log-phase cells (Fig. 4B). Yet stationary-phase cells do not transport any 63Ni2+ (Fig. 4C). The lack of uptake via CorA in the high-Mg stationary-phase cells is not due to a general lack of energy or transport since these cells exhibit a substantial rate of 54Mn2+ uptake via the MntH and SitABCD Mn2+ transporters (data not shown). Moreover, despite the lack of CorA-mediated transport in the stationary-phase cells, they contain much more intracellular Mg2+ than cells grown to stationary phase in other media (Fig. 2D).
Overall, these data show that changes in the levels of CorA protein in S. enterica serovar Typhimurium do not always result in corresponding changes in either the initial rate of cation influx via CorA or intracellular Mg2+ content. For example, even though CorA transport is completely undetectable in stationary-phase cells grown in high Mg, CorA protein is present in an amount comparable to that in the same culture at mid-log phase. Further, the lack of transport in the stationary-phase cells does not alter the total intracellular Mg2+ compared to log-phase cells. Taken together, these results strongly suggest that CorA function is regulated. Whether this regulation involves a posttranslational modification such as phosphorylation, interaction with another protein, allosteric modulation, or a combination will be the subject of future experiments.
DISCUSSION
The role of Mg2+ versus CorA.
A corA mutant strain (MM2242) is attenuated for virulence in the mouse and defective for invasion and replication within Caco-2 epithelial cells (22). We therefore conducted experiments with Caco-2 cells to examine potential reasons why loss of corA results in these phenotypes. The overall results indicate that the presence of an evolutionarily related and functional CorA protein itself is important for full virulence. Surprisingly, intracellular Mg2+ content does not seem to be related to the corA mutant strain's invasion and replication defect since total intracellular Mg2+ content was not significantly different between the wild type and a corA mutant. MgtE, a Mg2+ transporter from B. subtilis (MM3217), fails to complement the corA virulence phenotype, despite its robust transport in S. enterica serovar Typhimurium. Chronic or acute inhibition of CorA transport in a wild-type strain (MM2089) by high concentrations of the selective inhibitor Co(III) hexaammine did not elicit the invasion phenotype, suggesting that a significant amount of Mg2+ flux through CorA before or at the time of invasion is not essential. Thus, these results suggest that the CorA protein but neither intracellular Mg2+ nor Mg2+ influx via CorA is important for Salmonella interaction with epithelial cells.
While the overall funnel-shaped structure of T. maritima CorA is presumably conserved between the E. coli, M. jannaschii, and S. enterica serovar Typhimurium CorA proteins and the rest of the CorA family (12), only the E. coli (MM3218) and S. enterica serovar Typhimurium (MM2320) CorA proteins complement the invasion/replication phenotype. The S. enterica serovar Typhimurium and E. coli CorA proteins differ by only eight residues, whereas the membrane domain sequence of M. jannaschii is only 22% similar to that of S. enterica serovar Typhimurium and its soluble domain sequence is merely 12% similar. This implies that the protein has to look very much like S. enterica serovar Typhimurium CorA in surface detail and the spatial placement of individual residues to complement. A caveat to the complementation experiments is that more phylogenetically distant CorA proteins are not expressed as well and thus do not transport as much Mg2+ as S. enterica serovar Typhimurium CorA. For example, uptake of divalent cation by M. jannaschii CorA is about threefold greater than residual Mg2+ uptake via MgtA and MgtB in a corA mutant strain, but M. jannaschii CorA exhibits about 15% of the level of Mg2+ uptake of wild-type S. enterica serovar Typhimurium CorA (27). Despite this experimental limitation, a reasonable interpretation of the complementation studies is that rescue of the invasion defect appears to require a CorA protein that is phylogenetically closely related to S. enterica serovar Typhimurium CorA, in turn implying that the molecular details of the structure of CorA are necessary for complementation. This interpretation is supported by the B. subtilis MgtE complementation experiment, in which, despite significant transport activity in S. enterica serovar Typhimurium, the invasion defect of a corA mutant strain is not rescued.
We further addressed CorA function by examining efflux. CorA-mediated efflux elicited at high extracellular Mg2+ concentrations appears unnecessary for invasion and replication within Caco-2 epithelial cells since a strain carrying a mutation in the corB, corC, or corD gene (MM3238, MM3239, and MM3240) had no effect compared to the wild type (Fig. 3C).
The F266A and P269A mutant forms of CorA have 40 to 50% of the wild-type level of transport but appear to leak Mg2+, presumably because they cannot close properly. These mutant proteins (MM3227 and MM3228) do not rescue the invasion/replication defect (Fig. 3A), implying that CorA must not only be functional but also be able to close normally. Supporting this hypothesis is the lack of effect of either chronic or acute inhibition of CorA by Co(III) hexaammine, which leaves CorA functional but mimics a closed conformation. Wild-type cells (MM2089) treated with inhibitor invaded Caco-2 epithelial cells comparably to untreated wild-type cells (Fig. 3B). Therefore, our overall conclusions are that a CorA protein must be evolutionarily related, functional, and able to achieve a closed state to rescue the invasion/replication defect.
Our interpretation suggesting that CorA must be able to close properly to maintain the full virulence of Salmonella is supported by studies conducted by Sermon et al. (24, 25). Exposure to the host lactoperoxidase system induces the expression of corA in S. enterica serovar Typhimurium, and mutation of corA markedly sensitizes the bacteria to lactoperoxidase. The mechanism by which lactoperoxidase exposure induces corA expression is not known. The lactoperoxidase is produced as a defensive measure by immune cells. It oxidizes different substrates, primarily thiocyanate, with hydrogen peroxide to generate toxic hypothiocyanite, which is antibacterial. Uptake of Ni2+ via CorA markedly sensitizes S. enterica serovar Typhimurium to lactoperoxidase, an effect that is abolished by addition of Co(III) hexaammine. In this situation, although CorA is present, the inhibitor prevents the uptake of Ni2+ (and Mg2+). Thus, the presence of CorA protein, even though the channel is blocked by Co(III) hexaammine, protects S. enterica serovar Typhimurium from the host lactoperoxidase system.
Regulation of CorA.
On the basis of the above conclusions, we investigated the potential regulation of CorA transcription and translation. The level of corA transcription varied with the growth phase but did not vary in the different test media containing different levels of Mg2+ or between the wild type (MM2089) and a corA mutant strain (MM2242) (Fig. 4A). This regulation differs from that of mgtA and mgtB, where the Mg2+ concentration has a marked and biphasic effect on transcription (34). CorA protein levels measured in the wild-type strain varied about fourfold across test media (Fig. 4B). Although mRNA levels were consistently lower in stationary phase than in log phase, protein levels did not correspondingly decrease. Moreover, both mRNA content and the amount of CorA protein did not correlate either with the rate of CorA-mediated transport or with the Mg2+ content (Fig. 4B, C, and D).
One would expect that an increased level of CorA protein would be accompanied by a similar increase in the initial rate of Mg2+ influx, but the data are counter to this expectation. For example, in stationary-phase cells grown in high Mg, CorA is present but no Mg2+ influx can be detected (Fig. 4C). In sharp contrast, Mg2+ influx is very high during stationary phase in cells grown in LB broth, even though CorA protein levels and cell Mg2+ content are relatively low compared to the other conditions tested. Interpretation of these results is not confounded by the presence of wild-type alleles of the mgtA and mgtB Mg2+ transporters since in both high Mg and LB (30 to 50 μM total Mg2+), both genes are virtually completely repressed (32, 34, 35). Indeed, in low Mg, where mgtA and mgtB are expressed at low levels and would thus contribute to overall Mg2+ uptake, we have previously shown that the rate of uptake via CorA is still manyfold greater than uptake via MgtA or MgtB (31). Notwithstanding a small contribution from MgtA and MgtB, the rate of CorA-mediated Mg2+ uptake in low-Mg2+ medium is comparable and CorA protein levels are significantly higher than in cells grown in other media.
Mg2+ content also does not correlate with the expression of CorA. Although content is highest in cells grown in high Mg in both the log and stationary phases, CorA protein levels are relatively low. The increased intracellular Mg2+ content in high Mg is the result of CorA-mediated influx since the expression of MgtA and MgtB is completely repressed under this growth condition. Interestingly, these data also argue that the ability of the CorA Mg2+ channel to mediate Mg2+ efflux at high extracellular Mg2+ concentrations is not physiologically relevant. Since exposure to 10 mM extracellular Mg2+ should elicit significant efflux via CorA, cells grown in high Mg2+ concentrations might be expected to have decreased rather than markedly increased cellular Mg2+ content.
Finally, these results indicate that either translation of corA mRNA or CorA protein stability, as well as CorA function, is regulated in some manner. The regulation of expression must be at the translational or posttranslational level since, despite significantly different CorA protein content in cells grown in various media, both the pattern of mRNA expression and the relative amounts of mRNA are virtually identical under all of the conditions tested. However, current data do not speak to the mechanism by which translation and protein stability are regulated.
Functional regulation of CorA can be inferred from the mismatches among CorA protein content, CorA transport, and total intracellular Mg2+ content. In turn, this implies that CorA is subject to posttranslational modification, allosteric modification, interaction with another protein, or a combination of these regulatory mechanisms. Mg2+ itself can likely be eliminated as a potential allosteric modifier since there is no obvious correlation of function with the intracellular or extracellular Mg2+ concentration. This interpretation of the data further implies that CorA is a part of a broader signaling network within the cell. Our data in this report (also see reference 22) are all consistent with a role for CorA and Mg2+ homeostasis in several major pathways in S. enterica serovar Typhimurium, including some which impact virulence (22). Current experiments are focused on understanding this apparent regulation of CorA expression and function and delineating its connection to S. enterica serovar Typhimurium virulence.
Acknowledgments
K.M.P. was supported by National Institutes of Health training grant T32 AI-07024-28. These studies were supported by National Institutes of Health grant GM39447 (M.E.M.).
We thank John S. Gunn and Stephen J. Libby for their intellectual contributions to this research focus.
Footnotes
Published ahead of print on 1 August 2008.
REFERENCES
- 1.Alpuche Aranda, C. M., J. A. Swanson, W. P. Loomis, and S. I. Miller. 1992. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc. Natl. Acad. Sci. USA 8910079-10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bullas, L. R., and J. Ryu. 1983. Salmonella typhimurium strains which are r− m+ for all three chromosomally located systems of DNA restriction and modification. J. Bacteriol. 156471-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamnongpol, S., and E. A. Groisman. 2002. Mg2+ homeostasis and avoidance of metal toxicity. Mol. Microbiol. 44561-571. [DOI] [PubMed] [Google Scholar]
- 4.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García Véscovi, E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84165-174. [DOI] [PubMed] [Google Scholar]
- 6.Gibson, M. M., D. A. Bagga, C. G. Miller, and M. E. Maguire. 1991. Magnesium transport in Salmonella typhimurium: the influence of new mutations conferring Co2+ resistance on the CorA Mg2+ transport system. Mol. Microbiol. 52753-2762. [DOI] [PubMed] [Google Scholar]
- 7.Grubbs, R. D., M. D. Snavely, S. P. Hmiel, and M. E. Maguire. 1989. Magnesium transport in eukaryotic and prokaryotic cells using magnesium-28 ion. Methods Enzymol. 173546-563. [DOI] [PubMed] [Google Scholar]
- 8.Hattori, M., Y. Tanaka, S. Fukai, R. Ishitani, and O. Nureki. 2007. Crystal structure of the MgtE Mg2+ transporter. Nature 4481072-1075. [DOI] [PubMed] [Google Scholar]
- 9.Hmiel, S. P., M. D. Snavely, C. G. Miller, and M. E. Maguire. 1986. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J. Bacteriol. 1681444-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291238-239. [DOI] [PubMed] [Google Scholar]
- 11.Kucharski, L. M., W. J. Lubbe, and M. E. Maguire. 2000. Cation hexaammines are selective and potent inhibitors of the CorA magnesium transport system. J. Biol. Chem. 27516767-16773. [DOI] [PubMed] [Google Scholar]
- 12.Lunin, V. V., E. Dobrovetsky, G. Khutoreskaya, R. Zhang, A. Joachimiak, D. A. Doyle, A. Bochkarev, M. E. Maguire, A. M. Edwards, and C. M. Koth. 2006. Crystal structure of the CorA Mg2+ transporter. Nature 440833-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maguire, M. E. 2006. Magnesium transporters: properties, regulation and structure. Front. Biosci. 113149-3163. [DOI] [PubMed] [Google Scholar]
- 14.Maguire, M. E. 2006. The structure of the CorA magnesium transporter, a divalent cation channel. Curr. Opin. Struct. Biol. 4432-438. [DOI] [PubMed] [Google Scholar]
- 15.Maguire, M. E. 2007. Magnesium, manganese and divalent cation transport assays in intact cells, p. 289-306. In H. Schatten and A. Eisenstark (ed.), Salmonella: methods and protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 16.Maguire, M. E., and J. A. Cowan. 2002. Mg2+ chemistry and biochemistry. Biometals 15203-210. [DOI] [PubMed] [Google Scholar]
- 17.Maloy, S. R., V. J. Stewart, and R. K. Taylor. 1996. Genetic analysis of pathogenic bacteria: a laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor, NY.
- 18.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 865054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson, D. L., and E. P. Kennedy. 1971. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J. Biol. Chem. 2463042-3049. [PubMed] [Google Scholar]
- 20.Nelson, D. L., and E. P. Kennedy. 1972. Transport of magnesium by a repressible and a nonrepressible system in Escherichia coli. Proc. Natl. Acad. Sci. USA 691091-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papp-Wallace, K. M., and M. E. Maguire. 2007. Bacterial homologs of eukaryotic membrane proteins: the 2-TM-GxN family of Mg2+ transporters. Mol. Membr. Biol. 24351-356. [DOI] [PubMed] [Google Scholar]
- 22.Papp-Wallace, K. M., M. Nartea, D. G. Kehres, S. Porwollik, M. McClelland, S. J. Libby, F. C. Fang, and M. E. Maguire. 2008. The CorA Mg2+ channel is required for the virulence of Salmonella enterica serovar Typhimurium. J. Bacteriol. 1906517-6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romani, A. M., and M. E. Maguire. 2002. Hormonal regulation of Mg2+ transport and homeostasis in eukaryotic cells. Biometals 15271-283. [DOI] [PubMed] [Google Scholar]
- 24.Sermon, J., P. De Spiegeleer, K. Vanoirbeek, A. Aertsen, and C. W. Michiels. 2004. Characterization of lactoperoxidase stress response in Escherichia coli and involvement of corA in lactoperoxidase tolerance. Commun. Agric. Appl. Biol. Sci. 6939-42. [PubMed] [Google Scholar]
- 25.Sermon, J., E. M. Wevers, L. Jansen, P. De Spiegeleer, K. Vanoirbeek, A. Aertsen, and C. W. Michiels. 2005. CorA affects tolerance of Escherichia coli and Salmonella enterica serovar Typhimurium to the lactoperoxidase enzyme system but not to other forms of oxidative stress. Appl. Environ. Microbiol. 716515-6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith, R. L., J. L. Banks, M. D. Snavely, and M. E. Maguire. 1993. Sequence and topology of the CorA magnesium transport systems of Salmonella typhimurium and Escherichia coli. Identification of a new class of transport protein. J. Biol. Chem. 26814071-14080. [PubMed] [Google Scholar]
- 27.Smith, R. L., E. Gottlieb, L. M. Kucharski, and M. E. Maguire. 1998. Functional similarity between archaeal and bacterial CorA magnesium transporters. J. Bacteriol. 1802788-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith, R. L., M. L. Kaczmarek, L. M. Kucharski, and M. E. Maguire. 1998. Magnesium transport in Salmonella typhimurium: induction of MgtA and MgtCB expression during invasion of epithelial and macrophage cells. Microbiology 1441835-1843. [DOI] [PubMed] [Google Scholar]
- 29.Smith, R. L., M. A. Szegedy, C. Walker, R. M. Wiet, A. Redpath, M. L. Kaczmarek, L. M. Kucharski, and M. E. Maguire. 1998. The CorA magnesium transport protein of Salmonella typhimurium: mutagenesis of conserved residues in the third transmembrane segment identifies part of a Mg2+ pore. J. Biol. Chem. 27328663-28669. [DOI] [PubMed] [Google Scholar]
- 30.Smith, R. L., L. J. Thompson, and M. E. Maguire. 1995. Cloning and characterization of mgtE, a putative new class of Mg2+ transporter from Bacillus firmus OF4. J. Bacteriol. 1771233-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snavely, M. D., J. B. Florer, C. G. Miller, and M. E. Maguire. 1989. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J. Bacteriol. 1714761-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snavely, M. D., S. A. Gravina, T. T. Cheung, C. G. Miller, and M. E. Maguire. 1991. Magnesium transport in Salmonella typhimurium: Regulation of mgtA and mgtB expression. J. Biol. Chem. 266824-829. [PubMed] [Google Scholar]
- 33.Szegedy, M. A., and M. E. Maguire. 1999. The CorA Mg2+ transport protein of Salmonella typhimurium. Mutagenesis of conserved residues in the second membrane domain. J. Biol. Chem. 27436973-36979. [DOI] [PubMed] [Google Scholar]
- 34.Tao, T., P. F. Grulich, L. M. Kucharski, R. L. Smith, and M. E. Maguire. 1998. Magnesium transport in Salmonella typhimurium: biphasic time and magnesium dependence of the transcription of the mgtA and mgtCB loci. Microbiology 144655-664. [DOI] [PubMed] [Google Scholar]
- 35.Tao, T., M. D. Snavely, S. G. Farr, and M. E. Maguire. 1995. Magnesium transport in Salmonella typhimurium: mgtA encodes a P-type ATPase and is regulated by Mg2+ in a manner similar to that of the mgtB P-type ATPase. J. Bacteriol. 1772654-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Townsend, D. E., A. J. Esenwine, J. George III, D. Bross, M. E. Maguire, and R. L. Smith. 1995. Cloning of the mgtE Mg2+ transporter from Providencia stuartii and the distribution of mgtE in the eubacteria. J. Bacteriol. 1775350-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]