Abstract
Insulin and guanosine-5′-O-(3-thiotriphosphate) (GTPγS) both stimulate glucose transport and translocation of the insulin-responsive glucose transporter 4 (GLUT4) to the plasma membrane in adipocytes. Previous studies suggest that these effects may be mediated by different mechanisms. In this study we have tested the hypothesis that these agonists recruit GLUT4 by distinct trafficking mechanisms, possibly involving mobilization of distinct intracellular compartments. We show that ablation of the endosomal system using transferrin-HRP causes a modest inhibition (∼30%) of insulin-stimulated GLUT4 translocation. In contrast, the GTPγS response was significantly attenuated (∼85%) under the same conditions. Introduction of a GST fusion protein encompassing the cytosolic tail of the v-SNARE cellubrevin inhibited GTPγS-stimulated GLUT4 translocation by ∼40% but had no effect on the insulin response. Conversely, a fusion protein encompassing the cytosolic tail of vesicle-associated membrane protein-2 had no significant effect on GTPγS-stimulated GLUT4 translocation but inhibited the insulin response by ∼40%. GTPγS- and insulin-stimulated GLUT1 translocation were both partially inhibited by GST-cellubrevin (∼50%) but not by GST-vesicle-associated membrane protein-2. Incubation of streptolysin O-permeabilized 3T3-L1 adipocytes with GTPγS caused a marked accumulation of Rab4 and Rab5 at the cell surface, whereas other Rab proteins (Rab7 and Rab11) were unaffected. These data are consistent with the localization of GLUT4 to two distinct intracellular compartments from which it can move to the cell surface independently using distinct sets of trafficking molecules.
INTRODUCTION
Insulin stimulates glucose transport in muscle and adipose cells by virtue of the expression of a unique glucose transporter, GLUT4. Unlike other GLUT isoforms, in the absence of insulin >95% of GLUT4 is intracellularly sequestered but translocates rapidly to the cell surface in response to insulin stimulation (Rea and James, 1997). Although other molecules such as the transferrin receptor (TfR) and GLUT1 also undergo insulin-dependent movement to the cell surface, the magnitude of these effects is much smaller than that of GLUT4 (∼2-fold increase compared with ∼15- to 20-fold, respectively) (Tanner and Lienhard, 1987; Calderhead et al., 1990). This difference appears to be due to a unique intracellular trafficking event involving the sorting of GLUT4 from other endosomal traffic.
Although a proportion of GLUT4 is found in endosomes and the trans-Golgi network (Slot et al., 1991, 1997) a significant proportion (∼60%) is localized to a discrete vesicle pool (Herman et al., 1994; Livingstone et al., 1996; Martin et al., 1996; Malide et al., 1997). Two models have emerged to explain these data. The first suggests that this additional pool (termed Glut4 storage vesicles [GSVs]) is a specialized secretory compartment perhaps analogous to synaptic vesicles that fuses directly with the plasma membrane, an event that is somehow accelerated by insulin (Rea and James, 1997). The second model suggests that GLUT4 is in dynamic equilibrium between a slowly recycling and a more rapidly recycling compartment and that insulin influences one of the sorting steps that regulates the entry into or exit out of this compartment (Kandror and Pilch, 1998). Hence, an important distinction between this model and the first is that there is in fact no specialized insulin-sensitive secretory compartment (Pessin et al., 1999). Distinguishing between these models has been limited by the difficulty in segregating these different compartments. Although GLUT4 is enriched in this separate vesicular compartment, it is not excluded from endosomes and the trans-Golgi network (Slot et al., 1991, 1997; Martin et al., 1994). In fact most molecules in the endosomal system are widely distributed between multiple organelles making it difficult to define these compartments. Here, we have sought to dissect these different compartments by establishing conditions that would enable us to regulate the trafficking through one of these compartments without effecting the other.
The concept of two distinct intracellular GLUT4 secretory compartments has been suggested from studies involving exercise and insulin in skeletal muscle, because these two agonists appear to trigger GLUT4 translocation from discrete intracellular sites (Coderre et al., 1995; Aledo et al., 1997; Ploug et al., 1998). GLUT4 translocation in adipocytes can also be triggered by a variety of agonists, such as hyperosmolarity (Chen et al., 1997) and guanosine-5′-O-(3-thiotriphosphate) (GTPγS) (Baldini et al., 1991; Robinson et al., 1992). Like exercise-stimulated GLUT4 translocation in muscle, these agonists differ in their mode of action compared with insulin, raising the possibility that adipocytes also contain two separate routes to the cell surface that can be regulated independently. Consistent with this, we and others have shown the involvement of two different vesicle-associated soluble N-ethylmaleimide-sensitive factor–attached protein receptors (v-SNAREs) in the insulin regulation of membrane trafficking in adipocytes (Volchuk et al., 1995; Martin et al., 1996; Olson et al., 1997; Martin et al., 1998). We have shown that the majority of cellubrevin is localized to endosomes and a significant portion of vesicle-associated membrane protein-2 (VAMP2) is localized to GSVs (Martin et al., 1996) and demonstrated that a peptide corresponding to the unique N terminus of VAMP2 inhibits insulin-stimulated GLUT4 translocation by ∼35% but was without effect on insulin-stimulated GLUT1 translocation (Martin et al., 1998).
To test the hypothesis that there are two separate regulatable exit pathways for GLUT4 in adipocytes, we have studied the role of different v-SNARE proteins and the effects of treatments that disrupt endosomal function on GTPγS and insulin action. We show that a GST fusion protein encompassing the cytosolic tail of cellubrevin inhibited GTPγS-stimulated GLUT4 translocation by ∼40%, whereas GST-VAMP2 had no significant effect on GTPγS action. Conversely, the VAMP2 fusion protein inhibited insulin-stimulated GLUT4 translocation by ∼40%. Ablation of the recycling endosomal system caused almost quantitative inhibition of GTPγS-stimulated GLUT4 translocation but only partially reduced insulin-stimulated translocation. These data suggest that GTPγS selectively stimulates recycling of GLUT4 via the endosomal system in a process that is regulated by cellubrevin. In addition to regulating this pathway, insulin also stimulates the exocytosis of GLUT4 from a separate compartment (GSVs), which can be functionally distinguished by the unique role of VAMP2.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle’s medium (DMEM), Myoclone-Plus fetal calf serum, and antibiotics were from Life Technologies (Paisley, United Kingdom). [125I]Transferrin and [14C]sucrose were from Amersham International (Bucks, United Kindgom). α-Toxin and GTPγS were from Calbiochem (Nottingham, United Kingdom). Streptolysin O (SLO) was the generous gift of Dr. Robin Plevin (University of Strathclyde, Strathclyde, United Kingdom). All other reagents were as described (Livingstone et al., 1996; Martin et al., 1996, 1998).
Cell Culture
3T3-L1 fibroblasts were grown and differentiated as described (Frost and Lane, 1988) and used 8–12 d after differentiation and between passages 4 and 12. Before use, monolayers were washed once with serum-free DMEM and then incubated with serum-free DMEM for 2 h.
Permeabilization of 3T3-L1 Adipocytes
3T3-L1 adipocytes were washed twice with IC buffer (10 mM NaCl, 20 mM HEPES, 50 mM KCl, 2 mM K2HPO4, 90 mM potassium glutamate, 1 mM MgCl2, 4 mM EGTA, 2 mM CaCl2, pH 7.4) at 37°C and then incubated with 500 μl ICR buffer (IC buffer plus 4 mM MgATP, 3 mM sodium pyruvate, 100 μg/ml BSA, pH 7.4) containing α-toxin at 250 hemolytic units/ml for 5 min. The medium was removed, and the cells were covered with 500 μl of ICR buffer containing GTPγS, insulin, or vehicle (Herbst et al., 1995). SLO permeabilization was performed exactly as outlined for α-toxin, except SLO was used at 0.5 μg/ml and incubated with the cells for 10 min at 37°C. After washing with ICR buffer, cells were then incubated with GTPγS, insulin, and the test proteins as indicated in the figure legends.
Deoxyglucose Transport Measurements
The effect of GTPγS on [3H]2-deoxy-d-glucose (deGlc) transport (final concentration 50, μM and 0.5 μCi/well) was measured in α-toxin-permeabilized cells as described (Herbst et al., 1995). Uptake was carried out for 3 min. Nonspecific association of radioactivity (determined by simultaneous assay of [14C]sucrose association with the cells) amounted to <20% of the specific uptake under these conditions.
Plasma Membrane Lawn Assays for GLUT Translocation
After experimental manipulations, coverslips of adipocytes were rapidly washed in ice-cold buffer for the preparation of plasma membrane lawns exactly as described by Martin et al. (1998). Triplicate coverslips were prepared at each experimental condition, and 10 random images of plasma membrane lawns were collected from each. These were quantified using MetaMorph (Universal Imaging, West Chester, PA) software on a DAN personal computer (Noran Instruments, Surrey, United Kingdom).
Subcellular Fractionation of Adipocytes
Adipocytes were subjected to a differential centrifugation procedure as described previously (Martin et al., 1994; Martin et al., 1996). Briefly, cells were scraped and homogenized in ice-cold HES (20 mM HEPES, 1 mM EDTA, 250 mM sucrose, pH 7.4; 5 ml/10-cm plate) containing protease inhibitors (1 μg/ml pepstatin A, 0.2 mM diisopropylfluoro-phosphate, 20 μM l-transepoxysuccinyl-leucylamido-4-guanidinio-butane, 50 μM aprotinin). The homogenate was subjected to a differential centrifugation procedure to produce plasma membrane, low-density microsomes, high-density microsomes, and soluble protein as described (Martin et al., 1998). All fractions were snap frozen and stored at −80°C before use.
Fusion Protein Expression and Purification
The expression and purification of GST-cellubrevin and GST-VAMP2 were performed exactly as described (Martin et al., 1998)
Preparation and Use of HRP-conjugated Transferrin
TfHRP was prepared, purified, iron loaded, and used exactly as described (Livingstone et al., 1996; Stoorvogel et al., 1987, 1988). Briefly, after 2-h incubation in serum-free DMEM, adipocytes were incubated with 20 μg/ml TfHRP for 1 h. Cells were chilled by washing in ice-cold isotonic citrate buffer (150 mM NaCl, 20 mM sodium citrate, pH 5.0) to remove cell surface-attached TfHRP and kept on ice to prevent vesicle trafficking during the diaminobenzidine (DAB) cytochemistry reactions. The monolayer was washed in ice-cold PBS and DAB (2 mg/ml stock; 0.22 μm filtered) added at 100 μg/ml to all wells and H2O2 added to 0.02% (vol/vol) to one of each pair of wells. After a 1-h incubation at 4°C in the dark, the reaction was stopped by washing in PBS containing 5 mg/ml BSA. This was then aspirated, and the cells were used in experiments as outlined in the figure legends. In all experiments, duplicate plates were used, one of which was exposed to DAB and H2O2, the other only to DAB as a negative control.
To verify that the incubation conditions described above did not compromise the ability of the cells to respond to insulin, we performed similar experiments but selectively omitted TfHRP, DAB, or peroxide. At the end of the incubations, cells were warmed to 37°C, and insulin-stimulated glucose transport was assayed. The maximal rate of insulin-stimulated glucose transport was unaffected by any of the incubations used during this procedure (Table 1). However, and consistent with previous studies, the low-temperature incubation resulted in a slight elevation of basal transport rate (∼2- to 2.5-fold), such that the fold increase in response to insulin is diminished. It is important not to confuse this with blunted insulin responsiveness in these cells, because the extent of insulin-stimulated GLUT4 translocation was not reduced. Moreover, addition of peroxide alone was also found to slightly raise basal glucose transport rates, consistent with the partial insulinomimetic effect of this compound.
Table 1.
Effects of TfHRP ablation on deGlc uptake in 3T3-L1 adipocytes
| DeGlc transport rate (pmol/million cells/min)
|
|||
|---|---|---|---|
| Basal | Insulin or GTPγS stimulated | Fold increase | |
| Insulin stimulated | |||
| Control cells | 24 ± 2.1 | 320 ± 30 | 13.3 |
| TfHRP loaded | 40 ± 3.5a | 324 ± 21 | 8.1 |
| DAB treated | 39 ± 2.1a | 330 ± 19 | 8.5 |
| H2O2 treated | 45 ± 1.6a | 325 ± 3.9 | 7.2 |
| TfHRP + DAB | 41 ± 5.1a | 331 ± 18 | 8.0 |
| TfHRP + DAB + H2O2 | 46 ± 5.1a | 248 ± 24b | 5.5 |
| GTPγS stimulated | |||
| Control cells | 24 ± 2.1 | 149 ± 10 | 6.2 |
| TfHRP loaded | 40 ± 3.5a | 155 ± 6 | 3.9 |
| DAB treated | 39 ± 2.1a | 146 ± 12 | 3.7 |
| H2O2 treated | 45 ± 1.6a | 150 ± 4 | 3.3 |
| TfHRP + DAB | 41 ± 5.1a | 148 ± 20 | 3.6 |
| TfHRP + DAB + H2O2 | 46 ± 5.1a | 55 ± 9c | 1.2 |
Control cells were assayed after a 2-h incubation in serum-free media and immediate assay. All other assays were performed after treatment exactly as outlined for ablation, except that some or all of the additions were omitted. Thus, TfHRP loaded refers to cells loaded with TfHRP as outlined, but not incubated with DAB or peroxide. DAB treated refers to cells washed and treated exactly as for ablation, except TfHRP loading and peroxide addition were omitted. H2O2 treated cells were washed and treated exactly as for ablation, except TfHRP loading and DAB addition were omitted. TfHRP + DAB refers to cells loaded with TfHRP and incubated with DAB as outlined, except peroxide was omitted. Note that after ablation, insulin can still elicit a significant increase in deGlc uptake, whereas GTPγS cannot. Shown are data from a representative experiment (repeated two further times) in which each rate shown is the mean of triplicate determinations (± SEM). Also shown are the average fold increases observed in response to insulin from three separate experiments of this type on separate platings of cells.
Statistically significant elevation of basal rate compared with control cells, manifest in all cells subjected to low-temperature washes.
Significant reduction compared with control insulin-stimulated transport (p < 0.05 in this experiment).
Significant reduction in GTPγS-stimulated deGlc transport (p < 0.01 in this experiment).
Quantification of Cell Surface TfR Levels
TfRs present at the cell surface were quantified as outlined by Tanner and Lienhard (1987) and Jess et al. (1996). After washing in ice-cold buffer, cells were incubated with ∼3 nM [125I]transferrin for 2 h on ice. After this time, the monolayers were washed, and cells were solubilized in 1 M NaOH, and the radioactivity associated with each well was determined. To estimate nonspecific binding, duplicate plates were incubated exactly as above but in the presence of 1 μM transferrin. This was between 5 and 10% of the total counts per well.
TfR Externalization Assay
The rate of externalization of the TfR was measured as described (Tanner and Lienhard, 1987). 3T3-L1 adipocytes were incubated in serum-free DMEM containing 1 mg/ml BSA for 2 h at 37°C. [125I]Transferrin (2.2 μg/ml; 3 μCi) and TfHRP (20 μg/ml) were added to the media for the final 1 h. Cell surface Tf/TfHRP were removed by washing with ice-cold isotonic citrate buffer and then washed with PBS. DAB with or without hydrogen peroxide was added to each well and incubated on ice for 1 h in the dark. Cells were then washed in PBS at 4°C and twice in Krebs Ringer phosphate (KRP) containing 1 mg/ml BSA at 37°C and then incubated in 1 ml of KRP buffer containing unlabeled transferrin (1 μM) with or without insulin (1 μM) at 37°C for the times indicated on the figures to allow internalized [125I]transferrin to recycle out of the cells. After the required time, cell-associated radioactivity was determined as outlined above. Nonspecific binding was determined using duplicate plates that were incubated in the presence of 10 μM unlabeled transferrin and treated exactly as described above.
Cell Surface TfR Internalization and Externalization Assays
An adaptation of the above method was used to determine whether internalization of the TfR is observed after endosomal ablation. Duplicate wells of 3T3-L1 adipocytes were incubated with serum-free DMEM containing 1 mg/ml BSA for 2 h at 37°C. TfHRP was added for the final 1 h of this incubation, and ablation was carried out as described. The cells were washed once with KRP buffer at 37°C and then incubated with 3 nM [125I]transferrin in KRP containing 1 mg/ml BSA at 37°C for the times indicated. After this time, cell surface transferrin was removed, and cell-associated transferrin was determined as outlined above.
A further adaptation of the assay was used to determine whether constitutive recycling of the TfR could be observed after endosome ablation. Duplicate wells of 3T3-L1 adipocytes were incubated in serum-free DMEM and BSA for 2 h at 37°C. For the last hour of this incubation the cells were loaded with TfHRP only, and ablation was carried out as described above. The cells were then washed once with KRP buffer at 37°C and incubated in 1 ml of 3 nM [125I]transferrin in KRP buffer containing 1 mg/ml BSA at 37°C for 20 min (this period was shown to be sufficient for maximal loading of the intracellular TfR pool). Cells were washed in ice-cold citrate buffer and PBS as described above. Subsequently, cells were washed once with KRP buffer containing 1 mg/ml BSA at 37°C and then incubated in 1 ml of KRP containing unlabeled transferrin (1 μM) at 37°C for the times shown. Thereafter, cell-associated radioactivity and nonspecific Tf-binding were measured as described above.
Electrophoresis and Immunoblotting
Proteins were electrophoresed on SDS-polyacrylamide gels and transblotted onto nitrocellulose as described (Martin et al., 1998). Immunolabeled proteins were visualized using either 125I-labeled goat anti-rabbit or HRP-conjugated secondary antibody and quantitated by γ counting or densitometry.
Antibodies
Rabbit polyclonal antibodies specific for Rab proteins were from Dr. P. Chavrier (INSERM-CNRS, Marseille, France; Rab5 and Rab7), Dr. Peter van der Sluijs (University of Utrecht, Utrecht, The Netherlands; Rab4), and Dr. Rob Parton (University of Queensland; Rab11). The anti-vp165 antibodies were kindly provided by Dr. S. Keller (Dartmouth Medical School, Hanover, NH). The anti-Syntaxin 4 antibody has been described previously (Tellam et al., 1997).
RESULTS
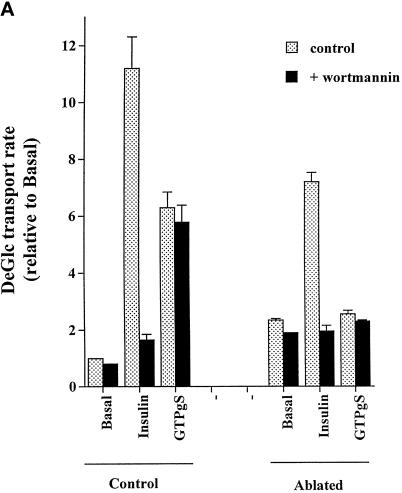
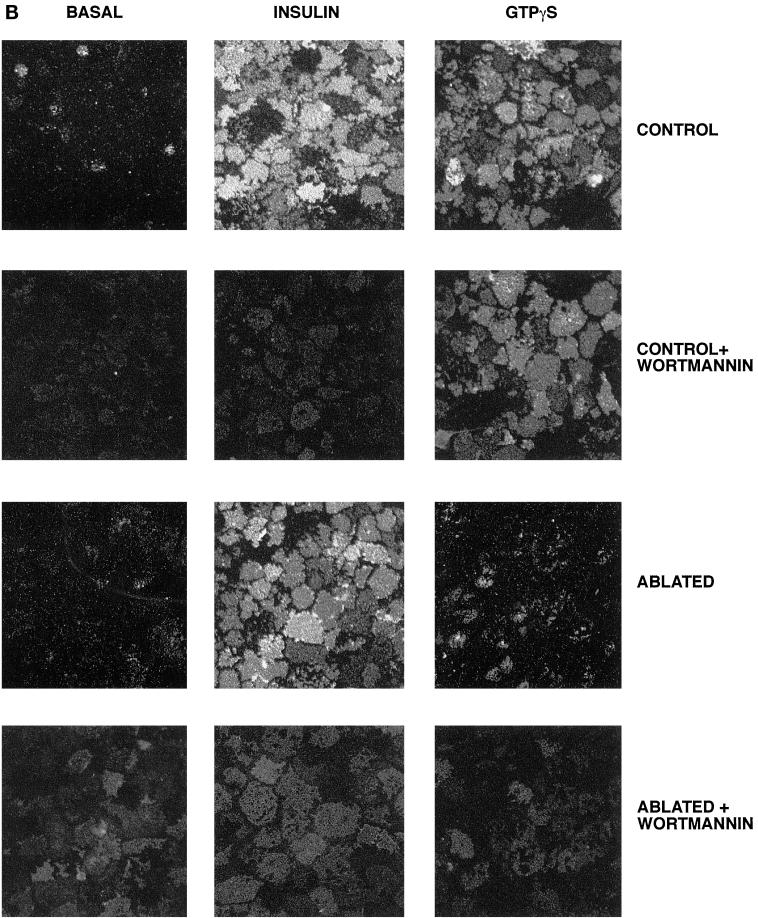
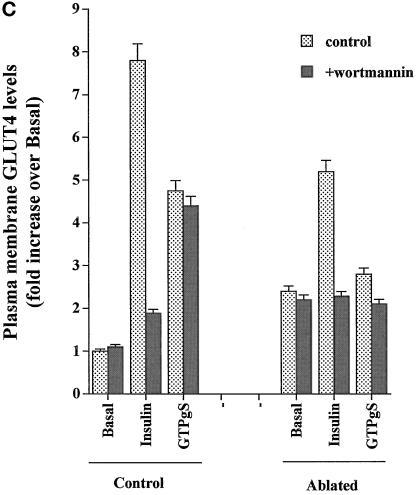
In this study we have compared the ability of insulin and GTPγS to stimulate glucose transport and GLUT4 translocation in 3T3-L1 adipocytes (Figure 1, A and B). The magnitude of the GTPγS effect was consistently less than that of insulin (Figure 1A), in agreement with previous studies (Baldini et al., 1991; Robinson et al., 1992; Clarke et al., 1994). We observed a maximal effect of GTPγS on glucose transport at a concentration of 100 μM (our unpublished data). At this concentration, the fold increase in glucose transport was ∼40% of that induced by insulin (Figure 1A). The PI3 kinase inhibitor wortmannin (100 nM) inhibited insulin-stimulated glucose transport but was without effect on GTPγS-stimulated deoxyglucose uptake (Figure 1A), consistent with previous studies (Clarke et al., 1994). Quantitatively similar results were observed using the plasma membrane lawn assay to quantify cell surface GLUT4 levels (Figure 1, B and C).
Figure 1.
DeGlc uptake and GLUT4 translocation in response to insulin and GTPγS: effects of ablation and wortmannin. In these experiments, 3T3-L1 adipocytes were incubated with TfHRP for 1 h and transferred to ice. After removal of cell surface TfHRP, DAB with or without hydrogen peroxide was added, and the DAB cytochemistry reaction progressed as outlined in MATERIALS AND METHODS. After ablation, cells were washed once in ice-cold PBS and twice in IC buffer at 37°C and then permeabilized with α-toxin as outlined. Cells were stimulated with GTPγS (100 μM) or insulin (1 μM) for 15 min before assay of deGlc uptake (A) or GLUT4 levels in plasma membrane lawns (B and C). In A, each point is the mean of triplicate determinations (± SEM) expressed as fold increase over basal transport rate. (B) Result of a typical experiment assaying GLUT4 levels by plasma membrane lawns. (C) Results of three experiments of this type, quantified as outlined in MATERIALS AND METHODS. In experiments examining the effect of wortmannin, this was added at 100 nM for 5 min before the addition of insulin or GTPγS.
To determine the role of the endosomal system on GTPγS versus insulin-stimulated GLUT4 translocation, we allowed adipocytes to internalize a TfHRP conjugate and then ablated the endosomes using the DAB polymerization technique (Livingstone et al., 1996; Stoorvogel et al., 1987, 1988). We then stimulated the cells with either insulin or GTPγS and measured either glucose transport or cell surface GLUT4 levels (Figure 1, A and B, and Table 1). This experimental regimen caused an approximately twofold increase in basal deGlc transport compared with control cells (Table 1). This is likely due to incubation of the cells at 4°C during the incubation with TfHRP rather than peroxide treatment, because there was no difference in deGlc transport between cells incubated with TfHRP and cells incubated with TfHRP plus hydrogen peroxide (Table 1). This is consistent with other studies in adipocytes, which have shown stimulatory effects of low temperature on glucose transport (Gibbs et al., 1991). The increase in transport caused by preincubation of the cells at low temperatures was relatively small (∼2-fold; Table 1) compared with that observed with insulin (∼13-fold) or GTPγS (∼6-fold) (Table 1). Ablation of the recycling endosomal system almost completely attenuated GTPγS-stimulated deGlc transport and GLUT4 translocation (Figure 1, A and B, and Table 1). This effect was specifically related to the endosomal ablation, because we only observed this inhibition in cells incubated with the combination of TfHRP, DAB, and hydrogen peroxide (Table 1). In contrast, a robust insulin effect on glucose transport and GLUT4 translocation was still evident after endosomal ablation (Figure 1, A and B, and Table 1). Intriguingly, the absolute magnitude of the ablation-inhibitable deGlc transport response was similar in the case of insulin and GTPγS (∼90–100 pmol/min per million cells; Table 1), consistent with the possibility that both insulin and GTPγS stimulate exit from endosomes, whereas only insulin stimulates GLUT4 translocation from GSVs.
Insulin- and GTPγS-stimulated Translocation Uses Distinct v-SNAREs
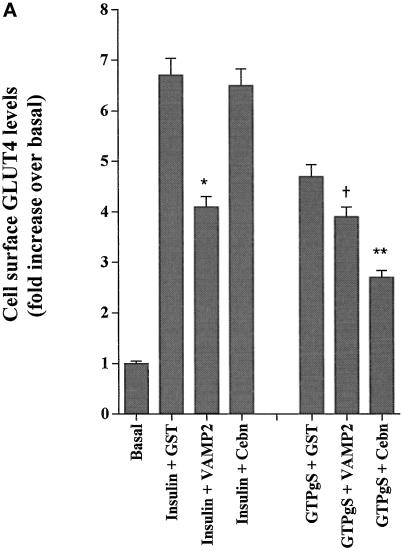
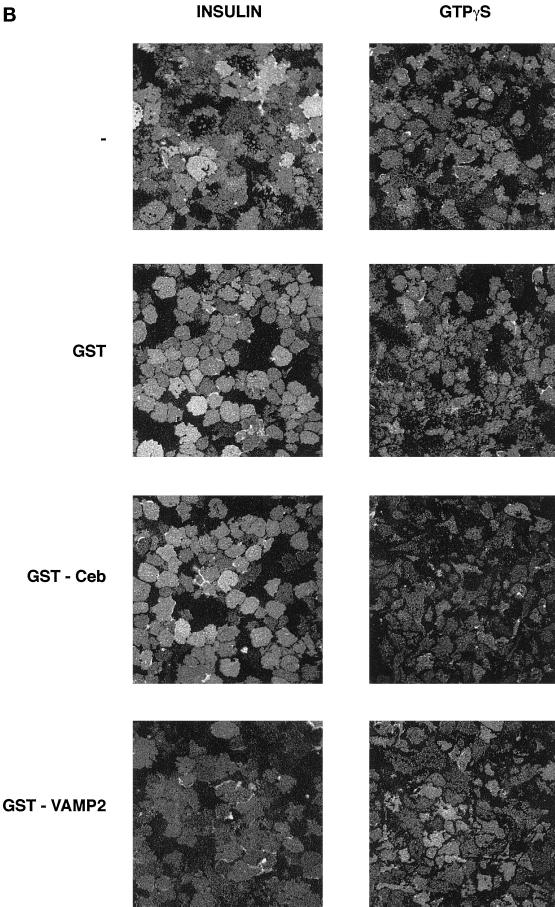
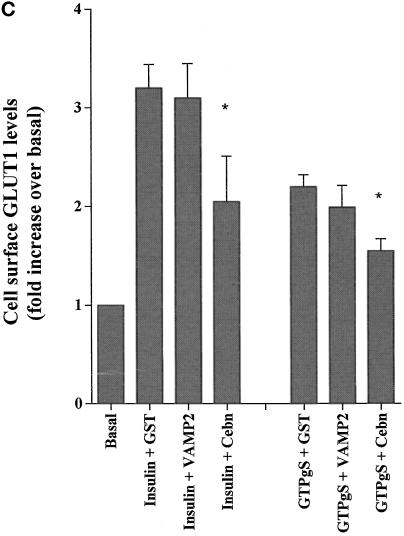
To further dissect the unique effects of GTPγS on GLUT4 trafficking, we studied the role of VAMP2 versus cellubrevin. It has previously been shown that v-SNARE cytosolic domains act as dominant negative inhibitors of trafficking (Volchuk et al., 1995; Olson et al., 1997; Martin et al., 1998). Thus, we incubated permeabilized adipocytes with either GST-VAMP2 or GST-cellubrevin and studied the effects of these agents on GTPγS versus insulin-stimulated deGlc transport. GST-cellubrevin inhibited GTPγS-stimulated GLUT4 translocation by 40–50%, whereas GST-VAMP2 had no significant effect (Figure 2, A and B). Conversely, GST-VAMP2 inhibited insulin-stimulated GLUT4 translocation by ∼40%, whereas GST alone or GST-cellubrevin had no significant effect on insulin action (Figure 2, A and B), consistent with previous findings (Martin et al., 1998).
Figure 2.
Effects of GST-v-SNARE fusion proteins on insulin- and GTPγS-stimulated GLUT4 and GLUT1 translocation. SLO-permeabilized 3T3-L1 adipocytes were incubated for 10 min with buffer containing GST alone or GST-VAMP2 or GST-cellubrevin fusion proteins, all at a concentration of 15 μg/ml. Cells were then stimulated with GTPγS (100 μM) or insulin (1 μM) for a further 15 min (still in the presence of fusion proteins) before preparation of plasma membrane lawns. (A) Effects of GST fusion proteins on GLUT4 translocation. (B) Representative experiment of this type. In B, cells were stimulated with GTPγS (100 μM) or insulin (1 μM) in the presence of GST alone (GST), GST-cellubrevin (GST-Ceb), or GST-VAMP2 or in the absence of any recombinant protein (−). (C) Effects of the same fusion proteins on GLUT1 translocation. In A and C, data are mean ± SEM of three independent experiments. † No significant difference compared with insulin alone. Significant inhibition is indicated: * p < 0.05; ** p ∼ 0.01.
To determine how specific these effects were with regard to GLUT4, we also performed similar experiments in which we assayed cell surface levels of GLUT1 (Figure 2C). Insulin and GTPγS stimulated cell surface GLUT1 levels by approximately three- and twofold, respectively. GST-cellubrevin inhibited both insulin- and GTPγS-stimulated GLUT1 translocation by ∼45%, whereas GST-VAMP2 and GST alone had no significant effect (Figure 2C). Collectively these data suggest that GTPγS has a selective effect on endosomal protein trafficking in adipocytes and that the docking and fusion of recycling endosomes with the cell surface is regulated by cellubrevin.
Effect of Insulin and GTPγS on the Subcellular Distribution of Rab Proteins in 3T3-L1 Adipocytes
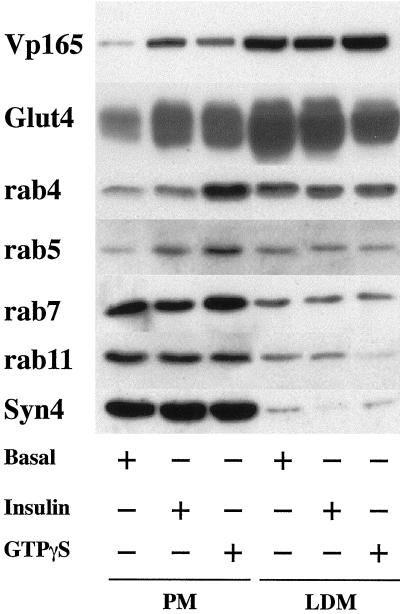
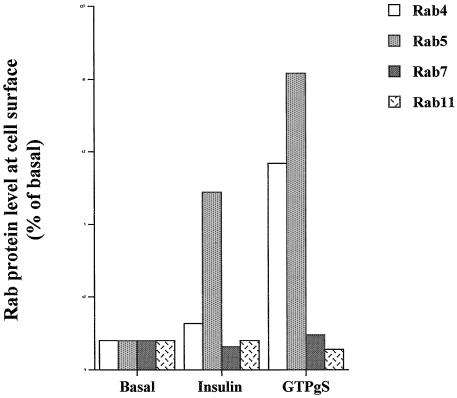
Recycling through the endosomal system may occur either via early endosomes or recycling endosomes (Ullrich et al., 1996; Sheff et al., 1999). These different organelles have been delineated based on the presence of unique Rab proteins at each location. Rabs 4 and 5 are predominantly localized to early endosomes (Bottger et al., 1996; Daro et al., 1996; Rybin et al., 1996; McLauchlan et al., 1998), whereas Rab 11 is targeted to recycling endosomes (Ullrich et al., 1996). To determine which of these organelles may be involved in the GTPγS response in adipocytes, we examined the subcellular distribution of these proteins with the view that GTPγS may cause the accumulation of different Rabs at their respective target organelles (Figure 3, A and B). In the absence of insulin, Rab4 and Rab5 were predominantly localized to intracellular (lwo-density microsome) membrane fractions, with much lower levels evident at the plasma membrane. Rab7 and Rab 11 were approximately equally distributed between the plasma membrane and intracellular membranes. In response to insulin, levels of Rab4, Rab7, and Rab11 at the plasma membrane were not significantly altered. By contrast, and in agreement with previous studies, Rab5 levels at the plasma membrane increased approximately sixfold (Figure 3, A and B) (Cormont et al., 1996). In response to GTPγS, the levels of both Rab4 and Rab5 were dramatically increased at the plasma membrane (∼7- and ∼10-fold, respectively). These data are consistent with the suggestion that GTPγS stimulates the fusion of vesicles derived from the early sorting endosome with the cell surface but not vesicles derived from the late endosome or the perinuclear recycling endosome.
Figure 3.
Effects of insulin and GTPγS on the subcellular distribution of Rab proteins in 3T3-L1 adipocytes. 3T3-L1 adipocytes were stimulated with GTPγS (100 μM) or insulin (1 μM) for 15 min and subjected to subcellular fractionation as outlined in MATERIALS AND METHODS. Aliquots (10 μg of protein) of isolated plasma membrane (PM) and low-density microsomes (LDM) were separated by SDS PAGE, transferred to nitrocellulose, and immunoblotted with antibodies against vp165, GLUT4, Rab4, Rab5, Rab7, or Rab11 and Syntaxin 4 as indicated. Shown are representative immunoblots from three experiments of this type. Quantification of this data is presented in B.
Insulin and GTPγS Regulation of TfR Levels at the Cell Surface
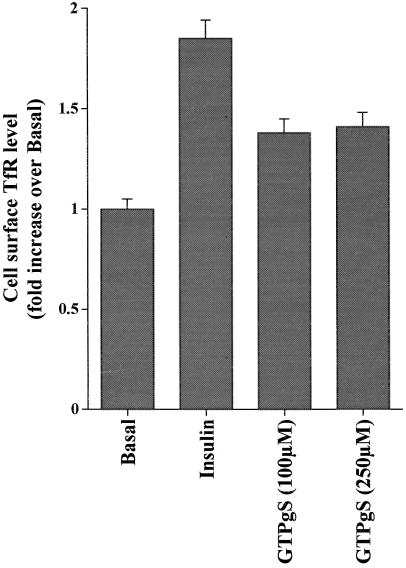
As well as stimulating GLUT4 translocation in adipocytes, insulin also causes an approximately twofold increase in cell surface TfR levels in 3T3-L1 adipocytes (Tanner and Lienhard, 1987). We have compared the effects of insulin and GTPγS on cell surface TfR levels. GTPγS induced a smaller increase in cell surface TfR levels (∼135%, average of two experiments) than insulin (185%, average of two experiments) (Figure 4).
Figure 4.
Effects of insulin and GTPγS on cell surface TfR levels. 3T3-L1 adipocytes were permeabilized with α-toxin as outlined in MATERIALS AND METHODS and incubated for 5 min before addition of insulin (1 μM) or GTPγS (100 μM) for 15 min at 37°C. Cells were then rapidly transferred to ice-cold buffer for assay of cell surface TfR levels. Shown are data from a representative experiment; each point is the mean of triplicate determinations (± SEM).
Is the Endosomal System Involved in Insulin-stimulated GLUT4 Translocation?
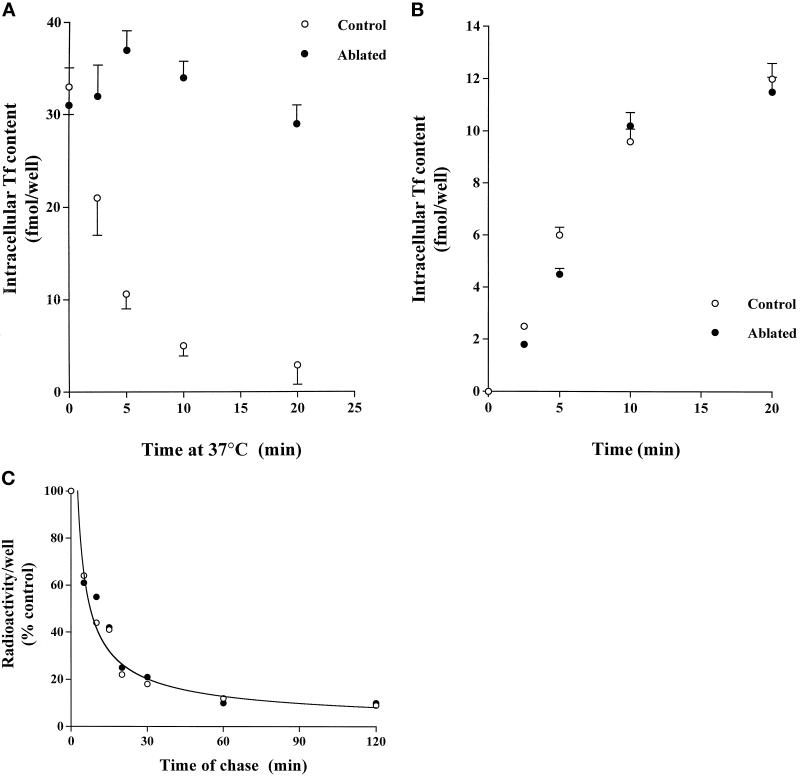
Whereas the effects of GTPγS on GLUT4 translocation were almost completely attenuated by endosome ablation, a robust effect of insulin was still observed under these conditions (Table 1 and Figure 1). The most likely interpretation of these data is that insulin stimulates the direct movement of GLUT4 to the plasma membrane from an intracellular compartment (such as GSVs) that is distinct from the endosomal system. However, it is also conceivable that new endosomes are regenerated during the incubation with insulin, and the GSVs may first fuse with this compartment en route to the cell surface. In an attempt to determine whether endosome regeneration could occur under the present experimental conditions, we performed the experiments outlined in Figure 5. We have previously shown (Martin et al., 1998) that endosome ablation inhibits subsequent recycling of the TfR in adipocytes (Figure 5A). However, a proportion of the total cellular TfR remains at the plasma membrane and thus will be unaffected by ablation. These receptors may be internalized and thus can be used as a marker for newly formed endosomes. To test this possibility, we determined 1) whether TfR present at the cell surface could internalize effectively after ablation, and 2) if so, whether these receptors could recycle back to the cell surface. The data presented in Figure 5B clearly show that internalization of TfR from the plasma membrane of 3T3-L1 adipocytes is essentially unaffected by endosome ablation and that the internalized receptors can subsequently recycle back to the plasma membrane (Figure 5C). Hence, it is not possible to use this experimental strategy to definitively rule out a role for the endosomal system in mediating at least some of the effects of insulin on GLUT4 translocation in these cells.
Figure 5.
TfR recycling in ablated cells indicates de novo endosome formation. (A) 3T3-L1 adipocytes were incubated with 2.2 μg/ml [125I]transferrin (3 μCi) together with TfHRP at 20 μg/ml. Subsequently, cell surface–attached Tf/TfHRP was removed, and cytochemistry using DAB with or without hydrogen peroxide was performed at 4°C as outlined. After incubation at 4°C for 1 h to complete the ablation reaction, cells were rewarmed to 37°C and incubated for the times shown to allow assay of the release of radiolabeled transferrin from the cells. Cell-associated transferrin was measured at the time shown in control (open circles) and ablated (filled circles) cells. Each point is the mean of three determinations, and data from a representative experiment are shown. (B) In these experiments, cells were loaded with TfHRP as outlined and then transferred to ice-cold buffer to stop membrane trafficking. Subsequently, cell surface–attached Tf/TfHRP was removed, and cytochemistry using DAB with or without hydrogen peroxide was performed at 4°C as outlined. After incubation at 4°C for 1 h to complete the ablation reaction, cells were rewarmed to 37°C, and the rate of uptake of 3 nM [125I]transferrin was measured as described. In these experiments, cells were incubated at 37°C with 3 nM [125I]transferrin for the times shown. Cells were then transferred to ice-cold buffer, cell surface–associated [125I]transferrin was removed by acid washing, and cell-associated transferrin was then determined as outlined. Shown are the data from a typical experiment (each point is the mean of triplicate determina-tions), repeated a further two times. Open circles represent uptake in control cells, and filled circles represent uptake in ablated cells. There was no detectable difference in the rate of Tf uptake under these conditions. (C) The experiment shown in B indicates that the TfR that remains at the cell surface after endosome ablation is still capable of binding ligand and internalizing it. This suggests that an endosomal system may reform de novo. To examine this possibility we next measured TfR recycling under the same conditions. Cells were loaded with TfHRP as outlined and then transferred to ice-cold buffer to stop membrane trafficking. Subsequently, cell surface–attached TfHRP was removed, and cytochemistry using DAB with or without hydrogen peroxide was performed at 4°C as outlined. After incubation at 4°C for 1 h to complete the ablation reaction, cells were rewarmed to 37°C, and 3 nM [125I]transferrin was added to the cells for 30 min at 37°C. Cells were then transferred to ice-cold buffer, and cell surface–associated [125I]transferrin was removed by acid washing. The cells were then rewarmed to 37°C, and the rate of externalization of transferrin was determined. Shown is the result of a typical experiment; each point is the mean of triplicate determinations at each condition. Open circles represent uptake in control cells, and filled circles represent uptake in ablated cells. There was no detectable difference in the rate of Tf release under these conditions.
DISCUSSION
In the present study we provide evidence demonstrating that adipocytes contain at least two regulatory recycling pathways that can be used to differentially regulate the composition of the cell surface. The first of these pathways represents the endosomal system, and the second is a nonendosomal pool of GLUT4, which we have referred to as GSVs. These pathways appear to be distinct in three major ways. First, they exhibit unique regulatory properties. Although insulin appears to stimulate both pathways, other agonists, in this case GTPγS, selectively regulate only one of these pathways. Second, these pathways appear to have distinct points of origin within the cell. The GTPγS-sensitive pathway is predominately derived from the endosomal system, whereas, in addition to this pathway, insulin also stimulates the movement of GLUT4 from a compartment that is distinct from the recycling endosomal system. Finally each pathway appears to carry a subset of unique cargo in that molecules such as GLUT1 appear to selectively use the endosomal route, whereas other proteins, such as GLUT4, appear to use both.
Insulin and GTPγS Access Distinct Intracellular GLUT4 Compartments
We present three lines of evidence to suggest that GTPγS selectively stimulates recycling via the endosomal system. First, it is almost completely blocked by endosomal ablation, whereas by contrast there is only a partial block of insulin action (Figure 1, A and B, and Table 1). Second, we have shown that GTPγS-stimulated GLUT4 translocation is selectively inhibited by GST-cellubrevin but not by GST-VAMP2 (Figure 2, A and B). Finally, other proteins, such as GLUT1, thought to recycle by the endosomal system are also regulated by GTPγS and are selectively blocked by GST-cellubrevin (Figure 2C). Insulin, on the other hand, appears to stimulate GLUT4 translocation from both endosomes and a separate compartment (GSVs). This separate compartment is defined on the basis of two experimental criteria. First, a robust effect of insulin on GLUT4 translocation was still observed in ablated cells, and second, the cytosolic tail of VAMP2 inhibited insulin-stimulated GLUT4 translocation by ∼40%.
Collectively, these findings have led us to propose the following model. We suggest that there are two separate GLUT4 compartments in adipocytes, one of which is endosomal in origin and the other nonendosomal (GSV). Both insulin and GTPγS mobilize the endosomal pool of GLUT4, but only insulin is capable of translocating GLUT4 from the GSV compartment. Some proteins, such as GLUT1 and the TfR, predominantly recycle back to the plasma membrane via recycling endosomes. This probably represents a generic pathway that is found in all cell types. Indeed, many growth factors have been shown to stimulate cell surface levels of GLUT1, TfR, and other recycling membrane proteins in a range of cell types (Oka et al., 1984; Tanner and Lienhard, 1987; Gould et al., 1994; Kandror and Pilch, 1996, 1998), and many of these proteins have been observed to colocalize in 3T3-L1 adipocytes (Tanner and Lienhard, 1989; Kandror and Pilch, 1998). Thus the endosomal pathway may be regulated via a variety of agonists to transiently increase generalized recycling. More specialized molecules, such as GLUT4 and insulin-responsive amino peptidase, may recycle via the GSVs, and this compartment may be uniquely regulated by insulin. This unique insulin-responsive compartment is likely to be cell type specific, and recent studies in 3T3-L1 adipocytes have provided evidence for the formation of such a compartment in these cells at an early stage of differentiation (El-Jack et al., 1999b). This pathway may serve two major purposes: 1) to exclude proteins such as GLUT4 and insulin-responsive amino peptidase from the recycling pathway in the absence of insulin, and 2) to act as a reservoir of GLUT4, which is mobilized after insulin stimulation. Neither of these properties is afforded by the endosomal pathway. The notion of different intracellular GLUT4 compartments has also been advanced in the context of exercise- and insulin-stimulated glucose transport in muscle (Coderre et al., 1995; Yeh et al., 1995; Aledo et al., 1997; Ploug et al., 1998). The data presented here suggest that exercise and GTPγS may represent analogous pathways of GLUT4 recruitment.
Implications of the Two-Compartment Model for GLUT4 Trafficking
One implication of this two-compartment model is that distinct signal transduction machinery may be involved in the mobilization of GLUT4 from these different compartments. We suggest that GSVs are uniquely sensitive to insulin presumably as a consequence of a novel aspect of intracellular signaling in response to activation of the insulin receptor. This model also implies that the trafficking machinery may be distinct. In this regard, we have shown that GTPγS-stimulated GLUT4 translocation primarily involves the generic endosomal v-SNARE cellubrevin (Figure 2) and early endosomal Rab proteins Rab4 and Rab5 (Figure 3). Conversely, the effect of insulin on GSVs is mainly regulated by the more specialized v-SNARE VAMP2 and presumably by a distinct Rab protein that remains to be identified.
One prediction of this model is that insulin and GTPγS should be additive for GLUT4 translocation. This is not the case, at least at maximal doses of these agonists (Robinson et al., 1992; Elmendorf et al., 1998). The most obvious explanation for this is that insulin and GTPγS may have similar effects on the endosomal system in these cells, which is consistent with our model. This highlights one difference between the model proposed here and that suggested to explain insulin- and exercise-stimulated GLUT4 translocation in skeletal muscle. In muscle, insulin and exercise are additive for GLUT4 translocation, whereas in adipocytes insulin and GTPγS are not.
A further prediction of our model is that if insulin stimulates GLUT4 translocation from both endosomes and GSVs, there should have been at least partial inhibition of the insulin effect in the presence of GST-cellubrevin. However, this was not the case (Figure 2A). Cellubrevin clearly plays a role in insulin-stimulated endosomal trafficking to the cell surface based on its effect on GLUT1 translocation (Figure 2C). We cannot exclude a role for cellubrevin in the insulin-regulated trafficking of GLUT4 in view of the quantitative limitations of our assay. GST-cellubrevin inhibited insulin-stimulated GLUT1 translocation by ∼40%. Assuming that all of the GLUT1 is recycling via early endosomes, this sets an upper limit to the degree of inhibition to be expected for GLUT4. However, we have estimated that only ∼40% of the total intracellular GLUT4 is targeted to endosomes (Livingstone et al., 1996). Hence, if GST-cellubrevin inhibited flux from the endosomal pool of GLUT4 by 40%, quantitatively this would have resulted in a 16% decrease in the total insulin response. A change of this magnitude would not be detected by our assay system.
The TfR is often considered to be the prototypical marker of the recycling endosomal system, in which case we would have expected to have observed a similar effect of insulin and GTPγS on the translocation of this protein to the cell surface. However, we found that insulin had a much greater effect than GTPγS on TfR exocytosis (Figure 4). The most likely explanation for this discrepancy is that a proportion of the TfR may be targeted to the GSVs thus rendering it more responsive to insulin than to GTPγS. In fact a recent study by El Jack et al. (1999a) has proposed that the TfR is targeted to GSVs in 3T3-L1 adipocytes. We argue that this is a minor fraction of the TfR, because ∼85% of the TfR is lost upon endosome ablation (Livingstone et al., 1996). Nevertheless, the nonablatable TfR may contribute to the additional effect of insulin observed in the present studies.
Another question raised by the present experiments is why GTPγS does not promote translocation of GLUT4 from the GSVs, thus mimicking insulin action. There may be several explanations for this. First, GSVs may first fuse with another compartment en route to the cell surface, and GLUT4 may accumulate in this compartment in response to GTPγS. Such a model is attractive because it can account for the inhibition of insulin-stimulated GLUT4 translocation observed in response to GDPβS at low doses of insulin (Elmendorf et al., 1998). We have attempted to exclude this possibility, but instead our data definitively show that a new endosomal compartment can reform after ablation with TfHRP (Figure 5), and so we cannot exclude this model. Finally, the model we currently prefer is that exit from the early endosome is a constitutive ongoing process, which is accelerated by activating a Rab protein, which increases docking of vesicles derived from this site with the plasma membrane. However, GSVs have a slow exocytic rate in the absence of insulin because of some kind of interaction with a factor that precludes recycling. Thus, to recruit GLUT4 from GSVs it may be necessary to overcome this interaction as well as to regulate docking of these vesicles at the cell surface. Insulin may be able to achieve both of these effects, whereas GTPγS may only activate the latter. This model is also consistent with the lack of additivity of GTPγS and insulin on transport.
Rab Proteins and GLUT4 Trafficking in Response to GTPγS
GTPγS caused an accumulation of both Rab4 and Rab5 in the plasma membrane, with no effect on either Rab7 or Rab11 (Figure 3, A and B). This is consistent with a role for Rab4 in regulating a recycling pathway from early endosomes to the cell surface and a role for Rab5 in regulating internalization from the plasma membrane (McLauchlan et al., 1998). Insulin stimulation increased Rab5 levels at the plasma membrane (Figure 3A) (Cormont et al., 1996) but had no effect on Rab4. The basis for the differential effect of insulin but not GTPγS on the accumulation of these proteins at the plasma membrane is not known. The prediction is that the Rab proteins will localize either to their target membrane or the step that defines their rate-limiting point of function in response to GTPγS. This will not be the case for insulin, because repeated cycles of GTP loading and hydrolysis will occur. Our observation that in response to GTPγS both Rab4 and Rab5 become plasma membrane localized argues that this is the likely target membrane for both of these Rabs. This is in contrast to data obtained in other cell types with GTP-locked mutants of these Rabs, which do not localize to the plasma membrane (van der Sluijs et al., 1992; Stenmark et al., 1995; Vitale et al., 1998). The difference may reflect the acute nature of our experiments in which we have studied the localization of the Rabs at 15 min after GTPγS loading. It is conceivable that long-term expression of a GTP-locked mutant may cause an accumulation at a different stage of the Rab cycle. As such, this approach is potentially interesting and may shed new light on the function of these proteins. Based on the data presented here, we propose that Rab4 and Rab5 regulate trafficking events directly at the plasma membrane, and that insulin may also regulate such events.
Limitations of the Two-Compartment Model
Many recent studies, the present one included, have proposed a relatively simple two-compartment model to explain the insulin-dependent trafficking of GLUT4. A number of issues remain to be resolved, however, concerning the validity of this model. First, do the GSVs move directly to the plasma membrane? We have tried to answer this question using endosomal ablation in living cells. However, we cannot exclude the possibility of endosomal reformation under the assay conditions used (Figure 5). Second, what is the communication between the GSVs and other compartments under basal conditions? At present, we view the GSVs as a very static compartment that once formed remains disconnected from the endosomal system; however, this may be an oversimplification, and these compartments may remain in dynamic equilibrium. Nevertheless, there is clearly a separate trafficking event for GLUT4 that is acutely regulated by insulin and that involves unique molecules such as VAMP2. Thus, the present studies suggest that there are at least two separate pathways via which GLUT4 can travel to the surface. Insulin appears to stimulate both of these pathways, but GTPγS only stimulates one of these. It is now of fundamental interest to determine the unique molecular characteristics of the insulin-specific pathway.
ACKNOWLEDGMENTS
We thank Drs. P. Chavrier, P. van der Sluijs, and R. Parton for the provision of antibodies used in this study. This work was supported by grants from The British Diabetic Association, The Wellcome Trust, and The Medical Research Council (to G.W.G.) and the National Health and Medical Research Council of Australia and Diabetes Australia (to D.E.J.). C.A.M. and G.R.X.H. thank The British Diabetic Association for Ph.D. studentships.
Abbreviations used:
- DAB
diaminobenzidine
- deGlc
2-deoxy-d-glucose
- DMEM
Dulbecco’s modified Eagle’s medium
- GLUT
glucose transporter
- GSV
GLUT4 storage vesicle
- GTPγS
guanosine-5′-O-(3-thiotriphosphate)
- SLO
streptolysin O, Tf-HRP, transferrin-HRP conjugate
- TfR
transferrin receptor
- VAMP2
vesicle-associated membrane protein-2
- v-SNARE
vesicle-associated soluble N-ethylmaleimide-sensitive factor–attached protein receptor
REFERENCES
- Aledo JC, Lavoie L, Volchuck A, Keller SR, Klip A, Hundal HS. Identification and characterization of two distinct intracellular GLUT4 pools in rat skeletal muscle: evidence for an endosomal and an insulin-sensitive GLUT4 compartment. Biochem J, 1997;325:727–732. doi: 10.1042/bj3250727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini G, Hohman R, Charron MJ, Lodish HF. Insulin and nonhydrolyzable GTP analogs induce translocation of GLUT4 to the plasma membrane in a-toxin-permeabilized rat adipose cells J. Biol Chem, 1991;266:4037–4040. [PubMed] [Google Scholar]
- Bottger G, Nagelkerken B, van der Sluijs P. Rab4 and Rab7 define distinct nonoverlapping endosomal compartments J. Biol Chem, 1996;271:29191–29197. doi: 10.1074/jbc.271.46.29191. [DOI] [PubMed] [Google Scholar]
- Calderhead DM, Kitagawa K, Tanner LI, Holman GD, Lienhard GE. Insulin regulation of the two glucose transporters in 3T3–L1 adipocytes J. Biol Chem, 1990;265:13800–13808. [PubMed] [Google Scholar]
- Chen D, Elmendorf JS, Olson AL, Li X, Earp HS, Pessin JE. Osmotic shock stimulates GLUT4 translocation in 3T3–L1 adipocytes by a novel tyrosine kinase pathway J. Biol Chem. 1997;272:27401–27410. doi: 10.1074/jbc.272.43.27401. [DOI] [PubMed] [Google Scholar]
- Clarke JF, Young PW, Yonezawa K, Kasuga M, Holman GD. Inhibition of translocation of GLUT1 and GLUT4 in 3T3–L1 cells by the phosphatidylinositol 3′-kinase inhibitor, wortmannin. Biochem J, 1994;300:631–635. doi: 10.1042/bj3000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre L, Kandror KV, Vallega G, Pilch PF. Identification and characterization of an exercise-sensitive pool of glucose transporters in skeletal muscle. J Biol Chem. 1995;270:27584–27588. doi: 10.1074/jbc.270.46.27584. [DOI] [PubMed] [Google Scholar]
- Cormont M, Van Obberghen E, Zerial M, Le Marchand-Brustel Y. Insulin induces a change in Rab5 subcellular localization in adipocytes independently of phosphatidylinositol 3-kinase activation. Endocrinology. 1996;137:3408–3415. doi: 10.1210/endo.137.8.8754768. [DOI] [PubMed] [Google Scholar]
- Daro E, van der Sluijs P, Galli T, Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci USA. 1996;93:9559–9564. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Jack AK, Hamm JK, Pilch PF, Farmer SR. Reconstitution of insulin-sensitive glycose transport in fibroblasts requires expression of both PPAR gamma and C/EBP alpha. J Biol Chem. 1999a;274:7946–7951. doi: 10.1074/jbc.274.12.7946. [DOI] [PubMed] [Google Scholar]
- El-Jack AK, Kandror K, Pilch PF. The formation of an insulin-responsive vesicular cargo compartment is an early event in 3T3–L1 adipocyte differentiation. Mol Biol Cell. 1999b;10:1581–1594. doi: 10.1091/mbc.10.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmendorf JS, Chen D, Pessin JE. Guanosine 5′-O-(3-triphosphate) (GTPγS) stimulation of GLUT4 translocation is tyrosine kinase dependent. J Biol Chem. 1998;273:13289–13296. doi: 10.1074/jbc.273.21.13289. [DOI] [PubMed] [Google Scholar]
- Frost SC, Lane MD. Evidence for the involvement of vicinal sulfydryl groups in insulin-activated hexose transport in 3T3–L1 adipocytes. J Biol Chem. 1988;260:2646–2652. [PubMed] [Google Scholar]
- Gibbs EM, Calderhead DM, Holman GD, Gould GW. Phorbol esters only partially mimic the effect of insulin on glucose transport and transporter distribution in 3T3–L1 adipocytes. Biochem J. 1991;275:145–150. doi: 10.1042/bj2750145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GW, Merrall NW, Martin S, Jess TJ, Campbell IW, Calderhead DM, Gibbs EM, Holman GD, Plevin RJ. Growth factor induced stimulation of hexose transport in 3T3–L1 adipocytes: evidence that insulin-induced translocation of GLUT4 is independent of activation of MAP kinase. Cell Signal. 1994;6:313–320. doi: 10.1016/0898-6568(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Herbst JJ, Andrews GC, Contillo LG, Singleton DH, Genereux PE, Gibbs EM, Lienhard GE. Effects of the activation of phosphatidylinositol 3′-kinase by a thiophosphotyrosine peptide on glucose transport in 3T3–L1 adipocytes. J Biol Chem. 1995;270:26000–26005. doi: 10.1074/jbc.270.43.26000. [DOI] [PubMed] [Google Scholar]
- Herman GA, Bonzelius F, Cieutat A-M, Kelly RB. A distinct class of intracellular storage vesicles, identified by expression of the glucose transporter GLUT4. Proc Natl Acad Sci USA. 1994;91:12750–12754. doi: 10.1073/pnas.91.26.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jess TJ, Belham CM, Thomson FJ, Scott PH, Plevin RJ, Gould GW. Phosphatidylinositol 3′-kinase, but not p70 ribosomal S6 kinase, is involved in membrane protein recycling: wortmannin inhibits glucose transport and down-regulates cell-surface transferrin receptor numbers independently of any effect on fluid-phase endocytosis in fibroblasts. Cell Signal. 1996;8:297–304. doi: 10.1016/0898-6568(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. The insulin-like growth factor II/mannose 6-phosphate receptor utilizes the same membrane compartments as GLUT4 for insulin-dependent trafficking to and from the rat adipocyte cell surface. J Biol Chem. 1996;271:21703–21708. doi: 10.1074/jbc.271.36.21703. [DOI] [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. Multiple endosomal recycling pathways in rat adipose cells. Biochem J. 1998;331:829–835. doi: 10.1042/bj3310829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone C, James DE, Rice JE, Hanpeter D, Gould GW. Compartment ablation analysis of the insulin responsive glucose transporter, GLUT4, in 3T3–L1 adipocytes. Biochem J. 1996;315:487–495. doi: 10.1042/bj3150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malide D, Dwyer NK, Blanchette-Mackie EJ, Cushman SW. Immunocytochemical evidence that GLUT4 resides in a specialized translocation postendosomal VAMP2-positive compartment in rat adipose cells in the absence of insulin J. Histochem Cytochem. 1997;45:1083–1095. doi: 10.1177/002215549704500806. [DOI] [PubMed] [Google Scholar]
- Martin L, Shewan A, Millar CA, Gould GW, James DE. Vesicle associated membrane protein-2 (VAMP2) plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3–L1 adipocytes. J Biol Chem. 1998;273:1444–1452. doi: 10.1074/jbc.273.3.1444. [DOI] [PubMed] [Google Scholar]
- Martin S, Reaves B, Banting G, Gould GW. Analysis of the colocalization of the insulin-responsive glucose transporter (GLUT4) and the trans Golgi network marker TGN38 within 3T3–L1 adipocytes. Biochem J. 1994;300:743–749. doi: 10.1042/bj3000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE. The glucose transporter (GLUT4) and vesicle-associated membrane protein (VAMP2) are segregated from recycling endosomes in insulin-sensitive cells. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe E. A novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- Oka Y, Mottola C, Oppenheimer CL, Czech MP. Insulin activates the appearance of insulin-like growth factor II receptors on the adipocyte cell surface. Proc Natl Acad Sci USA. 1984;81:4028–4032. doi: 10.1073/pnas.81.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AL, Knight JB, Pessin JE. Syntaxin 4, VAMP2, and/or VAMP3/Cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1997;17:2425–2435. doi: 10.1128/mcb.17.5.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin stimulated GLUT4 vesicle trafficking: location! location! location! J Biol Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- Ploug T, van Deurs B, Ai H, Cushman SW, Ralston E. Analysis of GLUT4 distribution in whole skeletal muscle fibers: Identification of distinct storage compartments that are recruited by insulin and muscle contractions J. Cell Biol. 1998;142:1429–1446. doi: 10.1083/jcb.142.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Pang S, Harris DS, Heuser J, James DE. Translocation of the glucose transporter (GLUT4) to the cell surface in permeablized 3T3–L1 adipocytes: effects of ATP, insulin and GTPγS and localization of GLUT 4 to clathrin lattices. J Cell Biol. 1992;117:1181–1196. doi: 10.1083/jcb.117.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybin V, Illrich O, Rubino M, Alexandrov K, Simon I, Seabrea MC, Goody R, Zerial M. GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature. 1996;383:266–269. doi: 10.1038/383266a0. [DOI] [PubMed] [Google Scholar]
- Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Garruti G, Martin S, Oorschot V, Posthuma G, Kraegen EW, Laybutt R, Thibault G, James DE. Glucose transporter (GLUT-4) is targeted to secretory granules in rat atrial cardiomyocytes. J Cell Biol. 1997;137:1243–1254. doi: 10.1083/jcb.137.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Geuze HJ, Griffith JM, Strous GJ. The pathways of endocytosed transferrin and secretory protein are connected in the trans-Golgi reticulum. J Cell Biol. 1988;106:1821–1829. doi: 10.1083/jcb.106.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Geuze HJ, Strous GJ. Sorting of endocytosed transferrin and asialoglycoprotein occurs immediately after internalization in HepG2 cells. J Cell Biol. 1987;104:1261–1268. doi: 10.1083/jcb.104.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner LI, Lienhard GE. Insulin elicits a redistribution of transferrin receptors in 3T3–L1 adipocytes through an increase in the rate constant for receptor externalization. J Biol Chem. 1987;262:8975–8980. [PubMed] [Google Scholar]
- Tanner LI, Lienhard GE. Localization of transferrin receptors and insulin-like growth factor II receptors in vesicles from 3T3–L1 adipocytes that contain intracellular glucose transporters J. Cell Biol. 1989;108:1537–1545. doi: 10.1083/jcb.108.4.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellam JT, Macaulay SL, McIntosh S, Hewish DR, Ward CW, James DE. Characterization of Munc-18c and Syntaxin-4 3T3–L1 adipocytes. J Biol Chem. 1997;272:6179–6186. doi: 10.1074/jbc.272.10.6179. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinsche S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs P, Hull M, Zahraouri A, Tavitian A, Goud B, Mellman I. The small GTP-binding protein rab4 controls and early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- Vitale G, Rybin V, Christofordis S, Thornqvist P-O, McCaffrey N, Stenmark H, Zerial M. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound rab4 and rab5. EMBO J. 1998;17:1941–1951. doi: 10.1093/emboj/17.7.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchuk A, Sargeant R, Sumitani S, Liu Z, He L, Klip A. Cellubrevin is a resident protein of insulin-sensitive GLUT4 glucose transporter vesicles in 3T3–L1 adipocytes. J Biol Chem. 1995;270:8233–8240. doi: 10.1074/jbc.270.14.8233. [DOI] [PubMed] [Google Scholar]
- Yeh JI, Gulve EA, Rameh L, Birnbaum MJ. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem. 1995;270:2107–2111. doi: 10.1074/jbc.270.5.2107. [DOI] [PubMed] [Google Scholar]