Abstract
In this report, we show that biofilm formation by Streptococcus pneumoniae serotype 19 gives rise to variants (the small mucoid variant [SMV] and the acapsular small-colony variant [SCV]) differing in capsule production, attachment, and biofilm formation compared to wild-type strains. All biofilm-derived variants harbored SNPs in cps19F. SCVs reverted to SMV, but no reversion to the wild-type phenotype was noted, indicating that these variants were distinct from opaque- and transparent-phase variants. The SCV-SMV reversion frequency was dependent on growth conditions and treatment with tetracycline. Increased reversion rates were coincident with antibiotic treatment, implicating oxidative stress as a trigger for the SCV-SMV switch. We, therefore, evaluated the role played by hydrogen peroxide, the oxidizing chemical, in the reversion and emergence of variants. Biofilms of S. pneumoniae TIGR4-ΔspxB, defective in hydrogen peroxide production, showed a significant reduction in variant formation. Similarly, supplementing the medium with catalase or sodium thiosulfate yielded a significant reduction in variants formed by wild-type biofilms. Resistance to rifampin, an indicator for mutation frequency, was found to increase approximately 55-fold in biofilms compared to planktonic cells for each of the three wild-type strains examined. In contrast, TIGR4-ΔspxB grown as a biofilm showed no increase in rifampin resistance compared to the same cells grown planktonically. Furthermore, addition of 2.5 and 10 mM hydrogen peroxide to planktonic cells resulted in a 12- and 160-fold increase in mutation frequency, respectively, and gave rise to variants similar in appearance, biofilm-related phenotypes, and distribution of biofilm-derived variants. The results suggest that hydrogen peroxide and environmental conditions specific to biofilms are responsible for the development of non-phase-variable colony variants.
Streptococcus pneumoniae is a leading cause of invasive and noninvasive bacterial diseases, including sinusitis, otitis media, bacteremia, pneumonia, and meningitis (10, 19). The ability to exist in these diverse host environments is dependent on the capability of S. pneumoniae to undergo spontaneous phase variation between transparent and an opaque colony phenotypes (17, 25). In comparison to the opaque colony variant, cells displaying a transparent phenotype have increased amounts of cell wall carbohydrate (C polysaccharide or teichoic acid) and reduced capsular polysaccharide. A reduction in capsule material has been shown to lead to enhanced adherence to human epithelial cells in vitro and to enhanced colonization of the nasopharynx in vivo in mammals (3, 7, 24). In contrast, cells displaying an opaque phenotype are more resistant to opsonophagocytosis, due to increased protection conferred by the capsular polysaccharide. Pneumococci isolated from normally sterile sites such as the blood and lungs typically form opaque colonies (25).
Published studies suggest that the cause of spontaneous opaque/transparent phase variation is the recombinant exchange and sequence duplication of pneumococcal capsule-specific genes, resulting in on-off switching of the genes encoding the capsular biosynthetic machinery (13, 31, 34, 35). Opaque/transparent phase variation occurs at frequencies of higher than 10−5 switches per cell per generation This process is distinct from spontaneous mutations, which occur at a frequency of approximately 10−7 mutations per cell per generation (13). Importantly, opaque/transparent phase variation in S. pneumoniae has been observed under both planktonic and surface-associated conditions, including biofilm growth (12, 13, 22, 34, 35).
Recently, mucoid variants of S. pneumoniae have been detected. These mucoid variants are unable to undergo phase variation, harbor a variety of mutations, and emerge only upon surface-associated growth on both biotic and abiotic surfaces. Thus, they are different from opaque transparent phase variants. Studies examining these novel mucoid variants include those by Hammerschmidt et al. (12), who demonstrated the emergence of mucoid or capsule polysaccharide variants following the initial phase of pneumococcal infections of lung epithelial cells. These variants exhibited reduced expression of capsular polysaccharide, up to a 105-fold-greater capacity to adhere to epithelial cells, and up to a 104-fold-greater capacity to invade epithelial cells compared to wild-type strains. In 44% of the 25 variants analyzed, a single base pair mutation generating a premature stop codon in cps3D, rendering the capsule biosynthetic protein Cps3D nonfunctional, was detected (12). More recently, McEllistrem et al. (22) noted that colony biofilms gave rise to nonrevertible unencapsulated variants harboring single nucleotide polymorphisms (SNPs) in the cps3D gene, an SNP at the −10 site on the promoter, and large deletions in the cps3D gene. The variants were detected at low frequency.
We recently observed the high-frequency emergence of an acapsular small-colony variant (SCV) during S. pneumoniae biofilm development. More than 80% of the bacterial biofilm population was observed to be composed of this acapsular variant soon after initial attachment. Two additional S. pneumoniae serotype 3 mucoid variants (the small mucoid variant [SMV] and the medium mucoid variant [MMV]) were detected in aging biofilms (2). The variants were detected only following growth as a biofilm. SCVs displayed distinct phenotypes compared to wild-type S. pneumoniae. SCVs autoaggregated in liquid culture, hyperadhered to solid surfaces, and formed biofilms with extensive three-dimensional structure. In contrast to the opaque and transparent phase variants, the acapsular SCV was not revertible. The molecular basis of SCV formation was attributed to a large deletion within the cps3DSU capsule operon (2). This indicated that phase variation was not the mechanism involved in SCV formation. To our knowledge, this is the first description of variants other than phase variants emerging at high frequency in S. pneumoniae.
Several studies have indicated that the spatial heterogeneity within a biofilm is responsible for the diversification of bacterial strains, including certain pneumococcal serotypes, into colony phenotypes (3, 22, 34, 35). While it is apparent that growth conditions may play a significant role in diversification and colony variant formation, the underlying mechanisms of the biofilm-derived colony variant formation are unknown. In this study, we explored the role of genetic diversity among S. pneumoniae serotypes and growth conditions in the emergence of colony variants. S. pneumoniae serotype 19 was chosen for this study due to the differences in capsule, biofilm architecture, and attachment capabilities compared to the previously characterized S. pneumoniae serotype 3 strain (1, 2). Furthermore, we addressed the issue of whether increased frequency of nonrevertible variant mutant formation is a result of a natural selection process for phenotypes better equipped for attachment and microcolony formation or due to increased mutation rates under biofilm growth conditions. In addition, we provide evidence that endogenous hydrogen peroxide plays an important role in the emergence of biofilm-derived S. pneumoniae variants.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Streptococcus pneumoniae strains and laboratory-derived colony morphology variants listed in Table 1 were grown in Todd-Hewitt broth or on Trypticase soy agar supplemented with 5% sheep's blood at 37°C in 5% CO2 as previously described (1, 2). The laboratory-derived colony morphology variants were generated, as described below, in a continuous flow tube reactor.
TABLE 1.
Strains used in this study
| Strain | Serotype | Colony description | Colony diam (mm) | Reference or source |
|---|---|---|---|---|
| ATCC 6303 | 3 | Large mucoid | 4-5 | 1 |
| BS71, serotype 3 | 3 | Large mucoid | 4-5 | 1 |
| BS75, serotype 19 | 19 | Medium mucoid | 1.5-2.5 | 1 |
| TIGR4 | 4 | Small mucoid | 1-1.5 | 30 |
| TIGR4 spxB | 4 | Small mucoid | 1-1.5 | 30 |
| Serotype 19 biofilm-derived variants | ||||
| MMV | 19 | Medium mucoid | 2.5-3 | This study |
| SMV | 19 | Small mucoid | 1-1.5 | This study |
| SCV | 19 | Small nonmucoid | 1 | This study |
Biofilm formation and evaluation of colony variance.
S. pneumoniae serotype 19 (BS75 clinical isolate) biofilms were grown in flow cells and tube reactors under flowing conditions in 0.2× diluted Todd-Hewitt medium (6 g/liter) at 37°C in 5% CO2 for 1, 3, 6, and 9 days as previously described (2). To evaluate and quantify the emergence of colony morphology variants associated with S. pneumoniae serotype 19 biofilm formation, biofilms were harvested at various time points, serially diluted, and plated onto blood agar. Colonies were scored as either mucoid or nonmucoid based on their appearance on blood agar and categorized according to diameter as medium or small variants (Table 1). Similarly, S. pneumoniae TIGR4 and a pyruvate oxidase mutant, TIGR4-ΔspxB, were evaluated for the emergence of colony variants during biofilm growth. Autoaggregation was determined by visual inspection of planktonic cultures. All experiments were done in triplicate.
To determine the role of hydrogen peroxide and/or oxidative stress in the emergence of colony variants, S. pneumoniae wild-type (BS75 and BS71) bacteria were grown in tube reactors as described above for 1 day in the presence of 10 mM sodium thiosulfate or catalase (4 U/ml; added every 4 h to the growth medium) to eliminate hydrogen peroxide. Following 1 day of growth, biofilms were removed from the biofilm reactor, homogenized, serially diluted, and plated onto blood agar to enumerate surviving bacteria and to evaluate the emergence of colony variants. Experiments were repeated at least three times. Untreated biofilms were used as controls. It is worth noting that addition of catalase and sodium thiosulfate did not affect the overall growth under planktonic conditions (not shown).
Initial attachment using a 96-well microtiter dish assay.
Initial biofilm formation was measured by using the microtiter dish assay system, as described previously (2, 33). Briefly, microtiter wells were inoculated with 20 μl of a S. pneumoniae culture grown in Todd-Hewitt broth to mid-logarithmic phase (turbidity of ∼0.5 at 600 nm). The cells were grown for 3, 6, and 12 h before they were stained with crystal violet and quantified.
Capsule quantification of S. pneumoniae colony variants.
The relative amounts of capsule for serotype 19 colony variants grown to exponential phase as determined by spectrophotometric measurements were determined using Stains-All reagent as described by Schrager et al. (32), with modifications as described by Allegrucci and Sauer (2). Absorbance values were compared to those of a standard curve generated with known concentrations of pneumococcal polysaccharide (ATCC, Manassas, VA). Protein concentrations were determined by the method of Lowry, as modified by Peterson (29).
Microbial adhesion to hydrocarbon (MATH) test.
To compare the relative hydrophobicities of S. pneumoniae BS75 and colony variants, cell adherence to hexadecane was determined in triplicate as described previously (2, 8).
Analysis of the cps19fEFGHIJK operon of serotype 19 wild-type and colony variants.
To verify the presence of capsule genes and gene aberrations within the serotype 19 capsule operon, PCR was performed as previously described using cps19fFGHIJKLMNO primers derived from the S. pneumoniae serotype 19f capsule locus (4, 9, 23). The identity of the PCR products was confirmed by DNA sequencing using a BigDye Terminator sequencing kit (Applied Biosystems) and an ABI Prism sequencer (Applied Biosystems). To determine the presence of SNPs in cps19fF, the gene was amplified from variants obtained from three independent experiments and subsequently sequenced as described above. A total of six variants per experiment were analyzed.
Transcript abundance of serotype 19 capsule biosynthetic genes determined by RT-PCR.
To determine the transcript abundance of cps19fEFGHIJK, reverse transcription-PCR (RT-PCR) was used as described previously (2, 33). The identities of the RT-PCR transcripts were confirmed by sequencing.
Reversion frequencies during planktonic growth and biofilm growth and upon antimicrobial challenge.
To determine whether the colony phenotype of each colony variant was revertible, reversion frequencies were determined for variants grown in plankton culture and as a biofilm. To grow the cultures, the wild-type and colony variants (MMV, SMV, and SCV) were subcultured six times in Todd-Hewitt medium and subsequently plated onto blood agar. For reversion during biofilm growth, colony variants (MMV, SMV, and SCV) were grown as biofilms for 3 days and the resulting populations analyzed as described above. Reversion frequencies for 3-day-old SCV biofilms were also determined following treatment with tetracycline (10 μg/ml) for 6 h. Experiments were done in triplicate; untreated biofilms were used as controls. To ensure that tetracycline was stable under the conditions tested, disk diffusion assays using Escherichia coli were performed as described previously (2).
Since SCVs were found to revert to SMV but not to wild-type (MMV) phenotypes, the frequency of reversion of SCVs to SMVs was determined as the total CFU per number of variants that reverted to SMVs. All experiments were done in triplicate.
Furthermore, S. pneumoniae wild-type (ATCC 6303) biofilms were grown in flow cells under flowing conditions as described previously (1, 2, 33) to monitor the effect of antimicrobial treatment on biofilm architecture and to visualize the zone of killing. Biofilms were grown for 6 days, after which time the biofilms were exposed to tetracycline (10 μg/ml). After 12 h, biofilms were stained using Live/Dead BacLight stain from Invitrogen (Carlsbad, CA) and viewed by confocal laser scanning microscopy.
Determination of mutation rates during planktonic and biofilm growth.
To determine mutation rates, rifampin resistance of S. pneumoniae strains (BS71, BS75, TIGR4, and TIGR4-ΔspxB) grown planktonically and as biofilms for 1 day was evaluated. Planktonic cultures were inoculated as previously described (1, 2), allowed to grow to mid-log phase for 3 h, and subsequently plated onto blood agar supplemented with 100 μg of rifampin/ml. Biofilm cells were harvested after 1 day of growth as described above, homogenized, serially diluted, and plated onto blood agar containing 100 μg of rifampin/ml. Total CFU numbers per milliliter were determined in parallel using blood agar. Mutation rates were calculated by determining the ratio of rifampin-resistant mutants to total CFU.
Evaluation of hydrogen peroxide generated colony variants during planktonic growth conditions.
To determine whether the addition of hydrogen peroxide to planktonic serotype 3 and 19 S. pneumoniae would generate colony variants, planktonic cells were treated with various concentrations of hydrogen peroxide (2.5 mM and 10 mM). Cultures were allowed to grow for 1 and 5 h in Todd-Hewitt medium, after which time they were treated with 20 mM sodium thiosulfate to neutralize hydrogen peroxide. Samples were plated onto blood agar and rifampin-containing blood agar. Colony variants were scored as described above.
RESULTS
We have previously shown that serotype 3 S. pneumoniae biofilm formation is correlated with the emergence of nonrevertible mucoid colony variants (2). To determine whether the formation of these variants was dependent on the serotype or biofilm growth conditions, and to gain further insight into the emergence of mucoid variants and the corresponding genetic mechanism, biofilm development of a S. pneumoniae serotype 19 clinical isolate (BS75) was investigated in greater detail. This serotype was selected for the following reasons. (i) S. pneumoniae serotype 19 wild-type cells are significantly less mucoid when grown on blood agar than serotype 3 cells and produce only medium-sized mucoid colonies approximately 1.5 to 2.5 mm in diameter (Table 1). (ii) Serotype 19 and serotype 3 S. pneumoniae strains differ significantly in initial attachment (2) and biofilm architecture (1). The S. pneumoniae serotype 19 biofilm architecture was formerly identified as characteristic of members of group III, whereas serotype 3 was placed in biofilm architectural group I (1). (iii) Serotype 19 has been implicated in pediatric diseases such as otitis media, whereas serotype 3 is the most frequent cause of invasive disease in adults worldwide (11, 14, 16).
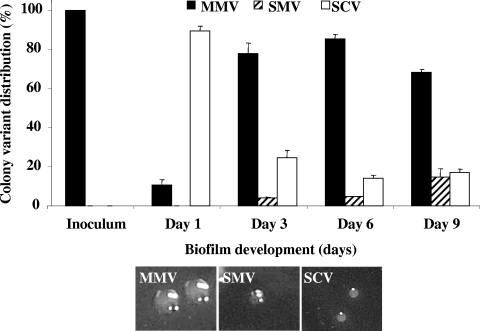
Colony variant formation by S. pneumoniae serotype 19 was assessed following 1, 3, 6, and 9 days of biofilm growth. Overall, serotype 19 wild-type strains gave rise to three different colony variants during biofilm growth, including a wild-type-like colony morphology with the appearance of an MMV, an SMV, and a nonmucoid SCV (Table 1; Fig. 1). SCV dominated during initial attachment (day 1) but decreased with respect to population distribution (based on total CFU) during mature biofilm stages (days 3 to 9). In contrast, SMVs were first detectable in 3-day-old biofilms and increased threefold in population size by day 9 (Fig. 1). Overall, the frequency and timing of serotype 19 variant formation during biofilm formation were similar to those previously described for S. pneumoniae serotype 3 biofilms (2).
FIG. 1.
The emergence of S. pneumoniae serotype 19 variants during biofilm development. Distribution of variants was determined from total CFU, colony size, and mucoidy of colonies on blood agar.
It is worth noting that under planktonic growth conditions, the serotype 19 wild type was found to give rise to only medium-sized mucoid colonies (MMV; Table 1), indicating that none of the variants (SMV or SCV) were present in the parental wild-type population or in the preparation used as an inoculum for the biofilms. The finding also suggests that environmental conditions specific to biofilms are responsible for development of SMVs and SCVs.
S. pneumoniae serotype 19-produced biofilm-derived colony morphology variants that exhibit biofilm-related phenotypes.
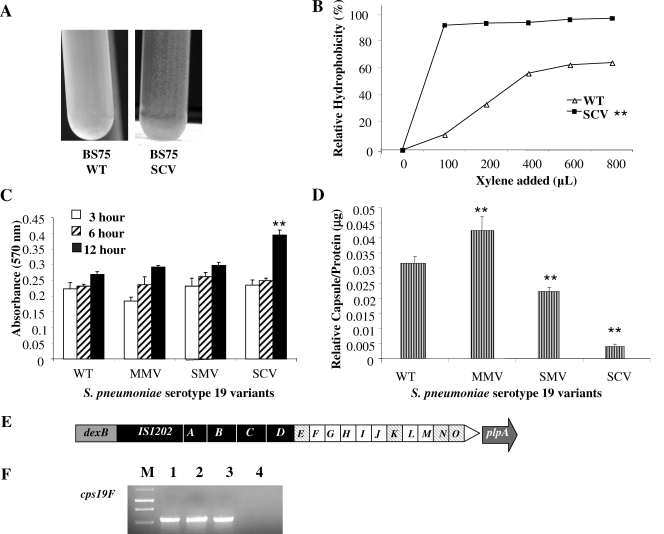
SCVs isolated from S. pneumoniae serotype 19 (BS75) biofilms were not only similar in size and colony morphology to those of the previously isolated serotype 3 SCVs (2) but also displayed similar biofilm-related phenotypes, including autoaggregation in liquid culture (Fig. 2A), increased hydrophobicity (Fig. 2B), hyperadherence to abiotic surfaces (Fig. 2C), and microcolony formation. In microtiter plate adhesion assays, BS75 SCVs showed a threefold increase in crystal violet-stainable biofilm biomass 12 h after initial attachment compared to wild type, MMV, and SMV (P < 0.05) (Fig. 2C). The increase in initial attachment correlated with decreased capsule production, with SCVs producing the least amount of capsule (Fig. 2D). The aggregative and adhesive nature of the serotype 19 SCVs suggested increased hydrophobicity relative to wild-type S. pneumoniae. When a standard hydrophobicity assay (MATH test) was used, the cell surfaces of the autoaggregative SCVs isolated from three independent experiments were shown to be more hydrophobic, with a relative hydrophobicity of 90%, than those of the corresponding wild-type strain, which exhibited a relative hydrophobicity of 60% (P < 0.05) (Fig. 2B). The relative hydrophobicity of mucoid variants was similar to that of the wild type (not shown). The hydrophobic and aggregative phenotype of SCVs coincided with enhanced formation of microcolonies ranging from 50 to 150 μm in diameter, as determined by confocal microscopy (see Fig. S1 in the supplemental material). In contrast, no microcolony formation was observed for biofilm-derived MMVs (see Fig. S1 in the supplemental material). Compared to wild-type and SCV biofilms, MMVs formed flat, unstructured biofilms.
FIG. 2.
Phenotypic and genotypic characteristics of S. pneumoniae serotype 19 wild-type and biofilm-derived variants. (A) Hyperautoaggregation of serotype 19 SCVs in liquid culture. The autoaggregative phenotype was not detected in serotype 19 wild-type (WT) liquid culture. Cultures were inoculated from a blood agar plate and were grown to mid-log phase. (B) Relative hydrophobicity (MATH) results for WT and SCV. Each datum point is based on the results from three replicate measurements. SCVs were significantly (**) more hydrophobic than WT (P < 0.05). (C) Microtiter plate adhesion assay of S. pneumoniae serotype 19 WT and colony variants after 3, 6, and 12 h of initial attachment. The SCV showed significantly (**) greater biofilm adhesion after 12 h than did the WT, MMV, or SMV (P < 0.05). (D) Relative capsule amounts of S. pneumoniae WT and colony variants as determined by using Stains-All reagent. Significantly (**) smaller capsule amounts were detected for SCVs than for wild-type and mucoid variants (P < 0.05). (E) Serotype 19-specific capsule operon and transcript abundance. Open squares indicate transcribed genes; striped squares indicate genes not detected by RT-PCR; filled squares, unknown, as previously described (4). (F) cps19F transcripts (267 bp) determined by RT-PCR were obtained from serotype 19 wild-type and colony variants. Lanes: 1, S. pneumoniae serotype 19 wild type [BS75]; 2, MMV; 3, SMV; 4, SCV; M, 1-kb DNA ladder.
Biofilm-derived variants and identification of SNPs.
To determine the genetic basis of S. pneumoniae serotype 19 biofilm-derived variants, we first used PCR to examine the capsule biosynthetic operon cps19E-cps19O of both the wild type and the variants for chromosome aberrations, including amplifications and deletions. No evidence of sequence duplications or deletions that might result in an on-off switch in capsule production as previously reported by Waite et al. and others (5, 34, 35) was detectable within the cps19E-cps19O operon (data not shown).
We next looked for differences in levels of cps19E-cps19O transcript abundance among S. pneumoniae wild-type and variant strains by use of semiquantitative RT-PCR. Under the conditions tested, RT-PCR did not yield detectable transcripts for cps19E, cps19K, cps19N, and cps19O, either in the wild type or in any of the colony variants (Fig. 2E). Of the remaining cps19 genes, cps19GHIJ and cps19LM were found to be present at similar levels in both the wild type and the variants (Fig. 2E and data not shown). The only difference was detected for cps19F, which encodes a UDP-N-acetylmannosamine transferase. The transcript was detected in the wild type and in both mucoid variants (MMV and SMV) but was absent in SCVs (Fig. 2F). We therefore examined the DNA sequence of cps19F as well as the upstream region in greater detail for the presence of SNPs. Sequence analysis revealed a SNP 323 bp downstream of the cps19F ATG start codon in both the SMV and the SCV (Table 2). All SMVs and SCVs obtained in three independent experiments (for a total of 18 variants each) showed the same mutation. The transition (T-C) resulted in an amino acid substitution (from a nonpolar phenylalanine to a polar serine) located within the conserved domain of UDP-N-acetylmannosamine transferase. The substitution may affect folding of the conserved region by altering beta, turn, coil, and hydrophilicity (Protean software; DNA Star Lasergene) (data not shown) as well as the enzymatic activity of Cps19F. In addition, a transition from an adenine to a guanine as well as a deletion was detected in the cps19F ribosome binding site of SCV (Table 2). The mutation was found in all tested SCVs obtained from three independent experiments.
TABLE 2.
SNPs associated with variants isolated from S. pneumoniae serotype 19 biofilms
| Strain(s) | Mutation | Mutation location | SNP(s) | Protein modification |
|---|---|---|---|---|
| SCV/SMV | Transition | 323 bpa (cps19F start codon) | T → C | F → S |
| SCV | Transition/deletion | Ribosomal binding site (cps19F) | GAAG → GGA- | None |
| Reverted SMV | Transition/deletion | Ribosomal binding site (cps19F) | GAAG → GGA- | None |
Location of mutation (in base pairs) is based on the location of the transcriptional start codon.
SCV-to-SMV reversion rate is increased upon biofilm growth and treatment with antibiotics.
Under planktonic growth conditions, none of the biofilm-derived variants (SCV or SMV) reverted to the wild-type colony phenotype. However, a gain in capsule production was observed for the acapsular variant (SCV), which gave rise to small capsular/mucoid colonies similar in appearance to those of SMVs. Interestingly, no reversion to the wild-type phenotype was observed. Analysis of the cps19F gene sequence indicated that reversion of SCVs to SMVs coincided with a transition event at position 323 bp, resulting in restoration of the wild-type cps19F sequence. No additional SNPs in the cps19F sequence or reversion of the transition/deletion detected in the cps19F ribosome binding site of SCV was observed (Table 2). The reversion frequency of SCVs was dependent on the set of growth conditions. The reversion (SCV to SMV) rate under planktonic conditions was ∼0.007, with only one out of six experiments yielding revertants. Reversion rates increased under biofilm growth conditions, with reversion rates of ∼0.02 (four out of six biofilm growth experiments yielded revertants).
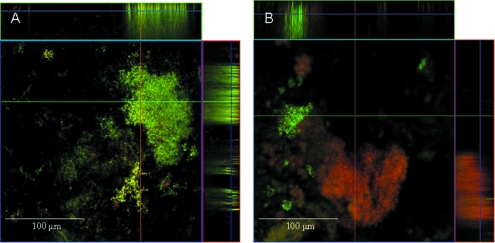
Bactericidal antibiotics such as tetracycline have been demonstrated to stimulate the production of deleterious hydroxyl radicals, which ultimately contribute to cell death via oxidative damage, as well as trigger the DNA damage response involving RecA (20), which may result in increased SCV-SMV reversion rates. Since spontaneous pneumococcal death during planktonic growth has been shown to be due to the production of endogenous hydrogen peroxide (H2O2) (30), we hypothesized that biofilm cells may be exposed to elevated (but nonlethal) levels of this oxidative stressor compared to planktonic cells. In support of this possibility, treatment of SCV biofilms with tetracycline (10 μg/ml) for 6 h resulted in a significant increase in the reversion rate to ∼0.5 (± 0.004 [P < 0.05]; six out of six biofilm growth experiments yielded revertants). Similar results were obtained upon treatment with tobramycin (data not shown). In contrast, no revertants were detected for serotype 19 SMVs under biofilm growth conditions (data not shown). Using confocal microscopy, we also visualized the effect of tetracycline treatment on SCV biofilms (Fig. 3). As indicated by live/dead staining, the areas of 6-day-old S. pneumoniae SCV biofilms most affected following 12 h of exposure to tetracycline were found to be within large microcolonies (Fig. 3B). In some cases, the perimeter of the microcolonies appeared to be unaffected by tetracycline whereas the interior portion of the microcolonies was stained in red, an indicator for the presence of dead cells (data not shown).
FIG. 3.
Tetracycline treatment and S. pneumoniae SCV biofilm viability. SCV biofilms after 6 days of growth (A) and after treatment with tetracycline (10 μg/ml) for 12 h (B). Flow cell experiments and image acquisition were performed as previously described (2).
Reduction of hydrogen peroxide coincides with reduction of biofilm-derived variant formation.
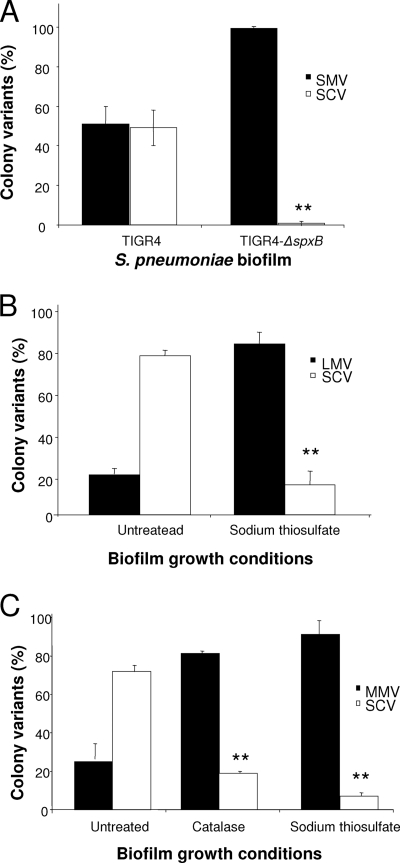
S. pneumoniae generates concentrations of hydrogen peroxide that may exceed 2 mM when cells are grown aerobically in liquid culture without the addition of exogenous catalase (26). The gene responsible for H2O2 production was identified as the suicide gene spxB, encoding pyruvate oxidase (28, 30). The possibility that endogenously generated H2O2 contributes to variant formation was examined by assessing the emergence of colony variants during biofilm growth of S. pneumoniae TIGR4 and its ΔspxB mutant, which is incapable of the endogenous production of H2O2 (30). Following 1 day of biofilm growth under continuous flow conditions, approximately 50% of the TIGR4 wild-type biofilm population was composed of acapsular SCVs (Fig. 4A). In contrast, the majority of the TIGR4-ΔspxB mutant biofilm population was composed of colonies having a small-colony phenotype (wild-type colony morphology) when grown on blood agar, with less than 0.5% of the biofilm population being composed of SCVs (Fig. 4A).
FIG. 4.
Role of the suicide gene spxB, catalase, and sodium thiosulfate in the emergence of variants in S. pneumoniae biofilms. (A) Distribution of colony variants in 1-day-old TIGR4 and TIGR4 spxB mutant biofilms. (B and C) Distribution of colony variants in biofilms of S. pneumoniae serotype 3 (BS71) (B) and serotype 19 (BS75) (C) grown for 1 day in the presence of sodium thiosulfate (10 mM) or catalase (4 U/ml; added every 4 h to the growth medium). Distribution of colony variants was determined from total CFU, colony size, and mucoidy of colonies on blood agar. Untreated biofilms were used as controls. Experiments were carried out in triplicate. Results with respect to the emergence of SCVs were significantly (**) different from those seen with untreated S. pneumoniae wild-type biofilms (P < 0.01).
To further confirm a role of hydrogen peroxide in the formation of biofilm-derived variants, we also pursued a chemical approach by supplementing the growth medium of S. pneumoniae serotype 3 and 19 biofilms with catalase or sodium thiosulfate to neutralize any H2O2 that may have been present in biofilms. In the presence of an exogenous source of catalase or sodium thiosulfate, there was a >10-fold reduction in SCV formation, representing as little as 7% of the biofilm population compared to 80% of the untreated S. pneumoniae serotype 19 biofilm population after 1 day of growth (Fig. 4C). In serotype 3 S. pneumoniae biofilms, supplementing the growth medium with sodium sulfate resulted in a more than fivefold reduction in the SCV population (Fig. 4B).
Effect of biofilm growth conditions on spontaneous mutation frequency.
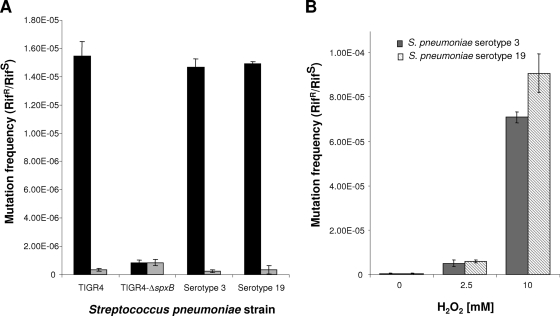
We next addressed the issue of whether the formation of biofilm-derived variants may be based on an increased mutation rate due to the presence of hydrogen peroxide or to the increased selection pressure inherent to biofilms. To distinguish between mutation and selection, the frequency of mutation to rifampin resistance was determined for S. pneumoniae grown planktonically and as 1-day-old biofilms. Rifampin resistance is caused by nucleotide substitutions in a well-defined central region of the gene that encodes the β-subunit of RNA polymerase (rpoB) (15). Since S. pneumoniae was not under rifampin selection pressure under the conditions tested, the emergence of rifampin resistance is therefore a good indicator of the general rate of mutation in the cell. The rifampin-sensitivity mutation frequency of S. pneumoniae strains grown planktonically ranged between 2.65 × 10−7 and 8.4 × 10−7 (Fig. 5A). Biofilm growth coincided with an up to 55-fold increase in mutation frequency for S. pneumoniae serotype 3 and serotype 19 and TIGR4. On average, the mutation frequency increased to 1.5 × 10−7 (Fig. 5A). However, unlike the results seen with S. pneumoniae strains capable of hydrogen peroxide production, no reduction of rifampin sensitivity occurred upon biofilm growth of TIGR4-ΔspxB mutants. The mutation frequency upon growth as planktonic or biofilm bacteria remained at ∼8.2 × 10−7 (Fig. 5A). Inactivation of spxB in TIGR4 has been previously demonstrated to eliminate detectable H2O2 in culture supernatants (30). The low mutation frequency noted in the absence of SpxB and thus, of endogenous hydrogen peroxide, could have been due to other types of oxidative stress, the minimal amounts of H2O2 still produced in the absence of pyruvate oxidase, or random background rates of substitutions.
FIG. 5.
Effect of growth conditions and exogenous hydrogen peroxide on the spontaneous mutation rate. The results represent the frequency of the spontaneous rate of mutation to rifampin resistance or sensitivity expressed as a mutation rate (rifampin resistant/rifampin sensitive [Rifr/Rifs]) for S. pneumoniae serotype 3, serotype 19, TIGR4, and TIGR4-ΔspxB on the basis of growth conditions (1-day-old biofilm, black bars; planktonic growth conditions, gray bars) (A) and the presence of 0 to 10 mM hydrogen peroxide (S. pneumoniae serotype 3, gray bars; S. pneumoniae serotype 19, striped bars) (B). For planktonic growth conditions, the frequency of the spontaneous rate of mutation to rifampin resistance or sensitivity in the absence or presence of hydrogen peroxide was calculated following growth to the mid-log phase and treatment for 60 min with H2O2 added at the concentration indicated. The frequency of spontaneous mutation of biofilms was calculated following 1 day of growth under biofilm growth conditions. Experiments were performed in triplicate for each set of conditions.
Effect of hydrogen peroxide on spontaneous mutation.
To confirm the effect of hydrogen peroxide on the mutation rate, exogenous H2O2 (0 to 10 mM) was added to planktonic S. pneumoniae serotypes 3 and 19 and the spontaneous mutation rate determined. Treatment with hydrogen peroxide resulted in a dose-related increase in the rate of spontaneous rifampin resistance, reaching 7.08 ×10−5 and 9.02 ×10−5 for S. pneumoniae serotypes 3 and 19, respectively, in the presence of 10 mM added H2O2 (Fig. 5B). Higher concentrations of exogenously added H2O2 exceeding 10 mM or prolonged treatment times (>5 h for 2.5 mM or >1 h for 10 mM H2O2) could not be tested because of cell death. Overall, exposure to 2.5 mM exogenous H2O2 resulted in a >12-fold increase in mutation rates whereas treatment with 10 mM H2O2 lowered the susceptibility to rifampin by more than 160-fold within 1 h of exposure (Fig. 5B).
Effect of hydrogen peroxide on variant formation under planktonic growth conditions.
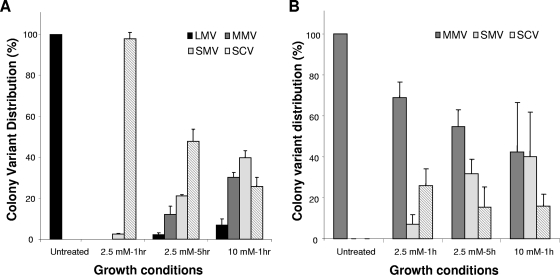
To further elucidate the role of hydrogen peroxide in colony variant formation, variant formation by 3 and serotype 19 S. pneumoniae serotype grown planktonically in the presence of hydrogen peroxide was assessed. We hypothesized that if the generation of hydrogen peroxide indeed plays a role in colony variant formation, such treatment would also result in the emergence of colony variants under planktonic growth conditions. Exogenous addition of H2O2 (0, 2.5, and 10 mM) resulted in a dose-related increase in the emergence and diversity of variants (Fig. 6). After 1 h of treatment with 2.5 mM H2O2, the majority (>90%) of serotype 3 S. pneumoniae planktonic cells were found to be acapsular (SCV), with a small percentage having an SMV phenotype (Fig. 6A). Continued exposure to 2.5 mM H2O2 for 5 h was found to result in the emergence of MMVs (10 to 15%) and wild-type-like large mucoid variant (LMVs) (<5%) (Fig. 6A). Addition of 10 mM H2O2 for 1 h resulted in the emergence of all four colony variants (SCV, SMV, MMV, and LMV). Under these conditions, approximately 40% of the planktonic population was composed of SMVs, with the remaining population made up of MMVs (30%), wild-type-like LMVs (5 to 10%), and SCVs (20 to 25%) (Fig. 6A). No colony variants were detected in untreated S. pneumoniae serotype 3 cells following 1 and 5 h of growth (Fig. 6A). Similar results were obtained when S. pneumoniae serotype 19 was used (Fig. 6B). Exposure of S. pneumoniae serotype 19 grown planktonically for 1 h in the presence of 2.5 mM H2O2 resulted in the emergence of both SMV and SCVs, making up >30% of the population. Continued exposure to 2.5 mM H2O2 or addition of 10 mM H2O2 for 1 h resulted in the majority of the population being composed of variants (40% SMV and 16% SCV).
FIG. 6.
The emergence of S. pneumoniae serotype 3 (A) and serotype 19 (B) variants upon treatment with hydrogen peroxide under planktonic conditions. Distribution of colony variants was determined from total CFU, colony size, and mucoidy of colonies on blood agar.
The SCVs obtained from planktonic S. pneumoniae upon treatment with hydrogen peroxide were phenotypically similar to the variants isolated during biofilm growth and displayed biofilm-related phenotypes similar to those of previously biofilm-derived SCVs, including autoaggregation in liquid culture and hyperadherence (see Fig. 2). Furthermore, the emergence of colony variants upon treatment with exogenous hydrogen peroxide treatment was similar to the emergence of colony variants during biofilm formation by S. pneumoniae serotype 3 (2) and serotype 19 (Fig. 1).
DISCUSSION
In S. pneumoniae, differences in colony phenotypes based on opacity have long been recognized and have been shown to emerge under numerous growth conditions, including planktonic and various biofilm growth conditions. Formation of phase-variable acapsular colony variants by S. pneumoniae was shown by Waite et al. to be based on tandem sequence duplications in cps3D and cap8E (34, 35). High-frequency phase variation of serotypes 9V, 18C, and 23F was found to be dependent on the presence of a BOX A-C element that was also essential for transformation activity (31). In contrast, non-phase-variable colony variants have only recently been described as emerging during biofilm growth by serotype 3 S. pneumoniae (2). The acapsular nonrevertible colony variants were found to harbor a deletion comprising the cps3DSU operon. Other mucoid variants detected during biofilm growth were found to harbor SNPs located in the cps3 promoter region (not shown). Here, we demonstrated that not only S. pneumoniae serotype 3 but also serotype 19, when grown as biofilms, generated both mucoid and acapsular non-phase-variable colony variants at high frequency. S. pneumoniae serotype 19 gave rise to three variants, depending on the age and maturity of the biofilm.
In contrast to opaque- and transparent-phase variants, the variants described here emerged only upon surface-associated growth, were nonrevertible, and were not based on tandem duplication or deletions of DNA located within the cps genes (Table 2). Reversion was observed only between SCV and SMVs. No reversion to the wild-type or medium mucoid colony phenotype was observed. Sequencing suggested the presence of only partial restoration of the wild-type cps19F sequence. SCV-to-SMV reversion coincided with restoration of the wild-type cps19F sequence at position 323 bp. However, revertants still harbored the transition/deletion in the RBS sequence of cps19F (Table 2). This finding suggests that additional mutations (that have not been detected in this study) may play a significant role in variant formation. Since the entire genomes of all the variants were not sequenced, it is therefore possible that the specific mutations detected in this study are only in part the genetic causes of the various phenotypes described here. Furthermore, biofilm growth conditions gave rise to more than two variant phenotypes, including acapsular SCVs, SMVs, and wild-type-like MMVs. The acapsular variants (SCVs) were detectable soon after initial attachment. Following 1 day of biofilm growth, ∼80% of the biofilm population consisted of SCVs whereas later stages were dominated by wild-type-like MMVs. Although the MMV appeared similar on solid medium to the wild type, MMVs isolated from biofilms showed phenotypic characteristics that were distinct from those of the wild type, including reduced initial attachment, increased capsule production, and a tendency to spread over surfaces and, thus, to form flat unstructured biofilms (see Fig. S1 in the supplemental material). Later biofilm developmental stages were also characterized by the emergence of SMVs, which remained at low levels even after extended biofilm growth (Fig. 1). In contrast to the diversity of variant phenotypes isolated from biofilms, phase variants have been shown previously to display only two phenotypes, opaque and transparent. Our findings clearly indicate that biofilm-derived, nonrevertible variants are unlike phase variants.
The biofilm-derived variants harbored SNPs in the cps19F gene as well as a SNP and a deletion located upstream of cps19F. Similar mutations were observed in S. pneumoniae acapsular variants isolated following surface-associated growth. McEllistrem et al. (22) noted that serotype 3 S. pneumoniae colony biofilms gave rise to revertible and nonrevertible acapsular variants after 4 to 7 days of growth. The latter harbored SNPs in the cps3D gene, an SNP in the −10 promoter, and large deletions in the cps3D gene (22). Hammerschmidt et al. (12) demonstrated phenotypic variation of the polysaccharide capsule in the initial phase of pneumococcal infections of lung epithelial cells. Variants with limited or no capsule expression exhibited an up to 105-fold-enhanced capacity to adhere and an up to 104-fold-enhanced capacity to invade epithelial cells. Similarly, biofilm-derived acapsular variants displayed an auto- and hyperaggregative phenotype with respect to abiotic surfaces (Fig. 2) (2). Furthermore, while approximately 50% of all tested variants were phase variable, the remaining variants were not (12). Nonrevertible variants were shown to harbor an SNP located within cps3D, resulting in a premature stop codon. Note that variant formation may result in reduced virulence, since biofilm-derived variants are unable to revert to the wild-type antiphagocytic capsule phenotype.
Overall, these findings suggest that the emergence of non-phase-variable colony variants is dependent on the presence of environmental conditions intrinsic to biofilms. Here, we demonstrate that hydrogen peroxide plays an important role in the formation of variants by S. pneumoniae biofilms. Variant formation was significantly reduced by eliminating/reducing hydrogen peroxide by supplementing the biofilm growth medium with catalase or sodium thiosulfate. Furthermore, when grown as a biofilm, the TIGR4-ΔspxB mutant, lacking hydrogen peroxide production, neither gave rise to acapsular variants at a high frequency nor resulted in increased mutation rates compared to the same organisms under planktonic growth. Thus, in addition to conferring advantages to S. pneumoniae by inhibiting growth of competitors in a particular niche and by increasing colonization in vivo (27, 28, 30), endogenous hydrogen peroxide production allows diversification among members of the biofilm community. SpxB and its product, hydrogen peroxide, therefore play a pivotal role in the emergence of biofilm-derived variants. In turn, addition of exogenous H2O2 at low concentrations to planktonic cells produced variants that were phenotypically similar to biofilm-derived variants.
Diversification of both S. pneumoniae serotypes into SCVs, SMVs, MMVs, and LMVs was found to be dependent on the H2O2 concentration (Fig. 6). Addition of 2.5 mM H2O2 to planktonic cells for 1 h gave rise to mostly acapsular SCVs, while continued exposure to 2.5 mM hydrogen peroxide (5 h) resulted in further diversification, as indicated by the emergence of mucoid variants (MMV and SMV) (Fig. 6). Similar results were observed at higher concentrations (10 mM). The percentage of SCVs present upon addition of 2.5 mM for 1 h was comparable to that observed for 1-day-old S. pneumoniae biofilms (Fig. 1) (2). Moreover, the rifampin-sensitivity mutation frequency observed for planktonic cells exposed to 2.5 mM hydrogen peroxide for 1 h was similar to that seen with 1-day-old biofilms (12- versus 55-fold increases, respectively) (Fig. 5). In addition, the phenotypic diversity observed in aging biofilms (3 to 9 days old) was comparable to that seen with the variant population present in the planktonic population at high concentrations of H2O2 or upon prolonged treatment with 2.5 mM H2O2 (Fig. 1 and 5) (2). The latter set of conditions resulted in a >160-fold increase in the rifampin-sensitivity mutation frequency compared to untreated planktonic cells (Fig. 5). Taken together, our results support a role of oxidative stress and hydrogen peroxide in the emergence of diversity and colony variance. Under biofilm growth conditions, hydrogen peroxide was implicated, being present at concentrations (potentially along with other oxidative agents) that exceed those found in planktonic cells grown to exponential phase. Such elevated hydrogen peroxide concentrations coincided with increased mutation rates in S. pneumoniae strains when grown as biofilms.
A similar link between hydrogen peroxide and increased mutation frequency was recently found by Pericone et al. (26). The authors characterized variants differing from the parental strains in opacity, optochin resistance, and autolysis upon in vitro and in vivo growth. The genotypic analysis of seven genes (pspA, spxB, xba, licD2, lytA, nanA, and atpC) revealed various mutations, including frameshift mutations, deletions, and substitutions of guanine residues. Since guanine bases are particularly susceptible to oxidative damage, the mutations were believed to be induced by the presence of endogenous hydrogen peroxide, which accounted for a frequency of spontaneous mutation as high as 1.9 × 10−5 (26). Since the study did not focus on capsule biosynthesis genes, it is unclear whether hydrogen peroxide affected capsule production or gave rise to nonrevertible variants such as acapsular SCVs at high frequency.
Hydrogen peroxide has been demonstrated to induce colony variance in studies of other species. In Pseudomonas aeruginosa, treatment with hydrogen peroxide resulted in mucoid conversion (21). The mucoid variants had mutations in mucA, which led to the deregulation of an alternative sigma factor (AlgT/AlgU) required for expression of the alginate biosynthetic operon. Interestingly, all of the mucoid variants tested showed a mutation in the mucA22 allele. Mucoid conversion was also observed in genetically engineered mucA22 mutants, suggesting that the only mutation incurred as a result of H2O2 treatment was in mucA (21). Similarly, in our study, all tested biofilm-derived variants were shown to harbor the same mutation located in or upstream of cps19F (Table 2). Based on the rifampin-sensitivity mutation rate study, treatment with hydrogen peroxide resulted in additional mutations. However, no additional mutations have been identified by us.
Biofilms confer many advantages to bacteria, including, among others, antimicrobial resistance and physical protection by the matrix (6, 7, 21). Furthermore, it has been suggested that the biofilm-related phenotypes associated with colony variants may provide a competitive advantage in a particular niche of a structured biofilm system (3, 18). Our findings provide evidence for the first time that the emergence of non-phase-variable, biofilm-derived colony variants at high frequency is not due to selection for variants better equipped for adherence and biofilm growth but instead is the result of increased mutation rates induced by oxidative stress conditions. SpxB and its product, hydrogen peroxide, play an important role in the formation of non-phase-variable S. pneumoniae variants. However, while the colony variants were not selected, they did provide a selective advantage under biofilm growth conditions, including enhanced attachment and biofilm formation. Hammerschmidt et al. (12) demonstrated that pneumococci in intimate contact with epithelial cells and in the process of entering the cells were devoid of a polysaccharide capsule. The finding suggests that loss of capsule and the formation of colony variants may occur only when in intimate contact with surfaces. Since invasive pneumococcal diseases coincide with tissue colonization and since biofilm but not planktonic growth conditions gave rise to colony variants, it is likely that nonrevertible colony variants may arise in vivo upon colonization. Moreover, Nelson et al. (24) demonstrated that unencapsulated S. pneumoniae mutants remained agglutinated within lumenal mucus compared to encapsulated strains. Thus, surface-induced formation of colony variance may be a mechanism used by S. pneumoniae to first transit unhindered through the lumenal mucus to the epithelial surface before stable colonization occurs. Once the cell surface has been reached, in vivo colony variance may be triggered by both surface-associated growth conditions and the presence of exogenous hydrogen peroxide released by polymorphonuclear leukocytes and other cells involved in the inflammatory response. An additional advantage of surface-induced variance may be an increased capacity to invade epithelial cells. Taken together, the findings concerning surface-induced colony variants may provide a link between adhesion and invasion of epithelial cells, biofilm formation, and the establishment of chronic pneumococcal infections.
Supplementary Material
Footnotes
Published ahead of print on 25 July 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Allegrucci, M., F. Z. Hu, K. Shen, J. Hayes, G. D. Ehrlich, J. C. Post, and K. Sauer. 2006. Phenotypic Characterization of Streptococcus pneumoniae Biofilm Development. J. Bacteriol. 1882325-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allegrucci, M., and K. Sauer. 2007. Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J. Bacteriol. 1892030-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boles, B. R., M. Thoendel, and P. K. Singh. 2004. Self-generated diversity produces “insurance effects” in biofilm communities. Proc. Natl. Acad. Sci. USA 10116630-16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caimano, M., G. G. Hardy, and J. Yother. 1998. Capsule genetics in Streptococcus pneumoniae and a possible role for transposition in the generation of the type 3 locus. Microb. Drug Resist. 411-23. [DOI] [PubMed] [Google Scholar]
- 5.Coffey, T. J., M. C. Enright, M. Daniels, J. K. Morona, R. Morona, W. Hryniewicz, J. C. Paton, and B. G. Spratt. 1998. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol. Microbiol. 2773-83. [DOI] [PubMed] [Google Scholar]
- 6.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49711-745. [DOI] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infection. Science 2841318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Déziel, E., Y. Comeau, and R. Villemur. 2001. Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J. Bacteriol. 1831195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillard, J. P., M. Caimano, T. Kelly, and J. Yother. 1995. Capsules and cassettes: genetic organization of the capsule locus of Streptococcus pneumoniae. Dev. Biol. Stand. 85261-265. [PubMed] [Google Scholar]
- 10.Faden, H., L. Duffy, and M. Boeve. 1998. Otitis media: back to basics. Pediatr. Infect. Dis. J. 171105-1112. [DOI] [PubMed] [Google Scholar]
- 11.Garcia, E., D. Llull, and R. Lopez. 1999. Functional organization of the gene cluster involved in the synthesis of the pneumococcal capsule. Int. Microbiol. 2169-176. [PubMed] [Google Scholar]
- 12.Hammerschmidt, S., S. Wolff, A. Hocke, S. Rosseau, E. Muller, and M. Rohde. 2005. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 734653-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson, I. R., P. Owen, and J. P. Nataro. 1999. Molecular switches—the ON and OFF of bacterial phase variation. Mol. Microbiol. 33919-932. [DOI] [PubMed] [Google Scholar]
- 14.Infante-Rivard, C., and A. Fernandez. 1993. Otitis media in children: frequency, risk factors, and research avenues. Epidemiol. Rev. 15444-465. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs, M. A., A. Alwood, I. Thaipisuttikul, D. Spencer, E. Haugen, S. Ernst, O. Will, R. Kaul, C. Raymond, R. Levy, L. Chun-Rong, D. Guenthner, D. Bovee, M. V. Olson, and C. Manoil. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 10014339-14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadioglu, A., S. Taylor, F. Iannelli, G. Pozzi, T. J. Mitchell, and P. W. Andrew. 2002. Upper and lower respiratory tract infection by Streptococcus pneumoniae is affected by pneumolysin deficiency and differences in capsule type. Infect. Immun. 702886-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, J. O., and J. N. Weiser. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177368-377. [DOI] [PubMed] [Google Scholar]
- 18.Kirisits, M. J., L. Prost, M. Starkey, and M. R. Parsek. 2005. Characterization of colony morphology variants isolated from Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 714809-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein, D. L. 1999. Pneumococcal disease and the role of conjugate vaccines. Microb. Drug Resist. 5147-157. [DOI] [PubMed] [Google Scholar]
- 20.Kohanski, M. A., D. Dwyer, J., B. Hayete, C. A. Lawrence, and J. J. Collins. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130797-810. [DOI] [PubMed] [Google Scholar]
- 21.Mathee, K., O. Ciofu, C. Sternberg, P. W. Lindum, J. I. A. Campbell, P. Jensen, A. Johnsen, M. M. Givskov, D. E. Ohman, S. Molin, N. Hoiby, and A. Kharazmi. 1999. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 1451349-1357. [DOI] [PubMed] [Google Scholar]
- 22.McEllistrem, M. C., J. V. Ransford, and S. A. Khan. 2007. Characterization of in vitro biofilm-associated pneumococcal phase variants of a clinically relevant serotype 3 clone. J. Clin. Microbiol. 4597-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morona, J. K., A. Guidolin, R. Morona, D. Hansman, and J. C. Paton. 1994. Isolation, characterization, and nucleotide sequence of IS1202, an insertion sequence of Streptococcus pneumoniae. J. Bacteriol. 1764437-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson, A. L., A. M. Roche, J. M. Gould, K. Chim, A. J. Ratner, and J. N. Weiser. 2007. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect. Immun. 7583-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul, R., S. Weiser, N. C. Amiot, C. Chan, T. Schirmer, B. Giese, and U. Jenal. 2004. Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 18715-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pericone, C. D., D. Bae, M. Shchepetov, T. McCool, and J. N. Weiser. 2002. Short-sequence tandem and nontandem DNA repeats and endogenous hydrogen peroxide production contribute to genetic instability of Streptococcus pneumoniae. J. Bacteriol. 1844392-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pericone, C. D., K. Overweg, P. W. Hermans, and J. N. Weiser. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 683990-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pericone, C. D., S. Park, J. A. Imlay, and J. N. Weiser. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J. Bacteriol. 1856815-6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson, G. L. 1977. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 83346-356. [DOI] [PubMed] [Google Scholar]
- 30.Regev-Yochay, G., K. Trzcinski, C. M. Thompson, M. Lipsitch, and R. Malley. 2007. SpxB is a suicide gene of Streptococcus pneumoniae and confers a selective advantage in an in vivo competitive colonization model. J. Bacteriol. 1896532-6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saluja, S. K., and J. N. Weiser. 1995. The genetic basis of colony opacity in Streptococcus pneumoniae: evidence for the effect of box elements on the frequency of phenotypic variation. Mol. Microbiol. 16215-227. [DOI] [PubMed] [Google Scholar]
- 32.Schrager, H. M., J. G. Rheinwald, and M. R. Wessels. 1996. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J. Clin. Investig. 981954-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Southey-Pillig, C. J., D. G. Davies, and K. Sauer. 2005. Characterization of temporal protein production in Pseudomonas aeruginosa biofilms. J. Bacteriol. 1878114-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waite, R. D., D. W. Penfold, J. K. Struthers, and C. G. Dowson. 2003. Spontaneous sequence duplications within capsule genes cap8E and tts control phase variation in Streptococcus pneumoniae serotypes 8 and 37. Microbiology 149497-504. [DOI] [PubMed] [Google Scholar]
- 35.Waite, R. D., J. K. Struthers, and C. G. Dowson. 2001. Spontaneous sequence duplication within an open reading frame of the pneumococcal type 3 capsule locus causes high-frequency phase variation. Mol. Microbiol. 421223-1232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.