Abstract
Study objective
To assess whether HIV RNA levels (log10 scale) in highly active antiretroviral therapy (HAART) treated population have a bimodal distribution, suggesting optimal or suboptimal response to HAART.
Methods
The study population from two ongoing cohort studies comprised 564 men (4785 person visits) and 1173 women (8675 person visits) with known dates of HAART initiation and with HIV RNA measurements before and after initiation. Values below detection limit of assays were treated in the analysis as left censored. Maximum likelihood methods were used to estimate parameters and to determine possible bimodality of HIV RNA distributions.
Results
A two component mixture model fitted HIV RNA levels significantly better than did a single component distribution at different years from HAART initiation in both therapy experienced and therapy naive patients. In the fifth year after HAART initiation, 32% of men and 44% of women had HIV RNA in the higher component with medians of 5247 and 9253 copies/ml, respectively, suggesting suboptimal virological response to HAART, which was associated with poor adherence and lower frequency of CCR5 heterozygous genotype.
Conclusion
The bimodal distribution of HIV RNA persisted during the years after HAART initiation. The high occurrence of suboptimal virological response at the fifth year after HAART initiation underscore the needs for careful monitoring and patient education about the importance of treatment adherence. This data analysis overcomes limitations of measurement techniques of observations having values below detection limits and serves to characterise the dynamics of the virological response to therapies.
Keywords: mixture model, left censoring, HIV RNA, HAART
The goal of highly active antiretroviral therapy (HAART) is to suppress HIV RNA to undetectable levels, providing conditions for CD4 cell counts to increase, thereby reducing HIV related morbidity and mortality.1,2,3,4 However, it is not always achievable as responses to HAART are heterogeneous.5,6 In some people, HIV RNA levels are not suppressed below 50 copies/ml (the limit of detection of current widely used assays) after HAART, and in others, the levels rebound after an initial suppression7,8; both scenarios constitute suboptimal virological response, which predicts poor HIV disease prognosis.9,10 Thus, it is important to investigate the extent of suboptimal virological response to HAART over time and to assess the extent of poor HIV disease prognosis.
In this study, the authors investigated whether the distribution of HIV RNA (log10 scale) is bimodal—that is, whether HIV RNA levels are composed of a mixture of two subpopulations reflecting different responses to HAART. If bimodality is identified in a HAART treated population, the subgroup with higher HIV RNA values would presumably represent suboptimal virological response. Bimodality of disease markers has been shown in other contexts. For example, Lim and coworkers11 showed the bimodality of blood glucose reporting that “diabetes is a distinct entity rather than an arbitrarily defined extreme end of a continuously distributed measurement”.
This report uses data from two ongoing cohort studies, the multicentre AIDS cohort study (MACS) and the women's interagency HIV study (WIHS), to examine (a) whether the distribution of HIV RNA (log10 scale) comprises two normal distributions; (b) assuming bimodality is identified, whether and how it would change over time from pre‐HAART to post‐HAART at different years, and how the distribution would differ between the MACS and WIHS; and (c) identification of exposures associated with suboptimal response to therapies. Here, we use statistical methods for appropriate handling of values below the limit of detection of current assays.
Methods
Population and study design
The MACS was started in 1984 to study the natural history of HIV‐1 infection among homosexual and bisexual men in the USA. The study design has been previously described.12,13 A total of 2785 infected participants were either HIV positive at enrolment (80%) or infected with HIV during follow up (20%). Of the 1437 men who were alive after 1995, a total of 750 men started HAART before 31 March 2003. The WIHS was started in 1993 and it is a multicentre prospective cohort study of the natural history of HIV‐1 infection in women. Methods and cohort characteristics of WIHS have been previously described.14 A total of 2072 infected women were either HIV positive at enrolment (99%) or infected with HIV during follow up (1%). Of which 1277 women started HAART before 31 March 2003. The MACS and WIHS study protocols were approved by institutional review boards of each of the participating centres, and informed consent was obtained from every participant.
Participants returned every six months for a detailed interview, a physical examination, and collection of biological specimens. In MACS, HIV RNA was determined using the Roche Amplicor RNA kit (Hoffman‐LaRoche, Nutley, NJ, USA) with a limit of detection (LD) of 400 copies/ml. If HIV RNA was not detected by this kit, the Roche Ultrasensitive RNA PCR assay (Hoffman‐LaRoche, Nutley, NJ, USA) was performed (LD of 50 copies/ml). In WIHS, HIV RNA was measured using the NASBA assay (Organon Teknika, Cambridge, UK) with an LD of 4000 copies/ml15 up to September 1998, and by the NucliSens assay (Organon Teknika) with an LD of 400 through March 1999 and 80 copies/ml beginning in April 1999.16 Self reported use of antiretroviral drugs at each visit was summarised to define whether participants were using HAART. HAART was defined according to the DHHS/Kaiser Panel guidelines.17 The date of HAART initiation was defined as the midpoint between the last visit without reporting HAART use (last no‐HAART) and the first visit at which HAART use was reported (first HAART).
MACS and WIHS data collected up to 31 March 2003 (end of semi‐annual visits 38th and 17th of MACS and WIHS, respectively) were included for the analysis, with further restriction that the interval between last no‐HAART date and first HAART date was ⩽ 1 year. We included all HIV RNA measurements if it was within a year before and the first five years after HAART initiation, and if HAART use was reported in the preceding six months.
Statistical method: a mixture model with left censoring
Analyses were conducted for MACS and WIHS separately to assess the presence and possible differences in the two cohorts. In addition, to determine the changes before and after starting HAART, analyses were performed for each of the following six intervals: (1) ⩾−1 and <0 year (that is, within one year before HAART initiation), (2) ⩾0 and <1 year, (3) ⩾1 and <2 years, (4) ⩾2 and <3 years, (5) ⩾3 and <4 years, and (6) ⩾4 and <5 years after HAART initiation. Furthermore, to assess whether the putative bimodality was present in participants who were naive to antiretroviral therapy (ART) at HAART initiation, we repeated the analysis on that subset of people.
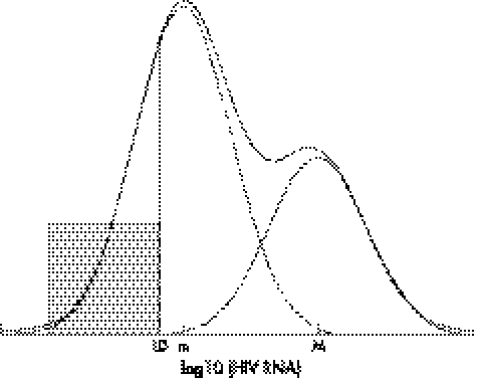
We conducted the analysis of HIV RNA in the log10 scale. Parameters for each cohort and each time interval were estimated using maximum likelihood methods. HIV RNA measurements below LD were treated as left censored. We allow different LD in analysis to accommodate the types of assays used over time. The primary purpose was to find out if the HIV RNA measurements in log scale were better described by a two component mixture distribution compared with a single component distribution. Let π be the probability HIV RNA measurement coming from the lower component of the mixture distribution, with its complement (1−π) the probability of the measurement coming from the higher component. As the proportion of post‐HAART HIV RNA measurements below LD was high, there might not be enough information for estimating both mean and variance in the lower component. Therefore, equal variances were assumed in the two components. The null hypothesis (that is, lack of heterogeneous response) corresponds to π = 0. Figure 1 provides an illustration of the expected shape of the HIV RNA distribution in log10 scale under the alternative hypothesis.
Figure 1 An illustration of the expected distribution of log10(HIV RNA)—a mixture of two normal distributions with equal variance. Solid and dashed lines denote the overall and component specific distributions, respectively. Shaded area represents the proportion below the limit of detection (LD).
As the null value of probability π falls on the boundary of parameter space under the null hypothesis, the standard regularity conditions of hypothesis testing by the generalised likelihood ratio test do not hold.18 Therefore, we selected the better fitting models by simulating the likelihood ratio statistic to obtain the p values.19,20 Technical details are described in the appendix. S‐plus 6.1 (Insightful Corporation, Seattle, WA) was used to perform all statistical analyses.
Results
The analysis included 564 men from the MACS (4785 person visits) and 1173 women from the WIHS (8675 person visits) who started HAART and had HIV RNA measurements. Among the 564 men, 475 (84%) were white, 53 (9%) African American, and 32 (6%) Latino. Among the 1173 women, 216 (18%) were white, 630 (54%) African American, and 297 (25%) Latino. By 31 March 2003, 60 (11%) of the 564 men were deceased, 38 (63% of 60) from AIDS; 191 (16%) of the 1173 women died, 83 (44% of 191) from AIDS. In both cohorts, the annual mortality rate of AIDS was 1%−2% in HIV infected persons receiving HAART over the years after HAART initiation. Before HAART initiation, 455 (81%) of the 564 men and 965 (82%) of the 1173 women had received ART with 412 (73%) men and 763 (65%) women having ART experience for more than one year before HAART initiation.
Table 1 shows descriptive statistics of study population by years from HAART initiation. The calendar years corresponding to person visits stratified by different years from HAART initiation for the two cohorts were similar. Compared with women in the WIHS, men in the MACS were about four to five years older, had 30−100 more CD4 cells/mm3 in each time period, and had higher HIV RNA levels before HAART initiation but lower HIV RNA levels after HAART.
Table 1 Descriptive statistics (median and interquartile ranges (IQR) based on person visits) of study populations stratified by years from HAART initiation.
| Years from HAART initiation | ⩾−1 and <0 year | ⩾0 and < 1 year | ⩾1 and <2 years | ⩾2 and <3 years | ⩾3 and <4 years | ⩾4 and <5 years |
|---|---|---|---|---|---|---|
| Men (MACS cohort, n = 565) | ||||||
| Number of persons | 533 | 528 | 476 | 436 | 388 | 354 |
| Number of person visits | 970 | 922 | 854 | 759 | 667 | 613 |
| Calendar year | ||||||
| Median | 1996.4 | 1997.4 | 1998.4 | 1999.4 | 2000.3 | 2001.3 |
| IQR | 1995.9−1997.3 | 1996.9−1998.3 | 1997.9−1999.2 | 1998.9−2000.1 | 1999.9−2001.0 | 2000.8−2001.9 |
| Age (years) | ||||||
| Median | 42.9 | 43.8 | 44.6 | 45.6 | 46.8 | 47.5 |
| IQR | 38.4−47.4 | 39.2−48.3 | 40.2−49.0 | 41.4−49.9 | 42.4−50.9 | 43.4−51.7 |
| CD4 (cells/mm3) | ||||||
| Median | 303 | 376 | 440 | 479 | 484 | 518 |
| IQR | 165−459 | 234−549 | 282−629 | 315−662 | 328−673 | 330−724 |
| HIV RNA (copies/ml) | ||||||
| Median | 26793 | 214 | 82 | <50 | <50 | <50 |
| IQR | 5113−96219 | <50−3115 | <50−4996 | <50−2504 | <50−1437 | <50−964 |
| Women (WIHS cohort, n = 1173) | ||||||
| Number of persons | 1143 | 1127 | 852 | 769 | 692 | 588 |
| Number of person visits | 2049 | 1834 | 1406 | 1260 | 1181 | 945 |
| Calendar year | ||||||
| Median | 1996.9 | 1997.9 | 1998.9 | 1999.8 | 2000.7 | 2001.6 |
| IQR | 1996.3−1998.1 | 1997.3−1999.2 | 1998.2−2000.0 | 1999.3−2000.6 | 2000.2−2001.4 | 2001.1−2002.2 |
| Age (years) | ||||||
| Median | 38.6 | 39.6 | 40.4 | 40.9 | 42.2 | 43.0 |
| IQR | 33.4−43.6 | 34.4−44.4 | 35.2−45.2 | 35.9−46.0 | 37.1−47.2 | 37.9−47.6 |
| CD4 (cells/mm3) | ||||||
| Median | 270 | 319 | 369 | 397 | 408 | 417 |
| IQR | 141−430 | 182−484 | 211−549 | 228−586 | 239−608 | 248−632 |
| HIV RNA (copies/ml) | ||||||
| Median | 16000 | 855 | 654 | 430 | 280 | 180 |
| IQR | <4000−86000 | <80−13000 | <80−9800 | <80−9900 | <80−6100 | <80−5700 |
The two component model fitted the log10(HIV RNA) significantly (p<0.005) better than the one component model for each time period in both cohorts. Table 2 summarises the parameter estimates of HIV RNA (log10 scale) as a mixture of two normal distributions. In the year before HAART initiation, there was an estimated 10% of men with median HIV RNA of 55 copies/ml and 90% with a median of 30 153 copies/ml; while an estimated 22% of women had median HIV RNA of 192 copies/ml and 78% had a median of 31 384 copies/ml.
Table 2 HIV RNA (log10 scale) as a mixture of two normal distributions*.
| Years from HAART initiation | Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of person visits | Lower component | Higher component | Number of person visits | Lower component | Higher component | |||||
| % | Median (copies/ml) | % | Median (copies/ml) | % | Median (copies/ml) | % | Median (copies/ml) | |||
| ⩾−1 and <0 | 970 | 10 | 55 | 90 | 30153 | 2049 | 22 | 192 | 78 | 31384 |
| ⩾0 and <1 | 922 | 52 | 19 | 48 | 3868 | 1834 | 38 | 9 | 62 | 6823 |
| ⩾1 and <2 | 854 | 58 | 10 | 42 | 7865 | 1406 | 43 | 12 | 57 | 6516 |
| ⩾2 and <3 | 759 | 64 | 10 | 36 | 8111 | 1260 | 47 | 13 | 53 | 7367 |
| ⩾3 and <4 | 667 | 65 | 4 | 35 | 5067 | 1181 | 49 | 7 | 51 | 6326 |
| ⩾4 and <5 | 613 | 68 | 6 | 32 | 5247 | 945 | 56 | 17 | 44 | 9253 |
*Observations below the limit of detection (LD) handled as censored. The two component model with equal variance fitted the log10 HIV RNA significantly (p<0.005) better than the one component model for each time period in both cohorts.
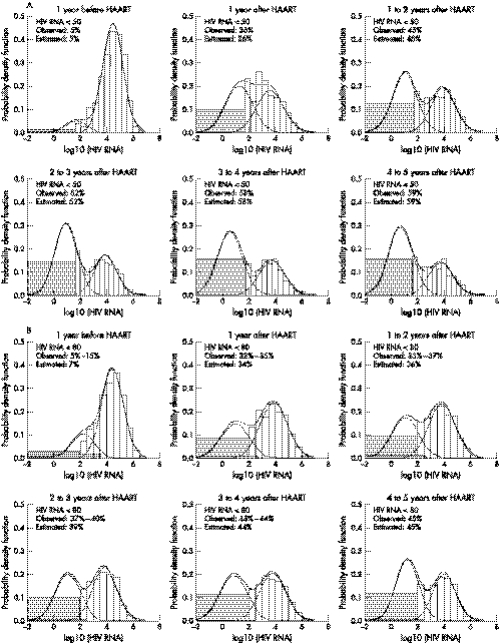
From the year before to the first year after HAART initiation, both the percentage of HIV RNA in the higher component of the mixture distribution and the median HIV RNA in that component decreased dramatically. Specifically, the percentages in the higher component decreased from 90% to 48% in men and from 78% to 62% in women; and the corresponding medians reduced by a factor of 8 and 5 for men and women, respectively. Table 2 also shows that from the first (0–1) year to fifth (4–5) year after HAART initiation measurements in the higher component of HIV RNA further decreased from 48% to 32% for men and from 62% to 44% for women, while the median of the higher component fluctuated between 3868 and 9253 HIV RNA copies/ml. In turn, the median of the lower component was estimated to be comparatively stable at <20 copies/ml after HAART initiation in both cohorts. Specifically, in the fifth year after HAART initiation, 68% of the measurements from men were estimated to have a median HIV RNA of 6 copies/ml with an interquartile range (IQR) from 2 to 22 copies/ml and 56% of the measurements from women were estimated to have a median HIV RNA of 17 copies/ml with an IQR from 5 to 62 copies/ml. The remaining 32% of men had a median of 5247 copies/ml (IQR: 1252–22 000 copies/ml) and the remaining 44% of women had a median of 9253 copies/ml (IQR: 2436–35 159 copies/ml). The goodness of fit for modelling the log10(HIV RNA) as a mixture of two normal distributions with equal variance is displayed in figure 2, showing that the two component model fits the log10 (HIV RNA) well.
Figure 2 Goodness of fit for modelling log10(HIV RNA) as a mixture of two normal distributions with equal variance for the MACS (panel A) and the WIHS (panel B), respectively. The histograms are observed data and the lines are fitted values. Shaded areas depict the observations below the limits of detection (LD). LD is 50 HIV RNA copies/ml (log10(50) = 1.7) in the MACS, and is 80 (log10(80) = 1.9) or 400 or 4000 HIV RNA copies/ml in the WIHS. The observed % below 80 in the WIHS ranges from the percentage <80 copies/ml alone to the sum of the percentages of undetected HIV RNA <80, or <400, or <4000 copies/ml.
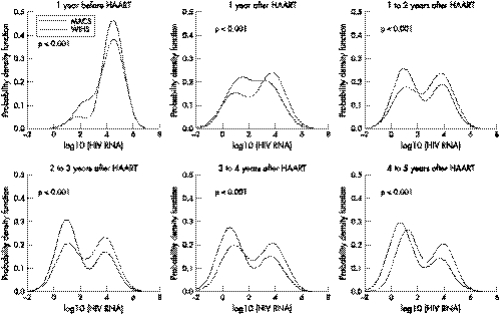
Figure 3 shows the superimposed estimated bimodal distributions of log10(HIV RNA) for the MACS and WIHS, suggesting that the mixture distributions of the two cohorts are significantly different (Likelihood ratio test: p<0.001) in each time interval. To find out if the bimodality was also present in the subset of people who were ART naive at HAART initiation, the analyses were repeated using only the data from this subgroup. The bimodality did persist at all periods from HAART initiation and in both men and women. Furthermore, after four years of HAART initiation, the median levels of the lower and higher components of HIV RNA were very similar to those reported in table 2. Specifically, for men ART naive at HAART initiation, the median HIV RNA levels of the lower and higher components were estimated to be 6 and 4514 copies/ml respectively; and, in women, they were 28 and 10034 copies/ml respectively. An important difference of the people who were ART naive at HAART initiation was that the percentage in the lower component after four years was higher. Specifically, 87% men (19% above the 68% in table 2) and 62% women (6% above the 56% on table 2) were estimated to be in the lower component of the bimodal distribution at the fifth year after HAART initiation.
Figure 3 Superimposed estimated bimodal distributions of log10(HIV RNA in copies/ml) for the MACS and WIHS by different years from HAART initiation. Negative values on the x axis represent less than 1 copy/ml (for example, −2 corresponds to 1 copy/100 ml). The solid line is for the MACS and the dashed line is for the WIHS. The p value in each panel was obtained from likelihood ratio test to examine whether the mixture distributions from the two cohorts were different.
As shown in figure 3, at the fifth (4–5) year after HAART in both cohorts, the two components of log10(HIV RNA) separated at about 2.5, which corresponded to 317 HIV RNA copies/ml. Therefore, we chose 300 HIV RNA copies/ml at the fifth year after HAART as the cut off point to partition our study population into two groups: (1) optimal virological responders—if their median HIV RNA measurements taken within the fifth year after HAART initiation <300 copies/ml, and (2) suboptimal virological responders—if their median HIV RNA ⩾300 copies/ml.
Using 300 copies/ml as the cut off value, we identified 517 optimal virological responders (54% of them women), and 423 suboptimal virological responders (72% of them women). Of the 517 subjects with optimal virological response, 51% were white, 25% African American, and 20% Hispanic; while among the 423 subjects with suboptimal virological response, 34% were white, 44% African American, and 21% Hispanic. The differences in the percentages of white people and African Americans between the two groups were because more women, most of whom were African Americans, were in the group with suboptimal virological response. Regarding AIDS restriction genes,21 CCR5 heterozygous genotype was found in 11.5% of subjects with optimal virological response, but in only 6.6% of those with suboptimal virological response (χ2 test p = 0.014).
Table 3 shows antiretroviral therapy use, adherence status, immunological and virological markers by time since HAART initiation for the two groups (HIV RNA <300 or ⩾300 copies/ml in the fifth year after HAART—that is, with optimal or suboptimal virological response). Before HAART initiation, the group with suboptimal virological response had lower CD4 cell count (p<0.001), higher HIV RNA level (p<0.001), and more exposure to ARTs before HAART (p = 0.029). After HAART the suboptimal virological response group had higher rate of poor adherence (<95%) to HAART (p<0.001 during both one to three and three to five years after HAART initiation). Furthermore, the rates of increase of CD4 cell count in the two groups (386−313 = 73 cells/mm3 in the optimal virological response group and 318−249 = 69 cells/mm3 in the suboptimal virological response group) were similar in the first year after HAART. However, the rate of increase of the group with suboptimal virological response was substantially lower (352−318 = 34 cells/mm3 compared with 470−386 = 84 cells/mm3; p<0.001) between one to three years after HAART; and the CD4 cell count of the group with suboptimal virological response actually declined afterwards (p<0.001).
Table 3 Antiretroviral therapy use, adherence, immunological and virological markers by time since HAART initiation stratified by HIV RNA ⩾ 300 or <300 copies/ml at the fifth year after HAART initiation.
| Years from HAART initiation | HIV RNA <300 copies/ml at fifth year after HAART (n = 517) | HIV RNA ⩾300 copies/ml at fifth year after HAART (n = 423) | ||||||
|---|---|---|---|---|---|---|---|---|
| ⩾−1 and <0 | ⩾0 and <1 | ⩾1 and <3 | ⩾3 and <5 | ⩾−1 and <0 | ⩾0 and <1 | ⩾1 and <3 | ⩾3 and <5 | |
| Number of person visits | 913 | 887 | 1660 | 1753 | 757 | 705 | 1307 | 1332 |
| NRTI, % | ||||||||
| = 0 | 31 | 0 | 0 | 0 | 26 | 0 | 0 | 0 |
| = 1 | 17 | 2 | 9 | 9 | 22 | 4 | 12 | 10 |
| ⩾2 | 52 | 98 | 91 | 91 | 52 | 96 | 88 | 90 |
| PI, % | ||||||||
| = 0 | 95 | 12 | 16 | 41 | 93 | 9 | 16 | 31 |
| = 1 | 4 | 76 | 68 | 43 | 7 | 75 | 61 | 45 |
| ⩾2 | 1 | 12 | 16 | 16 | <1 | 16 | 23 | 24 |
| NNRTI, % | ||||||||
| = 0 | >99 | 83 | 68 | 54 | 99 | 85 | 65 | 54 |
| = 1 | <1 | 17 | 32 | 45 | 1 | 15 | 34 | 44 |
| ⩾2 | 0 | 0 | <1 | 1 | 0 | 0 | 1 | 2 |
| Adherence, % | n = 125 | n = 1207 | n = 1740 | n = 77 | n = 902 | n = 1317 | ||
| 100 | NA | 39 | 42 | 40 | NA | 36 | 36 | 34 |
| 95–99 | NA | 40 | 45 | 47 | NA | 38 | 39 | 41 |
| 75–94 | NA | 15 | 9 | 12 | NA | 22 | 18 | 17 |
| <75 | NA | 6 | 4 | 1 | NA | 4 | 7 | 8 |
| CD4 (cells/mm3) | ||||||||
| Median | 313 | 386 | 470 | 528 | 249 | 318 | 352 | 339 |
| IQR | 178–461 | 246–550 | 306–656 | 351–728 | 150–400 | 199–461 | 213–532 | 196–541 |
| HIV RNA (copies/ml) | ||||||||
| Median | 13244 | 115 | <80 | <80 | 28000 | 2554 | 3500 | 4365 |
| IQR | 3744–64000 | <80–1551 | <50–505 | <50–80 | 5500–110000 | 200–19000 | 320–23000 | 690–23195 |
What this paper adds
The bimodal distribution of HIV RNA persisted during the years after HAART initiation. The high occurrence of suboptimal virological response at the fifth year after HAART initiation underscore the needs for careful monitoring and patient education about the importance of treatment adherence. Our approach overcomes limitations of measurement techniques of observations having values below detection limits and serves to characterise the dynamics of the virological response to treatments.
Discussion
Our data suggest that the overall distribution of log10 HIV RNA among HAART treated persons in the MACS and the WIHS might be better represented as a mixture of two normal distributions. Although it is well known that persons have heterogeneous responses to HAART, the significant finding of bimodality accentuates the classification of the population in two subgroups (that is—optimal and suboptimal responses). While most HAART users achieved optimal virological response (median HIV RNA <20 copies/ml), a substantial percentage of them (32% in men and 44% in women) had suboptimal response (median HIV RNA >5000 copies/ml) even in the fifth year after HAART initiation. These percentages with suboptimal response after prolonged use of HAART are the people on whom emergence of resistance may be seen in the future. In general, using the bimodal mixture model that we have proposed here for characterising failures (that is, progressing to disease under HAART) is likely to be a more efficient way in cohort studies following up persons under HAART for a long term.
Public policy implications
High percentages (32%–44%) of persons with moderate levels of HIV RNA after four years receiving HAART underscore the need for careful monitoring, as they have the potential for increasing resistance and consequently reducing treatment options over the long term. Given the relation of suboptimal response to lower adherence to HAART, patient education is needed to emphasise the importance of treatment adherence. Overall, the low levels of HIV RNA in people treated with HAART over four years, many of whom did not fully adhere to the recommended use, makes it imperative to offer HAART to people infected with HIV and who fulfilled the guidelines recommended for who should be receiving treatment.
This report presents the first characterisation of the distribution of HIV RNA using a mixture of parametric models with left censoring and relates it to the extent of suboptimal virological response to HAART at different years after HAART initiation, thereby providing a unique historical look at the distribution of HIV RNA levels in the HAART treated population.
In this analysis over 80% of the participants had received ART before HAART. Some of them might have had partial or no virological response to ART.22,23 This may account for the presence of bimodality of the distributions of HIV RNA levels in the year before HAART initiation. However, bimodality is not fully explained by antiretroviral experience before HAART initiation because, when we restricted the analysis to the subgroups who were ART naive at HAART initiation, the bimodality persisted with similar median values after four years from HAART initiation but with higher percentages in the lower component of HIV RNA for the ART naive HAART initiators. This is consistent with ART naive persons having a better response to treatment.
In our analyses we included only the visits where people reported receiving HAART in the previous six month interval, thereby eliminating the suboptimal virological response because of recent withdrawal of HAART. We also found that those with suboptimal virological response had more advanced HIV disease and ART exposure before HAART; and they had lower adherence after HAART, which is consistent with prior reports.24,25,26,27,28,29 In addition, we identified that there were fewer subjects with CCR5 heterozygous genotype among the suboptimal virological responders regardless of their level of adherence to HAART. This showed that poor adherence to HAART and lower frequency of CCR5 heterozygous genotype might be independently associated with suboptimal virological response.
In this analysis, the variances of two components were assumed to be equal because of a large proportion of censored data. As the left censored data (that is, below the LD) were unobservable, there is uncertainty about the actual number of components of the overall distribution and the actual distribution of the censored data. Although unlikely, it is not unfeasible that the true distribution could be unimodal with median below 50 and a heavy right tail to incorporate what here we have identified as the second component of the suboptimal responders. The inferences reported here could be validated once methods to detect HIV RNA below 50 and 80 copies/ml become widely available and properly standardised.
Most men in the MACS are white, and women in the WIHS are mostly African Americans, who differ in many social and demographic aspects. Despite these differences, it is comforting to note that the general shape and changes of the distributions of log10(HIV RNA) (fig 3) are present in both cohorts of HIV infected persons receiving HAART. The significant findings in figure 3 showing more suppression of HIV RNA in the cohort of men cannot be ascribed to sex only but have to be interpreted in the context of the differences between the two cohorts. Specifically, women participating in the WIHS cohort relative to the men in the MACS cohort are of substantially lower socioeconomic status with more difficulties to adhere to the complexities of HAART therapy.
In summary, high percentages (32%–44%) of persons with moderate levels of HIV RNA after four years receiving HAART underscore the need for careful monitoring, as they have the potential for increasing resistance and consequently reducing treatment options over the long term. Give the relation of suboptimal response to lower adherence to HAART, patient education is needed to emphasise the importance of treatment adherence. Overall, the low levels of HIV RNA in people treated with HAART over four years, many of whom did not fully adhere to the recommended use, makes it imperative to offer HAART to people infected with HIV and who fulfilled the guidelines recommended for who should be on treatment.
Acknowledgements
Data in this manuscript were collected by the multicentre AIDS cohort study (MACS) and the women's interagency HIV study (WIHS) collaborative study group with centres (principal investigators) located at:
MACS centres: The Johns Hopkins Bloomberg School of Public Health (Joseph Margolick); Howard Brown Health Center and Northwestern University Medical School (John Phair); University of California, Los Angeles (Roger Detels); University of Pittsburgh (Charles Rinaldo); and Data Analysis Center (Lisa Jacobson). The MACS is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute; and the National Heart, Lung, and Blood Institute: U01‐AI‐35042, 5‐M01‐RR‐00052 (GCRC), U01‐AI‐35043, U01‐AI‐37984, U01‐AI‐35039, U01‐AI‐35040, U01‐AI‐37613, and U01‐AI‐35041.
WIHS centres: New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Analysis Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases with supplemental funding from the National Cancer Institute, and the National Institute on Drug Abuse (U01‐AI‐35004, U01‐AI‐31834, U01‐AI‐34994, U01‐AI‐34989, U01‐AI‐34993, and U01‐AI‐42590). Funding is also provided by the National Institute of Child Health and Human Development (U01‐HD‐32632) and the National Center for Research Resources (M01‐RR‐00071, M01‐RR‐00079, M01‐RR‐00083).
Abbreviations
HAART - highly active antiretroviral therapy
ART - antiretroviral therapy
LD - limit of detection
MACS - multicentre AIDS cohort study
WIHS - women's interagency HIV study
Appendix
Simulating the likelihood ratio statistic
For normal component densities, McLachlan19 proposed a Monte Carlo approach for the calculation of a p value of the likelihood ratio test when testing whether the number of components G is 1 under the null hypothesis compared with 2 under the alternative hypothesis (that is, a mixture of two normal distributions with common variance). In this report, we use McLachlan's approach to assess the p value of the likelihood ratio test for π = 0 under the null hypothesis compared with π>0 under the alternative hypothesis. Based on the data
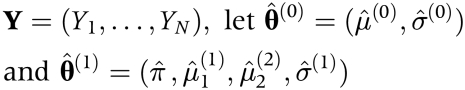 |
are the maximum likelihood estimators of the parameters under H0 (that is, π = 0) and H1 (that is, π > 0), respectively. The distribution of the likelihood ratio statistic to test the null hypothesis π = 0 compared with the alternative of π > 0 can be simulated as follows. Under the null hypothesis, a simulated sample
replications, each with sample size N is generated from the density
. Let
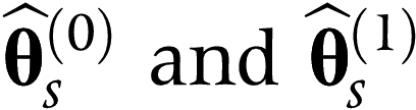 |
be the MLEs for θ under H0 and H1 based on the simulated sample
, respectively. It is expected that
be very close to
, and
will encode the variability of
under H1 when H0 is true. In particular, the variance of
will represent the variability of the likelihood ratio statistic (LRS)
when H0 is true. If the value of the LRS from the original data is between the (j−1)th and the jth smallest values of its S replications, then the p value for the test that rejects H0 is approximately 1−j/(S+1). A total of S = 200 simulations were run to provide the p values reported here.
Footnotes
Competing interests: none declared.
References
- 1.Gulick R M, Mellors J W, Havlir D.et al Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med 1997337734–739. [DOI] [PubMed] [Google Scholar]
- 2.Palella F J, Jr, Delaney K M, Moorman A C.et al Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998338853–860. [DOI] [PubMed] [Google Scholar]
- 3.Detels R, Muñoz A, McFarlane G.et al Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA 19982801497–1503. [DOI] [PubMed] [Google Scholar]
- 4.Detels R, Tarwater P, Phair J P.et al Effectiveness of potent antiretroviral therapies on the incidence of opportunistic infections before and after AIDS diagnosis. AIDS 200115347–355. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita T E, Phair J P, Muñoz A.et al Immunologic and virologic response to highly active antiretroviral therapy in the multicenter AIDS cohort study. AIDS 200115735–746. [DOI] [PubMed] [Google Scholar]
- 6.Tarwater P M, Margolick J B, Jin J.et al Increase and plateau of CD4 T‐cell counts in the 3(1/2) years after initiation of potent antiretroviral therapy. J Acquir Immune Defic Syndr 200127168–175. [DOI] [PubMed] [Google Scholar]
- 7.Ledergerber B, Egger M, Opravil M.et al Clinical progression and virological failure on highly active antiretroviral therapy in HIV‐1 patients: a prospective cohort study. Swiss HIV cohort study. Lancet 1999353863–868. [DOI] [PubMed] [Google Scholar]
- 8.Abgrall S, Duval X, Joly V.et al Clinical and immunologic outcome in patients with human immunodeficiency virus infection, according to virologic efficacy in the year after virus undetectability, during antiretroviral therapy. Clin Infect Dis 2003371517–1526. [DOI] [PubMed] [Google Scholar]
- 9.Anastos K, Barron Y, Cohen M H.et al The prognostic importance of changes in CD4+ cell count and HIV‐1 RNA level in women after initiating highly active antiretroviral therapy. Ann Intern Med 2004140256–264. [DOI] [PubMed] [Google Scholar]
- 10.Tarwater P M, Gallant J E, Mellors J W.et al Prognostic value of plasma HIV RNA among highly active antiretroviral therapy users. AIDS 2004182419–2423. [PubMed] [Google Scholar]
- 11.Lim T O, Bakri R, Morad Z.et al Bimodality in blood glucose distribution: is it universal? Diabetes Care 2002252212–2217. [DOI] [PubMed] [Google Scholar]
- 12.Dudley J, Jin S, Hoover D.et al The multicenter AIDS cohort study: retention after 9 1/2 years. Am J Epidemiol 1995142323–330. [DOI] [PubMed] [Google Scholar]
- 13.Kaslow R A, Ostrow D G, Detels R.et al The multicenter AIDS cohort study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987126310–318. [DOI] [PubMed] [Google Scholar]
- 14.Barkan S E, Melnick S L, Preston‐Martin S.et al The women's interagency HIV study. WIHS Collaborative Study Group. Epidemiology 19989117–125. [PubMed] [Google Scholar]
- 15.Yen‐Lieberman B, Brambilla D, Jackson B.et al Evaluation of a quality assurance program for quantitation of human immunodeficiency virus type 1 RNA in plasma by the AIDS Clinical Trials Group virology laboratories. J Clin Microbiol 1996342695–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowicki M J, Benning L, Bremer J W.et al Longitudinal variability of human immunodeficiency virus type 1 RNA viral load measurements by nucleic acid sequence‐based amplification and NucliSens assays in a large multicenter study. J Clin Microbiol 2001393760–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institutes of Health Guidelines for the use of antiretroviral agents among HIV‐infected adults and adolescents. http://aidsinfo.nih.gov/guidelines/
- 18.McLachlan G J, Peel D.Finite mixture models. New York: Wiley, 2000
- 19.McLachlan G J. On bootstrapping the likelihood ratio test statistics for the number of components in a normal mixture. Applied Statistics 198736318–324. [Google Scholar]
- 20.Chu H, Moulton L H, Mack W J.et al Correlating two continuous variables subject to detection limits in the context of mixture distributions. Applied Statistics 200554831–845. [Google Scholar]
- 21.Silverberg M J, Smith M W, Chmiel J S.et al Fraction of cases of acquired immunodeficiency syndrome prevented by the interactions of identified restriction gene variants. Am J Epidemiol 2004159232–241. [DOI] [PubMed] [Google Scholar]
- 22.Piketty C, Castiel P, Belec L.et al Discrepant responses to triple combination antiretroviral therapy in advanced HIV disease. AIDS 199812745–750. [DOI] [PubMed] [Google Scholar]
- 23.Deeks S G, Barbour J D, Martin J N.et al Sustained CD4+ T cell response after virologic failure of protease inhibitor‐based regimens in patients with human immunodeficiency virus infection. J Infect Dis 2000181946–953. [DOI] [PubMed] [Google Scholar]
- 24.d'Arminio Monforte A, Testa L, Adorni F.et al Clinical outcome and predictive factors of failure of highly active antiretroviral therapy in antiretroviral‐experienced patients in advanced stages of HIV‐1 infection. AIDS 1998121631–1637. [DOI] [PubMed] [Google Scholar]
- 25.Wit F W, van Leeuwen R, Weverling G J.et al Outcome and predictors of failure of highly active antiretroviral therapy: one‐year follow‐up of a cohort of human immunodeficiency virus type 1‐infected persons. J Infect Dis 1999179790–798. [DOI] [PubMed] [Google Scholar]
- 26.Paredes R, Mocroft A, Kirk O.et al Predictors of virological success and ensuing failure in HIV‐positive patients starting highly active antiretroviral therapy in Europe: results from the EuroSIDA study. Arch Intern Med 20001601123–1132. [DOI] [PubMed] [Google Scholar]
- 27.Grabar S, Pradier C, Le Corfec E.et al Factors associated with clinical and virological failure in patients receiving a triple therapy including a protease inhibitor. AIDS 200014141–149. [DOI] [PubMed] [Google Scholar]
- 28.Dionisio D, Vivarelli A, Zazzi M.et al Extent of human immunodeficiency virus type 1 drug resistance as a predictor of virological failure after genotype‐guided treatment switch. Clin Infect Dis 200133706–709. [DOI] [PubMed] [Google Scholar]
- 29.Masuhr A, Mueller M, Simon V.et al Predictors of treatment failure during highly active antiretroviral therapy (racing trial). Eur J Med Res 20027341–346. [PubMed] [Google Scholar]