Abstract
Korean isolates of the Mycobacterium chelonae-Mycobacterium abscessus group, which had been isolated from two different hospitals in South Korea, were identified by PCR restriction analysis (PRA) and comparative sequence analysis of 16S rRNA genes, rpoB, and hsp65 to evaluate the proportion of four closely related species (M. chelonae, M. abscessus, M. massiliense, and M. bolletii). Of the 144 rapidly growing mycobacterial strains tested, 127 strains (88.2%) belonged to the M. chelonae-M. abscessus group. In this group, M. chelonae, M. abscessus, M. massiliense, and M. bolletii accounted for 0.8% (n = 1), 51.2% (n = 65), 46.5% (n = 59), and 1.6% (n = 2), respectively. Two isolates which showed discordant results, M. massiliense by rpoB sequence analysis and M. abscessus by hsp65 sequence analysis, were finally identified as M. massiliense based on the additional analysis of sodA and the 16S-23S internal transcribed spacer. M. abscessus group I isolates previously identified by hsp65 PRA were all found to be M. abscessus, whereas group II isolates were further identified as M. massiliense or M. bolletii by sequencing of rpoB and hsp65. Smooth, rough, or mixed colonies of both M. abscessus and M. massiliense isolates were observed. M. massiliense strains that were highly resistant to clarithromycin had a point mutation at the adenine at position 2058 (A2058) or 2059 (A2059) in the peptidyltransferase region of the 23S rRNA gene.
Rapidly growing mycobacteria (RGM), which include members of the Mycobacterium fortuitum complex, the M. chelonae complex, and the M. smegmatis complex, are defined as mycobacteria that grow and form visible colonies on solid agar media within 7 days. Because of their low virulence, they usually cause respiratory tract or disseminated infections in persons with predisposing factors or who are immunosuppressed (27, 29, 32). However, recently, infections in immunocompetent persons have been reported more frequently. These cases are soft-tissue infections associated with trauma and injection and epidemics or pseudoepidemics that have occurred in hospitals (6, 12). Ninety-five percent of soft-tissue RGM infections are caused by members of the M. chelonae complex (7), which comprises M. chelonae, M. abscessus, and M. immunogenum. However, the situation is complex for the clinical microbiologist who must rapidly and correctly identify isolates because there are reports of heterogeneity in M. abscessus isolates. Two M. abscessus groups (groups I and II) were reported on the basis of hsp65 PCR-restriction fragment length polymorphism (RFLP) (11) and sequence analysis (21) of hsp65. High heterogeneity of M. abscessus isolates was also shown by rpoB analysis (2). Meanwhile, new RGM species were reported: M. massiliense (4) and M. bolletii (1). These were very closely related to M. abscessus and M. chelonae but showed different susceptibility to clarithromycin, and their pathogenic potentials were shown by infections in immunocompetent and immunocompromised hosts (13, 19).
RGM infections are also common in South Korea (24, 27, 34). M. abscessus is the most frequently isolated nontuberculous mycobacterium (NTM) in South Korea, second to the M. avium complex (20), in contrast to other countries such as the United States, Japan, and Sweden (9, 28, 39). In addition, there was an epidemic associated with intramuscular injection of an antimicrobial agent, which seemed to be caused by M. abscessus, in 2005 at Icheon, South Korea. However, the causative microorganism was finally identified as M. massiliense based on rpoB and hsp65 sequence analysis (19). Thus, because of the close relationship between M. abscessus and two recently reported species, M. massiliense and M. bolletii, we speculated that these were not newly found in the mycobacterial populations but rather had been collectively identified as M. abscessus or M. chelonae-M. abscessus group species in previous studies.
In the present study, we investigated several issues related to the heterogeneity of the M. chelonae-M. abscessus group. One is the isolation rates of four closely related species, M. chelonae, M. abscessus, M. massiliense, and M. bolletii in Korean clinical isolates. The others are colony morphology and susceptibility to clarithromycin. The first might be related to virulence (8, 14), and the last is important for treating M. abscessus infection (7). We reidentified a group of M. abscessus isolates (Asan Collection) that had been previously isolated and identified by hsp65 PCR restriction analysis (PRA) and prospectively identified another group of RGM isolates (Samsung Collection), which were mainly isolated from respiratory specimens collected over 2 years, using hsp65 and rpoB sequence analysis (19, 38).
MATERIALS AND METHODS
Bacterial strains.
Two different groups of RGM isolates obtained from two hospitals in Seoul, South Korea, were used. One group (42 strains of M. abscessus), which had been previously isolated from patients with respiratory infection and identified by hsp65 PRA, was provided by Tae Sun Shim, Asan Medical Center, South Korea. These isolates were used for reidentification. The other group comprised 102 RGM strains isolated from 99 patients with respiratory infection and was provided by Nam Yong Lee, Samsung Medical Center. Isolates in the Samsung Collection were collected from June 2005, just after recognition of an injection-associated M. massiliense epidemic (19), until May 2007. The type strains of M. abscessus (ATCC 19977), M. massiliense (CIP 108297), and M. bolletii (CIP 108541) were used for comparison. RGM isolates cultivated on Ogawa media were subcultured on blood agar plates at 37°C and 5% CO2 for 4 days to observe colony morphology and purity and then used for species identification based on hsp65 PRA (11) and PCR sequencing of rpoB (19) and hsp65 (38).
DNA extraction and PCR.
Total DNAs were extracted from cultured colonies using the bead beater-phenol extraction method (17) and used as templates for PCR. The following primer pairs were used: 285 (5′-GAG AGT TTG ATC CTG GCT CAG-3′) and 244 (5′-CCC ACT GCT GCC TCC CGT AG-3′) for 16S rRNA gene (351 bp) (36), RGMF (5′-GAC GAC ATC GAC CAC TTC GG-3′) and RGMR (5′-GGG GTC TCG ATC GGG CAC AT-3′) for rpoB PCR (365 bp) (19), and Tb11 (5′-ACC AAC GAT GGT GTG TCC AT-3′) and Tb12 (5′-CTT GTC GAA CCG CAT ACC CT-3′) for hsp65 (441 bp) (38). Template DNA (ca. 50 ng) and 20 pmol of each primer were added to a PCR mixture tube (AccuPower PCR PreMix; Bioneer, Daejeon, South Korea) containing 1 unit of Taq DNA polymerase, 250 μM of deoxynucleotide triphosphate, 10 mM Tris-HCl (pH 8.3), 10 mM KCl, 1.5 mM MgCl2, and gel loading dye. The final volume was adjusted to 20 μl with distilled water, and the reaction mixture was then amplified as previously described (19, 36, 38) using a model 9700 Thermocycler (Perkin-Elmer Cetus). Sequencing of sodA and the 16S-23S rRNA gene internal transcribed spacer (16S-23S ITS) and 23S rRNA gene sequencing were performed to resolve discrepancies in identification by rpoB and hsp65 analysis and to identify mutations associated with clarithromycin resistance, respectively (3, 25, 30).
hsp65 PRA.
PRA was performed as previously described (11). Briefly, 10 μl of the amplified PCR products was transferred to fresh microcentrifuge tubes and digested with HaeIII (TaKaRa, Shiga, Japan) or BstEII (Promega, Madison, WI) according to the supplier's instructions. Following digestion, the mixtures were electrophoresed on 3% agarose gel (at 100 V for 30 min). The DNA bands were visualized by ethidium bromide staining and photographed. For those strains that did not show clear PRA results, sizing of the DNA fragments on the basis of their determined hsp65 sequences was performed by MapDraw (version 3.14; DNASTAR, Madison, WI) and compared to the previous report (11).
Nucleotide sequencing.
PCR products were purified using Qiaex II gel extraction kits (Qiagen, Hilden, Germany) and were sequenced directly using forward and reverse primers with an Applied Biosystems automated sequencer (model 377) and BigDye Terminator cycle sequencing kits (Perkin-Elmer Applied Biosystems, Warrington, United Kingdom). Both strands were sequenced as a cross-check.
Sequence analysis.
Determined partial 16S rRNA gene, rpoB, and hsp65 sequences (306 bp, 306 bp, and 401 bp, respectively) were aligned using the ClustalW algorithm in MEGA4 (37). Phylogenetic trees were inferred from partial rpoB and hsp65 nucleotide sequences of Korean isolates in this study, and those of RGM type strains were retrieved from GenBank using the neighbor-joining method in MEGA4 (37) and median network in SplitsTree4 (15).
Susceptibility testing for clarithromycin.
MICs of clarithromycin were determined by the broth dilution method in microtiter plates (16) with slight modification. Nine randomly selected strains of M. abscessus, nine strains of M. massiliense, and two M. bolletii isolates were used for clarithromycin susceptibility testing. M. abscessus ATCC 19977T, M. massiliense CIP 108297T, and M. bolletii CIP 108541T, whose susceptibilities to clarithromycin are known, were used as controls. Briefly, pure cultured colonies were transferred to 7H9 broth (Becton Dickinson, Piscataway, NJ) with 0.02% Tween 80 (Junsei Chemical, Tokyo, Japan) and 0.2% glycerol (Sigma) in a tube and vigorously vortex mixed to a density equivalent of 0.5 on the McFarland scale. Bacterial suspensions (150 μl) were transferred to the wells of microtitration plates, which contained clarithromycin (Boryung, Seoul, South Korea) (150 μl) solubilized in 20% dimethyl sulfoxide and twofold serially diluted with 7H9 broth from 256 to 0.06 μg/ml. The plates were incubated at 37°C for 72 h. Bacterial growth was observed, and the MICs were determined. The susceptibility was determined according to the breakpoints recommended by the Clinical and Laboratory Standards Institute (CLSI) (10).
Separately, the 23S rRNA gene sequences of these 20 isolates and 3 type strains were analyzed (25) to observe any point mutation at the adenine at position 2058 (A2058) or at A2059 in the peptidyltransferase region of the 23S rRNA gene.
Nucleotide sequence accession numbers.
Determined rpoB and hsp65 sequences that were different from reference strains have been deposited in GenBank under accession no. EU732712 to EU732723.
RESULTS
Identification by hsp65 PRA.
Of the 42 reidentified M. abscessus strains (Asan Collection), 28 (66.7%) and 14 strains (33.3%) belonged to M. abscessus groups I and II, respectively (Table 1). Of the 102 strains in the Samsung Collection, 85 strains (83.3%) belonged to the M. chelonae-M. abscessus group, and these included 37 (36.2%) strains of M. abscessus group I, 47 strains (46.1%) of M. abscessus group II, and 1 strain (1.0%) of M. chelonae (Table 1). In addition, M. fortuitum (nine strains, 8.8%), M. peregrinum (four strains, 3.9%), and M. porcinum (three strains, 2.9%) were identified among the remaining mycobacteria.
TABLE 1.
Distribution of M. chelonae-M. abscessus group isolates identified by hsp65 PCR restriction analysis in this study
| Species and group | No. (%) of strains in collection:
|
Total no. of strains | |
|---|---|---|---|
| Asan | Samsung | ||
| M. abscessus | |||
| I | 28 (66.7) | 37 (43.5) | 65 |
| II | 14 (33.3) | 47 (55.3) | 61 |
| M. chelonae | 0 (0.0) | 1 (1.2) | 1 |
| Total | 42 (100.0) | 85 (100.0) | 127 |
Identification by gene sequence analysis.
Species identification of the RGM strains was accomplished by BLAST search to measure the similarities and infer the constructed phylogenetic trees. The M. chelonae-M. abscessus group (42 Asan and 85 Samsung strains) identified by hsp65 PRA were separated into four different species (M. chelonae, M. abscessus, M. massiliense, and M. bolletii) (Table 2). Based on the rpoB sequence analysis, all of the M. abscessus group I strains (65 strains) were identified as M. abscessus. Their rpoB sequence similarities to the reference sequences were 99.3 to 100%. The 61 M. abscessus group II strains were identified as M. massiliense (59 strains) and M. bolletii (2 strains). These strains showed rpoB sequences identical to those of M. massiliense and M. bolletii, respectively, but relatively low (97.3 to 98.7%) sequence similarity to that of the M. abscessus reference strain. Interestingly, two strains (1.4%) showed discordant results between rpoB and hsp65 sequence analysis. These strains were M. massiliense based on the rpoB sequences but were M. abscessus based on hsp65 sequences analysis. However, gene analysis of sodA (441 bp) and the 16S-23S ITS (216 bp) indicated that these two strains were M. massiliense (similarity of 100%) (Table 2).
TABLE 2.
Comparison of identification results for M. chelonae-M. abscessus group isolates using hsp65 PCR-RFLP and sequence analysis of rpoB and hsp65
| Species and group by hsp65 PCR-RFLP | No. (%) | Result by PCR sequencing of:
|
|||
|---|---|---|---|---|---|
|
rpoB
|
hsp65
|
||||
| Species | No. (%) | Species | No. (%) | ||
| M. abscessus group I | 65 (51.2) | M. abscessus | 65 (51.2) | M. abscessus | 67 (52.8) |
| M. abscessus group II | 61 (48.0) | M. massiliense | 59 (46.5) | M. massiliense | 57 (44.9) |
| M. bolletii | 2 (1.6) | M. bolletii | 2 (1.6) | ||
| M. chelonae | 1 (0.8) | M. chelonae | 1 (0.8) | M. chelonae | 1 (0.8) |
| Total | 127 (100.0) | 127 (100.0) | 127 (100.0) | ||
The other 17 mycobacterial strains were identified as M. fortuitum (7 strains), M. peregrinum (4 strains), M. porcinum (2 strains), M. senegalense (1 strain), and M. mageritense (1 strain) by rpoB and hsp65 sequence analysis. However, two strains could not be identified exactly. One of these two strains was genetically close to M. septicum (rpoB, 98.7%; hsp65, 98.3%), and one was close to M. peregrinum (rpoB, 98.7%; hsp65, 98.3%). By additional 16S rRNA gene and ITS sequence analysis these were finally identified as M. septicum and M. peregrinum (Table 2).
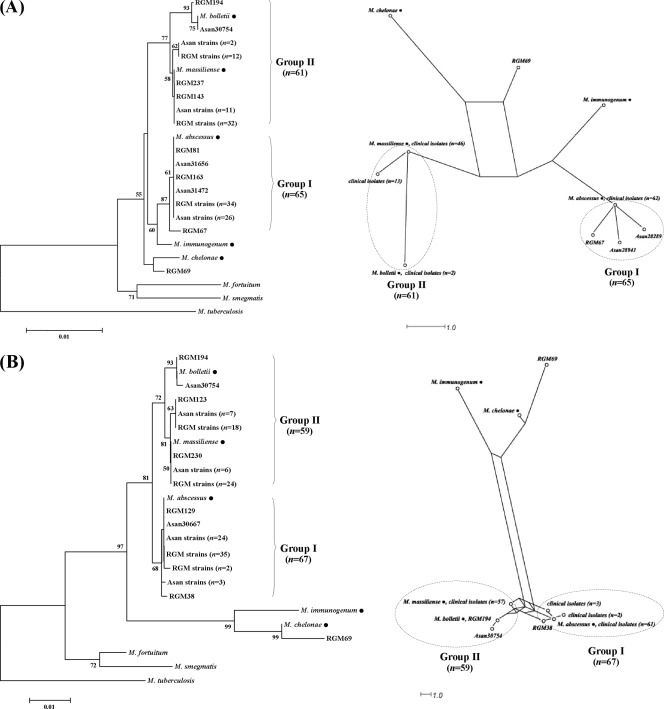
The relationships between tested RGM isolates and the type strains of M. chelonae, M. abscessus, M. immunogenum, M. massiliense, and M. bolletii are shown by the rpoB and hsp65 trees (Fig. 1). The M. chelonae-M. abscessus group formed distinct clusters in both trees, showing their high similarity in terms of rpoB and hsp65 sequences. All the M. abscessus group I and II strains were separated from M. chelonae and M. immunogenum by long branches and formed two clusters (groups I and II in Fig. 1). The M. abscessus type strain and isolates formed group I, whereas M. massiliense and M. bolletii type strains and isolates formed group II. All the strains of hsp65 PRA groups I and II belonged to groups I and II in both trees. The bootstrap values supporting their relationships were high (87 and 98% for each tree).
FIG. 1.
Relationships between the type strains (•) of M. chelonae, M. immunogenum, M. abscessus, M. massiliense, and M. bolletii and 127 Korean M. chelonae-M. abscessus group isolates (Asan, Asan Collection; RGM, Samsung Collection) inferred from partial rpoB (A) and hsp65 sequences (B). Trees were constructed by the neighbor-joining (NJ) method (left) and median network analysis (right). Most of the isolates, which were identified as M. abscessus by hsp65 PRA, are separated into two large groups, groups I and II. Group I includes the type strain and some isolates identified as M. abscessus, whereas group II comprises M. massiliense and M. bolletii type strains and isolates identified as such. Numbers in parentheses stand for isolates with identical sequences. In both NJ trees, M. tuberculosis (rpoB, AF057454; hsp65, AY299144) was used as the out-group to root the tree and the bootstrap values presented at corresponding branches were determined from 1,000 replications; those less than 50% are not indicated.
Colony morphology.
Subcultured colonies of 85 M. chelonae-M. abscessus group isolates (Samsung Collection) were analyzed on blood agar plates. In general, isolates with rough colonies (52 strains, 61.2%) were more frequently isolated than those with smooth colonies (24 strains, 28.2%). The proportions of smooth colonies in M. abscessus and M. massiliense isolates were similar (27.1% and 28.2%, respectively). Most of the isolates showed one of the two types. However, mixed colonies of seven strains (8.2%) of M. abscessus from seven different patients were observed (Table 3).
TABLE 3.
Morphological variation of colonies observed among 85 M. chelonae-M. abscessus group isolates
| Colony morphology | No. (%) of strains
|
||||
|---|---|---|---|---|---|
| M. chelonae | M. abscessus | M. massiliense | M. bolletii | Total | |
| Smooth | 1 (100.0) | 10 (27.0) | 13 (28.3) | 1 (100.0) | 24 (28.2) |
| Rough | 0 (0.0) | 20 (54.1) | 33 (71.7) | 0 (0.0) | 52 (61.2) |
| Mixed | 0 (0.0) | 7 (18.9) | 0 (0.0) | 0 (0.0) | 7 (8.2) |
| Total | 1 (100.0) | 37 (100.0) | 46 (100.0) | 1 (100.0) | 85 (100.0) |
Susceptibility to clarithromycin.
MICs for randomly selected M. abscessus (9 strains), M. massiliense (9 strains), and M. bolletii isolates (2 strains) were compared with those for their type strains. MICs for the 10 M. abscessus strains ranged from 0.25 to 64 μg/ml. Four susceptible, four resistant, and two intermediate strains were determined on the basis of CLSI criteria (10). Three M. bolletii strains were resistant to clarithromycin (MICs, 8 to 16 μg/ml). Interestingly, M. massiliense strains were either markedly susceptible (MICs, 0.125 to 0.5 μg/ml) or highly resistant (MICs, >256 μg/ml) to clarithromycin. There was no intermediate group as for M. abscessus. In addition, three highly resistant M. massiliense strains had a point mutation at the adenine at position 2058 (A2058) or 2059 (A2059) in the peptidyltransferase region of the 23S rRNA gene. However, those mutations were not found in the four clarithromycin-resistant isolates of M. abscessus and two M. bolletii isolates tested in this study (Table 4).
TABLE 4.
Susceptibility of M. chelonae, M. abscessus, and M. bolletii isolates to clarithromycin
| Species (n) | Strain | MIC (μg/ml) | Susceptibilitya | Mutation |
|---|---|---|---|---|
| M. abscessus (10) | ATCC19977T | 2 | S | Nb |
| RGM81 | 0.5 | S | N | |
| RGM109 | 8 | R | N | |
| RGM130 | 4 | I | N | |
| RGM157 | 0.5 | S | N | |
| RGM174 | 4 | I | N | |
| RGM185 | 64 | R | N | |
| RGM220 | 0.25 | S | N | |
| RGM239 | 64 | R | N | |
| RGM240 | 64 | R | N | |
| M. massiliense | CIP 108297T | 0.5 | S | N |
| (10) | RGM65 | 0.125 | S | N |
| RGM70 | 0.25 | S | N | |
| RGM145 | >256 | R | A2058G | |
| RGM234 | 0.5 | S | N | |
| RGM247 | >256 | R | A2059G | |
| RGM250 | 0.25 | S | N | |
| RGM264 | >256 | R | A2058G | |
| RGM270 | 0.25 | S | N | |
| RGM279 | 0.5 | S | N | |
| M. bolletii (3) | CIP 108541T | 8 | R | N |
| Asan30754 | 8 | R | N | |
| RGM231 | 16 | R | N |
S, susceptible; R, resistant; I, intermediate.
N, none.
DISCUSSION
Recently, reports on the rapidly growing mycobacterial infections in various clinical situations have greatly increased. Among the RGM species, the M. chelonae-M. abscessus group is the most common causative agent of soft-tissue infections (7). Though there have been continuous taxonomic changes of M. chelonae and M. abscessus, M. abscessus is not considered a subspecies of M. chelonae (26) but rather a separate species (22). However, another genotype analysis, such as hsp65 PRA (11), and sequence analysis of hsp65 (21) and rpoB (2) again revealed genetic heterogeneity in the M. abscessus population. Furthermore, making things more complex, M. massiliense and M. bolletii were newly reported and are very closely related to M. abscessus (1, 4). Human infections and intracellular survival, which showed their pathogenic potential, were reported (1, 4, 40).
When we used sequence analysis to identify mycobacteria, we also found several RGM isolates that were closely related but could not be identified as M. abscessus or M. chelonae. In addition, after the identification of the causative agent in an epidemic associated with intramuscular injection (19), the possibility that M. massiliense and M. bolletii could have been identified as M. abscessus was raised. By confirming that M. abscessus hsp65 PRA group II strains are M. massiliense by sequence analysis, we could in part simplify the heterogeneous structure of the M. abscessus population in this study. M. massiliense accounted for a large proportion of clinical RGM isolates. Nearly one-half of M. abscessus isolates could not be properly identified prior to the different phenotype and genotype reports on M. massiliense and M. bolletii as new species. The rate of isolation of M. massiliense in South Korea is much higher than that in a recent report (33). It was not evaluated by a nationwide surveillance study. However, because the two groups of clinical isolates used in this study were collected from two different hospitals (Asan and Samsung) where the NTM infections are mainly reported (18, 20, 23), our results may in part represent the proportions of M. chelonae, M. abscessus, M. massiliense, and M. bolletii isolates in South Korea.
In order to manage infectious diseases, it is important to correctly identify the causative agents and to know their susceptibility to antimicrobial agents since different treatment regimens are required for each infectious disease. Although several unique phenotypes that can differentiate M. massiliense and M. bolletii from M. abscessus and M. chelonae have been identified (1, 4, 22), they are not easy to characterize in routine clinical laboratories. In general, molecular methods are preferred over phenotype analysis. For genotype identification of these causative agents, PCR or PCR-linked methods have been used. Although PRA is a rather simple and rapid method, PCR sequencing is more useful for species identification, as shown above. Since the early 1990s, genotype analysis, especially PCR sequencing, has greatly contributed to the classification and identification of mycobacterial species. Nucleotide sequence information for the 16S rRNA genes has been widely used to find and define new species of mycobacteria (31, 35). However, 16S rRNA gene sequence analysis has critical limitations in that the pathogenic M. kansasii cannot be distinguished from nonpathogenic M. gastri. In the first report on M. massiliense, because its 16S rRNA gene sequence was identical to that of M. abscessus, other gene sequences (hsp65, rpoB, and 16S-23S ITS, etc.) were also presented to demonstrate the genotype difference between the two species. Thus, we did not apply 16S rRNA gene sequence analysis in the early stage of our study.
Interestingly, two strains showed discordant results in the rpoB and hsp65 analysis. Two M. massiliense strains based on rpoB analysis were identified as M. abscessus by hsp65 analysis as described in a previous report (33). However, additional gene (sodA and 16S-23S ITS) analyses indicated that these two strains were M. massiliense. In such situations, although these strains may represent a very small proportion (1.4%), complementation with other gene analyses may be useful for precise identification. Either sodA or the 16S-23S ITS may be suitable for this purpose because these are frequently used and there are abundant sequence data.
Aside from the precise identification of M. abscessus and its related species, what led to our interest in these four species was their susceptibility to clarithromycin, which is administered orally. Among the many antimicrobial susceptibility profiles, an especially low MIC of clarithromycin (0.125 μg/ml) was observed for M. massiliense (4, 19). Based on the susceptibility testing, clarithromycin was used to treat patients who suffered injection-associated abscess formation without any combination (19). In addition, M. bolletii was reported to be naturally resistant to clarithromycin (1). Although we tested only 23 strains and more-comprehensive studies are needed, we observed some interesting results. Some of our results were concordant with previous reports in that seven M. massiliense strains, which were in the susceptible range of CLSI criteria, showed very low MICs (0.125 to 0.5 μg/ml). In addition, although only three strains were used, all M. bolletii strains were resistant to clarithromycin (MICs, 8 to 16 μg/ml). However, unlike the three highly resistant (MICs > 256 μg/ml) M. massiliense strains that had a mutation (A2058 or A2059) in the 23S rRNA gene, there was no mutation in resistant M. abscessus and M. bolletii strains. Furthermore, there was an “intermediate” group among M. abscessus strains. These results suggest that there are other mechanisms besides 23S rRNA gene mutation that confer clarithromycin resistance in these strains. Reports of a broad range of MICs (0.125 to 64 μg/ml) and of clarithromycin-resistant M. abscessus (41, 42) might have been brought about, in part, by such heterogeneity in the M. abscessus population, which comprises three strains with different susceptibilities to clarithromycin. Because we randomly selected strains and analyzed only two strains of M. bolletii in this study, we cannot comprehensively know the exact clarithromycin resistance rate of these three species at this moment. In addition, we cannot generalize that all M. bolletii strains are naturally resistant to clarithromycin. Thus, because the susceptibility depends on each strain, every isolate should be subjected to susceptibility testing.
The pathogenicity of M. massiliense was carefully described in an earlier report (4). We also suggest two lines of evidence that M. massiliense is pathogenic. First, 46 M. massiliense isolates of the Samsung Collection were collected from 99 patients over almost 2 years. There were 19 patients who gave repeated culture-positive results (from two to seven times). Although detailed information on these patients is not available and is out of the scope of this study, repeated positive cultures from respiratory specimens fulfill the basic criteria for diagnosis of NTM infection (5). Second, repeated incision and drainage could not eradicate M. massiliense in patients in the “injection-associated epidemic” (19). This reminds us of the intra-amoebal survival and growth of M. massiliense. If this mycobacterium can survive in tissue macrophages, simple curettage may not be sufficient to eradicate it. Intracellular survival and growth can play an important role, as in tuberculosis.
In conclusion, we evaluated the proportion of M. chelonae, M. abscessus, M. massiliense, and M. bolletii strains among Korean M. chelonae-M. abscessus group isolates by PCR sequencing and compared these results to hsp65 PCR-RFLP results. M. massiliense and M. bolletii accounted for the M. abscessus group II strains identified by hsp65 PRA and accounted for 46.5% and 1.6% of the M. chelonae-M. abscessus group strains analyzed, respectively. Repeated positive cultures from the patient's respiratory infection specimens suggest that there are considerable numbers of patients with M. massiliense pulmonary infection, which may be diagnosed as M. abscessus (or M. chelonae-M. abscessus group) infection, in South Korea.
Acknowledgments
This work was supported by a 2007 grant from the SNUH Research fund (04-2007-012-0). H.-Y. Kim, Y.-J. Yun, and C. G. Park were supported by the second stage of the Brain Korea 21 Project.
Footnotes
Published ahead of print on 27 August 2008.
REFERENCES
- 1.Adékambi, T., P. Berger, D. Raoult, and M. Drancourt. 2006. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int. J. Syst. Evol. Microbiol. 56133-143. [DOI] [PubMed] [Google Scholar]
- 2.Adékambi, T., P. Colson, and M. Drancourt. 2003. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J. Clin. Microbiol. 415699-5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adékambi, T., and M. Drancourt. 2004. Dissection of phylogenetic relationships among 19 rapidly growing Mycobacterium species by 16S rRNA, hsp65, sodA, recA and rpoB gene sequencing. Int. J. Syst. Evol. Microbiol. 542095-2105. [DOI] [PubMed] [Google Scholar]
- 4.Adékambi, T., M. Reynaud-Gaubert, G. Greub, M. J. Gevaudan, B. La Scola, D. Raoult, and M. Drancourt. 2004. Amoebal coculture of “Mycobacterium massiliense” sp. nov. from the sputum of a patient with hemoptoic pneumonia. J. Clin. Microbiol. 425493-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Thoracic Society. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156S1-S25. [DOI] [PubMed] [Google Scholar]
- 6.Brantley, J. S., A. L. Readinger, and E. S. Morris. 2006. Cutaneous infection with Mycobacterium abscessus in a child. Pediatr. Dermatol. 23128-131. [DOI] [PubMed] [Google Scholar]
- 7.Brown-Elliott, B. A., and R. J. Wallace, Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15716-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catherinot, E., J. Clarissou, G. Etienne, F. Ripoll, J. F. Emile, M. Daffé, C. Perronne, C. Soudais, J. L. Gaillard, and M. Rottman. 2007. Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infect. Immun. 751055-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1999. Nontuberculous mycobacteria reported to the Public Health Laboratory Information System by state public health laboratories in the United States, 1993-1996. NTM Report 1-51. Centers for Disease Control and Prevention, Atlanta, GA.
- 10.Clinical and Laboratory Standards Institute. 2003. Susceptibility testing of Mycobacteria, Nocardia, and other aerobic actinomycetes. Approved standard M24-A. National Committee for Clinical Laboratory Standards, Wayne, PA. [PubMed]
- 11.Devallois, A., K. S. Goh, and N. Rastogi. 1997. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J. Clin. Microbiol. 352969-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dytoc, M. T., L. Honish, C. Shandro, P. T. Ting, L. Chui, L. Fiorillo, J. Robinson, A. Fanning, G. Predy, and R. P. Rennie. 2005. Clinical, microbiological, and epidemiological findings of an outbreak of Mycobacterium abscessus hand-and-foot disease. Diagn. Microbiol. Infect. Dis. 5339-45. [DOI] [PubMed] [Google Scholar]
- 13.Freudenberger, R. S., and S. M. Simafranca. 2006. Cutaneous infection with rapidly-growing mycobacterial infection following heart transplant: a case report and review of the literature. Transplant. Proc. 381526-1529. [DOI] [PubMed] [Google Scholar]
- 14.Howard, S. T., E. Rhoades, J. Recht, X. Pang, A. Alsup, R. Kolter, C. R. Lyons, and T. F. Byrd. 2006. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 1521581-1590. [DOI] [PubMed] [Google Scholar]
- 15.Huson, D. H., and D. Bryant. 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23254-267. [DOI] [PubMed] [Google Scholar]
- 16.Inderlied, C. B., and G. E. Pfyffer. 2003. Susceptibility test methods: mycobacteria, p. 1149-1177. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 17.Kim, B. J., S. H. Lee, M. A. Lyu, S. J. Kim, G. H. Bai, G. T. Chae, E. C. Kim, C. Y. Cha, and Y. H. Kook. 1999. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB). J. Clin. Microbiol. 371714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, E. K., T. S. Shim, C.-M. Lim, S. D. Lee, Y. Koh, W. S. Kim, W. D. Kim, and D. S. Kim. 2003. Clinical manifestations of pulmonary infection due to rapidly growing nontuberculous mycobacteria. Tuberc. Respir. Dis. 54283-294. [Google Scholar]
- 19.Kim, H. Y., Y. J. Yun, C. G. Park, D. H. Lee, Y. K. Cho, B. J. Park, S. I. Joo, E. C. Kim, Y. J. Hur, B. J. Kim, and Y. H. Kook. 2007. Outbreak of Mycobacterium massiliense infection associated with intramuscular injections. J. Clin. Microbiol. 453127-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh, W. J., O. J. Kwon, K. Jeon, T. S. Kim, K. S. Lee, Y. K. Park, and G. H. Bai. 2006. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 129341-348. [DOI] [PubMed] [Google Scholar]
- 21.König, B., I. Tammer, V. Sollich, and W. König. 2005. Intra- and interpatient variability of the hsp65 and 16S-23S intergenic gene region in Mycobacterium abscessus strains from patients with cystic fibrosis. J. Clin. Microbiol. 433500-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusunoki, S., and T. Ezaki. 1992. Proposal of Mycobacterium peregrinum sp. nov., nom. rev., and elevation of Mycobacterium chelonae subsp. abscessus (Kubica et al.) to species status: Mycobacterium abscessus comb. nov. Int. J. Syst. Bacteriol. 42240-245. [DOI] [PubMed] [Google Scholar]
- 23.Lee, H. W., M.-N. Kim, T. S. Shim, G. H. Bai, and C. H. Pai. 2002. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients. Tuberc. Respir. Dis. 53173-182. [Google Scholar]
- 24.Lee, W. J., T. W. Kim, K. B. Shur, B. J. Kim, Y. H. Kook, J. H. Lee, and J. K. Park. 2000. Sporotrichoid dermatosis caused by Mycobacterium abscessus from a public bath. J. Dermatol. 27264-268. [DOI] [PubMed] [Google Scholar]
- 25.Meier, A., P. Kirschner, B. Springer, V. A. Steingrube, B. A. Brown, R. J. Wallace, Jr., and E. C. Böttger. 1994. Identification of mutations in 23S rRNA gene of clarithromycin-resistant Mycobacterium intracellulare. Antimicrob. Agents. Chemother. 38381-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metchock, B., F. S. Nolte, and R. J. Wallace, Jr. 1999. Mycobacterium, p. 399-437. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, DC.
- 27.Oh, W. S., K. S. Ko, J. H. Song, M. Y. Lee, S. Y. Ryu, S. Taek, K. T. Kwon, J. H. Lee, K. R. Peck, and N. Y. Lee. 2005. Catheter-associated bacteremia by Mycobacterium senegalense in Korea. BMC Infect. Dis. 5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrini, B. 2006. Non-tuberculous mycobacterial infections. Scand. J. Infect. Dis. 38246-255. [DOI] [PubMed] [Google Scholar]
- 29.Pulcini, C., E. Vandenbussche, I. Podglajen, W. Sougakoff, C. Truffot-Pernot, A. Buu-Hoï, E. Varon, and J. L. Mainardi. 2006. Hip prosthesis infection due to Mycobacterium wolinskyi. J. Clin. Microbiol. 443463-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth, A., U. Reischl, A. Streubel, L. Naumann, R. M. Kroppenstedt, M. Habicht, M. Fischer, and H. Mauch. 2000. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S-23S rRNA gene spacer and restriction endonucleases. J. Clin. Microbiol. 381094-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schröder, K. H., L. Naumann, R. M. Kroppenstedt, and U. Reischl. 1997. Mycobacterium hassiacum sp. nov., a new rapidly growing thermophilic mycobacterium. Int. J. Syst. Bacteriol. 4786-91. [DOI] [PubMed] [Google Scholar]
- 32.Sermet-Gaudelus, I., M. Le Bourgeois, C. Pierre-Audigier, C. Offredo, D. Guillemot, S. Halley, C. Akoua-Koffi, V. Vincent, V. Sivadon-Tardy, A. Ferroni, P. Berche, P. Scheinmann, G. Lenoir, and J. L. Gaillard. 2003. Mycobacterium abscessus and children with cystic fibrosis. Emerg. Infect. Dis. 91587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmon, K. E., J. I. Pounder, J. N. Greene, F. Walsh, C. M. Anderson, S. Cohen, and C. A. Petti. 2007. Identification of an emerging pathogen, Mycobacterium massiliense, by rpoB sequencing of clinical isolates collected in the United States. J. Clin. Microbiol. 451978-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song, J. Y., J. W. Sohn, H. W. Jeong, H. J. Cheong, W. J. Kim, and M. J. Kim. 2006. An outbreak of post-acupuncture cutaneous infection due to Mycobacterium abscessus. BMC Infect. Dis. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Springer, B., E. C. Böttger, P. Kirschner, and R. J. Wallace, Jr. 1995. Phylogeny of the Mycobacterium chelonae-like organism based on partial sequencing of the 16S rRNA gene and proposal of Mycobacterium mucogenicum sp. nov. Int. J. Syst. Bacteriol. 45262-267. [DOI] [PubMed] [Google Scholar]
- 36.Springer, B., L. Stockman, K. Teschner, G. D. Roberts, and E. C. Böttger. 1996. Two-laboratory collaborative study on identification of mycobacteria: molecular versus phenotypic methods. J. Clin. Microbiol. 34296-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 241596-1599. [DOI] [PubMed] [Google Scholar]
- 38.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Böttger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukamura, M., N. Kita, H. Shimoide, H. Arakawa, and A. Kuze. 1988. Studies on the epidemiology of nontuberculous mycobacteriosis in Japan. Am. Rev. Respir. Dis. 1371280-1284. [DOI] [PubMed] [Google Scholar]
- 40.Viana-Niero, C., K. V. Lima, M. L. Lopes, M. C. Rabello, L. R. Marsola, V. C. Brilhante, A. M. Durham, and S. C. Leão. 2008. Molecular characterization of Mycobacterium massiliense and Mycobacterium bolletii in isolates collected from outbreaks of infections after laparoscopic surgeries and cosmetic procedures. J. Clin. Microbiol. 46850-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallace, R. J., Jr., A. Meier, B. A. Brown, Y. Zhang, P. Sander, G. O. Onyi, and E. C. Böttger. 1996. Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacterium abscessus. Antimicrob. Agents. Chemother. 401676-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang, S. C., P. R. Hsueh, H. C. Lai, L. J. Teng, L. M. Huang, J. M. Chen, S. K. Wang, D. C. Shie, S. W. Ho, and K. T. Luh. 2003. High prevalence of antimicrobial resistance in rapidly growing mycobacteria in Taiwan. Antimicrob. Agents Chemother. 471958-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]