Abstract
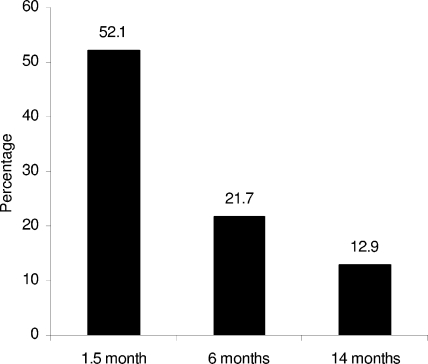
Serial nasal swabs were collected at the ages of 1.5, 6, and 14 months from 443 infants in the Generation R Study. The objective was to study the dynamics and determinants of Staphylococcus aureus nasal carriage in the first year of life. The prevalence of S. aureus carriage decreased in the first year of life, from 52.1% at the age of 1.5 months to 12.9% at 14 months. Persistent carriage, defined as continuous carriage of the same S. aureus strain at the three sampling moments, was rarely detected in early infancy.
Staphylococcus aureus is a human commensal as well as a cause of a wide range of infections (5, 8, 19). A significant fraction of the human population is colonized with S. aureus on epithelial surfaces, of which the anterior nares are the most frequent carriage sites (3, 11, 13, 19, 20). Longitudinal studies distinguish three carriage patterns among healthy adult individuals (1, 6, 15, 18, 21). Persistent carriage occurs in about 20% of the adult population, 30% are intermittent carriers, and 50% of individuals are noncarriers (10, 13, 15, 19). Persistent carriers usually carry the same strain for extended periods of time, whereas intermittent carriers tend to host different strains over time (13, 18, 19).
Children and adolescents under 20 years of age seem to have higher persistent carriage rates than adults (1, 4, 14, 19). Ten percent of children from 0 to 9 years old and 24% of children from 10 to 19 years old were found to be persistent carriers (1). The highest S. aureus carriage rate was observed in infants aged 3 months or younger (17).
Several determinants have been suggested to influence the carriage rate in healthy children. The number of older siblings, family size (fewer than five v. five or more people), breastfeeding, and passive smoking were found to be associated with S. aureus nasal carriage (2, 16).
The objective of the present investigation was to study the dynamics of S. aureus nasal carriage, as well as its human and microbial determinants, in the first year of life.
This project was embedded in the Generation R Study, a population-based prospective cohort study of pregnant women and their children from fetal life onward. Detailed assessments of fetal and postnatal growth and development were conducted with 1,232 pregnant Dutch women and their children. Of all approached pregnant women and their partners, 79% participated. All children were born between February 2003 and August 2005.
The Medical Ethics Committee of the Erasmus Medical Center, Rotterdam, The Netherlands, has approved the study. Written, informed consent was obtained from all participants . In total, 1,232 women were enrolled in the focus cohort study during pregnancy. Three infants died perinatally. The remaining mothers gave birth to 1,244 infants, of whom 138 were not included in the cohort of analysis as the consent was withdrawn after birth. Twins (n = 27) were excluded from this analysis since they are related, leaving 1,079 infants in the group of postnatal participants. The infants visited the Generation R focus study research center at the ages of 1.5 months (n = 884), 6 months (n = 882), and 14 months (n = 863). During these visits, 627 infants had swabs taken at 1.5 months, 832 at 6 months, and 757 at 14 months; 758 infants attended all visits, and 443 provided us with three swabs each to use for longitudinal analysis. Infants with antibiotic usage in the preceding 48 h were excluded from nasal sampling (7, 9).
Trained research nurses obtained a nasal swab (Dacron swabs; Copan, Brescia, Italy) for S. aureus isolation from each infant at each visit. Nasal samples were taken using sterile transport swabs suitable for isolating aerobes and anaerobes. Each swab was rubbed gently through both nostrils, transported in Amies transport medium to the medical microbiology laboratory of the Erasmus Medical Center within 6 h of sampling, put directly in phenol red mannitol broth, and kept at 35°C for 5 days. Material from tubes that turned yellow was plated on a blood agar plate with 5% sheep blood for 1 day at 35°C to isolate S. aureus. Infants with antibiotic usage in the preceding 48 h were excluded from nasal sampling (n = 0).
S. aureus strains from samples from infants who were positive two or more times were genotyped using pulsed-field gel electrophoresis (PFGE). The plugs for PFGE were prepared in a 1% low-melting-point agarose gel and kept for 3 to 4 h at 37°C in the presence of lysostaphin. The plugs were deproteinized using proteinase K. One-sixth of a plug was then put into restriction buffer and incubated for 4 hours with the endonuclease SmaI (50 U). After digestion of the DNA, PFGE, performed using a Chef Mapper (Bio-Rad, Veenendaal, The Netherlands), was used to separate the DNA into fragments in a 1% agarose gel at 14°C. The gels were stained for 30 min with ethidium bromide in distilled water and photographed. A dendrogram was made using BioNumerics (Applied Maths, Belgium) to visualize strain relatedness.
Information related to determinants of carriage was obtained from midwives, hospital registries (gender, birth weight, and gestational age), and parent-retrieved questionnaires at the infant ages of 6 and 12 months (breast-feeding, educational level of the mother, maternal smoking [pre- and postpartum], day-care attendance, and presence of siblings).
Multinomial logistic regression analysis was performed to report on the association of S. aureus carriage patterns with gender, birth weight, gestational age, breast-feeding, educational level of the mother, maternal smoking (pre- and postpartum), day-care attendance, and presence of siblings. We used all variables as determinants of longitudinal carriage and confirmed independence by adjusting for each variable with multivariate multinomial logistic regression analysis. The statistical analyses were performed using the Statistical Package of Social Sciences version 11.0 for Windows (SPSS Inc, Chicago, IL).
Of all the mothers of the noncolonized infants, 223 (63.9%) attended institutions of higher education compared to 63 (70.8%) mothers of the colonized infants. The median birth weights were 3,580 g (5 to 95% range, 2,751 to 4,305 g) for the noncolonized infants and 3,520 g (5 to 95% range, 2,385 to 4,605 g) for the colonized infants. The median gestational ages were the same for the noncolonized and colonized infants at 40.3 weeks. Of the noncolonized infants, 170 (48.0%) were male compared to 55 (61.8%) in the colonized group. Of the noncolonized infants, 39.5% (n = 131) had at least one sibling compared to 40.9% (n = 36) of the colonized group. Of all the infants in the noncolonized group, 30.9% (n = 106) received breast-feeding up until the age of 6 months compared to 36.4% (n = 32) in the colonized group. Two hundred thirty-one (77.5%) noncolonized infants attended day care in the first year of life compared to 54 (68.4%) of the colonized infants (Table 1).
TABLE 1.
Determinants of Staphylococcus aureus carriage in the first year of life
| Parameter | Value for infantsa:
|
|||
|---|---|---|---|---|
| Not colonized (0-1) (n = 354) | Colonized (≥2) (n = 89) | OR (95% CI) | aOR (95% CI) | |
| Gender | ||||
| Female | 184 (52.0) | 34 (38.2) | 1.00 | 1.00 |
| Male | 170 (48.0) | 55 (61.8) | 1.75 (1.09-2.82) | 2.09 (1.17-3.72) |
| Gestational age (mo) | 40.3 (37.7-42.0) | 40.3 (37.0-42.4) | 0.91 (0.78-1.06) | 0.94 (0.76-1.17) |
| Birth wt (g) | 3,580 (2,751-4,305) | 3,520 (2,385-4,605) | 1.00 (1.00-1.00) | 1.00 (1.00-1.00) |
| Breast-feeding at 6 mo | ||||
| No | 237 (69.1) | 56 (63.6) | 1.00 | 1.00 |
| Yes | 106 (30.9) | 32 (36.4) | 1.28 (0.78-2.09) | 1.36 (0.75-2.47) |
| Mother's educational level | ||||
| Higher education | 223 (63.9) | 63 (70.8) | 1.00 | 1.00 |
| Lower/intermediate education | 126 (36.1) | 26 (29.2) | 0.73 (0.44-1.21) | 0.52 (0.26-1.05) |
| Mother's prenatal smoking | ||||
| No | 319 (94.4) | 79 (94) | 1.00 | 1.00 |
| Yes | 19 (5.6) | 5 (6) | 1.06 (0.34-2.93) | 5.35 (0.86-33.40) |
| Mother's postnatal smoking | ||||
| No | 260 (87.8) | 70 (88.6) | 1.00 | 1.00 |
| Yes | 36 (12.2) | 9 (11.4) | 0.93 (0.43-2.02) | 0.25 (0.05-1.33) |
| Siblings | ||||
| No | 201 (60.5) | 52 (59.1) | 1.00 | 1.00 |
| Yes | 131 (39.5) | 36 (40.9) | 1.06 (0.66-1.71) | 1.03 (0.57-1.87) |
| Day-care attendance | ||||
| No | 67 (22.5) | 25 (31.6) | 1.00 | 1.00 |
| Yes | 231 (77.5) | 54 (68.4) | 0.63 (0.36-1.08) | 0.53 (0.27-1.01) |
Values are given as number (%) of infants unless indicated otherwise. Values are means or medians (5 to 95% range) for variables with skewed distribution. A total of 443 infants provided nasal swabs at all three collection moments. Data were missing on breast-feeding (n = 12), mother's educational level (n = 5), mother's prenatal smoking (n = 21), mother's postnatal smoking (n = 68), siblings (n = 23), and day-care attendance (n = 66).
The prevalence of S. aureus carriage significantly decreased from 52.1% (231 of 443 infants) at the age of 1.5 months to 21.7% (96 of 443 infants) at the age of 6 months and 12.9% (57 of 443 infants) at the age of 14 months (P < 0.001) (Fig. 1).
FIG. 1.
S. aureus carriage in the first year of life. We found a significant decrease in S. aureus nasal carriage in the first year of life. The P value is <0.001 for the difference between S. aureus carriage rates at 1.5, 6, and 14 months of age.
One group of infants, 36.3% (161 of 443), was never found positive for S. aureus; 2.9% (13 of 443) were found positive at all three collection moments. The largest group (43.6%; 193 of 443) consisted of infants with one positive swab. Seventy-six (17.2%) infants had two nasal swabs test positive for S. aureus.
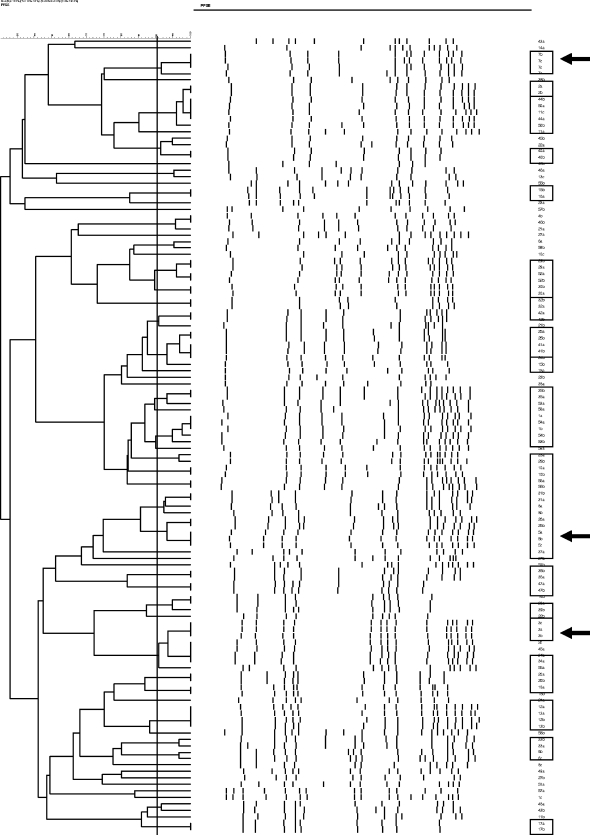
We genotyped the S. aureus strains from infants with two or more positive swabs. All three strains were available for further research for 10 of 13 infants who were found positive at all three collection moments (three samples were missing: one lab number was missing, one swab was lost, and one sample did not grow properly). Only 3 of these 10 infants seemed to carry the same S. aureus genotype over time; 6 carried two different strains, leaving 1 infant with three different strains for each of the three swabs. We genotyped the strains from 45 infants with two positive swabs in a row, of whom 29 (63%) carried the same strain. We did not observe large genetic clusters of S. aureus; rather, we observed a great variety of genotypes (Fig. 2).
FIG. 2.
S. aureus dendrogram. PFGE analysis was performed for all strains derived from infants with two or more cultures that tested positive for S. aureus. The banding patterns are shown in the central portion of the figure. On the left, the percentage of strain relatedness is indicated in the form of a BioNumerics-generated dendrogram. A cut-off percentage of 90% (vertical solid line) is used to identify similar to identical strains. Strains meeting this criterion and derived from the same infant are indicated by boxing in the right column, which identifies children by study number and by the first (a), second (b), and third (c) culture moment. In three cases, the strains isolated at the three culture moments were identical (arrow).
Boys have a significantly higher risk than girls to be positive two or more times (adjusted odds ratio [aOR], 2.09; 95% confidence interval [CI], 1.17 to 3.72; P value, 0.012). We did not find a significant association between S. aureus carriage and the presence of siblings (aOR, 1.03; 95% CI, 0.57 to 1.87; P value, 0.919) or with breast-feeding, day-care attendance, maternal smoking (pre- and postpartum), birth weight, or gestational age (Table 1).
We documented a significant decrease in the prevalence of S. aureus carriage in the first year of life (P < 0.001), which is in line with literature data (2, 16). A possible explanation for this drop may be found in the competition between S. aureus and Streptococcus pneumoniae. Bogaert et al. found an inverse prevalence of these pathogens in slightly older children; the same could occur in young infants (2). In our cohort, Labout et al. found an increased level of pneumococcal carriage in the first year of life (12). The significant decrease in the prevalence of S. aureus carriage in the first year of life might be explained by pneumococcal competition or bacterial interference with other organisms present in the nasopharynges of these children (unpublished data).
In our study, persistent nasal carriage as defined by bacterial genotyping of S. aureus is extremely rare in infancy. In the small group of infants with two or more positive swabs in a row, we rarely found infants carrying the same strain over time. Previous studies show a higher prevalence of persistent carriage among older children and adolescents up to the age of 20 than among adults (1, 4, 14). However, we studied infants in the first year of life and found the majority of them to be intermittent carriers. The apparent close match between pathogen and host as documented for adult persistent carriers may be an explanation as to why there are barely any persistent carriers among infants: the optimal match between pathogen and host may still be absent. Extensive staphylococcal dynamics seems to occur in the nasal cavity of infants, with staphylococcal elimination rather than acquisition being the main feature. In adults, by contrast, persistent carriers host the same strain over time by definition. Redefining the nature of carriage may be necessary to describe the dynamics in the anterior nares during infancy.
Of the 45 infants with two positive swabs in a row, 29 (63%) carried the same strain. This suggests that active colonization with a new genotype during the first year happens less frequently among these infants. This rather high frequency of 63% in the infants might also be explained by recolonization with the strain from the mother or other family members, who might be persistent carriers in 20% of the cases (10, 19).
The main difference between our findings and the studies on determinants of carriage by Bogaert et al. and Peacock et al. is our failure to identify family size, passive smoking, or breast-feeding as significant determinants of carriage (2, 16). However, our data on breast-feeding seems to be more precise than those of the earlier studies, with very little missing data; furthermore, our data cover a larger cohort of children in the same age group (first year of life) than do the two previously mentioned studies.
We conclude that S. aureus carriage among young infants is clearly different from that among adults. Long-term persistent carriage rarely occurs among infants, and the incidence of carriage drops enormously in the first year of life. Whether these differences are a result of immunomodulation or other biological phenomena is subject to further investigation.
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Center, Rotterdam, The Netherlands, in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, the Rotterdam Homecare Foundation, and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contributions of general practitioners, hospitals, midwives, and pharmacies in Rotterdam. We thank Ad Luijendijk for technical supervision at the Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam. Héléne Boelens and Deborah Kreft from the same department are acknowledged for support with PFGE analyses and the BioNumerics calculations.
The first phase (until the last child turns 4 years old) of the Generation R Study was made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam, and The Netherlands Organization for Health Research and Development (Zon Mw).
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Armstrong-Esther, C. A. 1976. Carriage patterns of Staphylococcus aureus in a healthy non-hospital population of adults and children. Ann. Hum. Biol. 3221-227. [DOI] [PubMed] [Google Scholar]
- 2.Bogaert, D., A. van Belkum, M. Sluijter, A. Luijendijk, R. de Groot, H. C. Rumke, H. A. Verbrugh, and P. W. Hermans. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 3631871-1872. [DOI] [PubMed] [Google Scholar]
- 3.Casewell, M. W., and R. L. Hill. 1986. Elimination of nasal carriage of Staphylococcus aureus with mupirocin (′pseudomonic acid')—a controlled trial. J. Antimicrob. Chemother. 17365-372. [DOI] [PubMed] [Google Scholar]
- 4.Cunliffe, A. C. 1949. Incidence of Staphylococcus aureus in the anterior nares of healthy children. Lancet ii411-414. [DOI] [PubMed] [Google Scholar]
- 5.El Helali, N., A. Carbonne, T. Naas, S. Kerneis, O. Fresco, Y. Giovangrandi, N. Fortineau, P. Nordmann, and P. Astagneau. 2005. Nosocomial outbreak of staphylococcal scalded skin syndrome in neonates: epidemiological investigation and control. J. Hosp. Infect. 61130-138. [DOI] [PubMed] [Google Scholar]
- 6.Eriksen, N. H., F. Espersen, V. T. Rosdahl, and K. Jensen. 1995. Carriage of Staphylococcus aureus among 104 healthy persons during a 19-month period. Epidemiol. Infect. 11551-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofman, A., V. W. Jaddoe, J. P. Mackenbach, H. A. Moll, R. F. Snijders, E. A. Steegers, F. C. Verhulst, J. C. Witteman, and H. A. Büller. 2004. Growth, development and health from early fetal life until young adulthood: the Generation R Study. Paediatr. Perinat. Epidemiol. 1861-72. [DOI] [PubMed] [Google Scholar]
- 8.Iwatsuki, K., O. Yamasaki, S. Morizane, and T. Oono. 2006. Staphylococcal cutaneous infections: invasion, evasion and aggression. J. Dermatol. Sci. 42203-214. [DOI] [PubMed] [Google Scholar]
- 9.Jaddoe, V. W., J. P. Mackenbach, H. A. Moll, E. A. Steegers, H. Tiemeier, F. C. Verhulst, J. C. Witteman, and A. Hofman. 2006. The Generation R Study: design and cohort profile. Eur. J. Epidemiol. 21475-484. [DOI] [PubMed] [Google Scholar]
- 10.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kluytmans, J. A., and H. F. Wertheim. 2005. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 333-8. [DOI] [PubMed] [Google Scholar]
- 12.Labout, J. A., L. Duijts, L. Arends, V. W. Jaddoe, A. Hofman, R. de Groot, P. W. M. Hermans, and H. A. Moll. Risk factors for pneumococcal carriage in healthy Dutch infants. The Generation R Study. J. Pediatr., in press. [DOI] [PubMed]
- 13.Moss, B., J. R. Squire, and E. Topley. 1948. Nose and skin carriage of Staphylococcus aureus in patients receiving penicillin. Lancet 251320-325. [DOI] [PubMed] [Google Scholar]
- 14.Noble, W. C., H. A. Valkenburg, and C. H. Wolters. 1967. Carriage of Staphylococcus aureus in random samples of a normal population. J. Hyg. 65567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nouwen, J. L., A. Ott, M. F. Kluytmans-Vandenbergh, H. A. Boelens, A. Hofman, A. van Belkum, and H. A. Verbrugh. 2004. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule.” Clin. Infect. Dis. 39806-811. [DOI] [PubMed] [Google Scholar]
- 16.Peacock, S. J., A. Justice, D. Griffiths, G. D. I. de Silva, M. N. Kantzanou, D. Crook, K. Sleeman, and N. P. J. Day. 2003. Determinants of acquisition and carriage of Staphylococcus aureus in infancy. J. Clin. Microbiol. 415718-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regev-Yochay, G., R. Dagan, M. Raz, Y. Carmeli, B. Shainberg, E. Derazne, G. Rahav, and E. Rubinstein. 2004. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA 292716-720. [DOI] [PubMed] [Google Scholar]
- 18.VandenBergh, M. F. Q., E. P. F. Yzerman, A. van Belkum, H. A. M. Boelens, M. Sijmons, and H. A. Verbrugh. 1999. Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. J. Clin. Microbiol. 373133-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wertheim, H. F., D. C. Melles, M. C. Vos, W. van Leeuwen, A. van Belkum, H. A. Verbrugh, and J. L. Nouwen. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5751-762. [DOI] [PubMed] [Google Scholar]
- 20.Williams, R. E. 1946. Skin and nose carriage of bacteriophage types of Staphylococcus aureus. J. Pathol. Bacteriol. 58259-268. [DOI] [PubMed] [Google Scholar]
- 21.Williams, R. E. O. 1963. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol. Rev. 2756-71. [DOI] [PMC free article] [PubMed] [Google Scholar]