Abstract
Infections with human parechoviruses (HPeVs) are prevalent in young children and have been associated with mild gastroenteritis and, less frequently, with meningitis and neonatal sepsis. To investigate the involvement of these viruses in respiratory disease, a highly sensitive nested PCR was used to screen a large archive of respiratory specimens, collected between January and December 2007. Respiratory samples had previously been tested for eight respiratory viruses, including respiratory syncytial virus and adenovirus, by PCR. HPeV was detected in 34 of 3,844 specimens, representing 27 of 2,220 study subjects (1.2%). HPeV types were identified by sequencing the VP3/VP1 junction amplified by PCR directly from clinical specimens. The assay could amplify all HPeV types examined with high sensitivity (types 1 and 3 to 6) and also identified HPeV types in all but one of the screen-positive study specimens (25 HPeV1 and eight HPeV6 specimens). Infections with both HPeV1 and HPeV6 were seasonal, with highest frequencies in July and August, and restricted to children aged between 6 months and 5 years. Other respiratory viruses were frequently codetected in HPeV-positive specimens, with significant overrepresentation of adenovirus coinfections (37%). Most HPeV-positive specimens were referred from emergency departments, although no association with specific respiratory symptoms or disease was found. In summary, the low frequency of detection and lack of clear disease associations indicate that HPeV1 and -6 are not major pathogens in individuals presenting with respiratory disease. However, the screening and typing methods developed will be of value in further HPeV testing, including testing for meningitis cases and other suspected HPeV-associated disease presentations.
Human parechoviruses (HPeVs) are single-stranded, positive-sense RNA viruses in the Parechovirus genus within the large family of Picornaviridae. The prototype strains, HPeV1 and -2, were previously designated echovirus 22 and 23 in the Enterovirus genus but were renamed and reclassified into their own genus in 1999 based on evident differences in genome organization and structure, as well as divergence of encoded proteins and biological properties (17, 27). Although these first HPeV types were discovered during a summer diarrhea outbreak in children over 50 years ago (31), it was only recently that four additional HPeV types were discovered. To date, HPeV3, -4, -5, and -6 have been identified in Japan, The Netherlands, the United States, Canada, and Germany (3, 4, 6, 18). HPeV infections are commonly observed to occur in the general population; at least 95% of the adult population are seropositive for HPeV1-specific antibodies (20, 28). In addition, it has been shown that HPeV infections are often acquired in early childhood, with the median age of acquiring HPeV1 infection being 18 months and with 20% of children being infected after the first year of life (28).
HPeV1 infections have generally been associated with mild gastrointestinal and respiratory infections (13, 15, 31), as well as with occasional cases of necrotizing enterocolitis (10), encephalitis (21), and Reye's syndrome (30). Although HPeV2 has been associated mostly with gastroenteritis, these infections seem to be very rare. In addition to HPeV2, HPeV6 has been suggested as another causative agent of children's diarrhea (4). An association between HPeV3 infection and sepsis-like illness, including central nervous system involvement in neonates, has recently been established (1, 7, 11), whereas the role of HPeV4 and -5 as human pathogens is still poorly understood (6, 12).
Although HPeVs are frequently isolated from patients with respiratory infection and are thought to be associated with disease (13, 15), HPeVs are not yet commonly included in respiratory screening. Currently, PCR-based screening for well-established pathogenic viruses, including respiratory syncytial virus (RSV), parainfluenza viruses 1 to 3 (PIV1 to PIV3), influenza A and B viruses (FLUA and FLUB, respectively), adenovirus (AdV), and, more recently, human metapneumovirus (HMPV) and human bocavirus (HBoV) (a new parvovirus), detects virus infection in a majority of often-severe respiratory infections (19). However, a substantial proportion of viral respiratory infections are thought to remain undiagnosed, not least because several other viruses associated with upper and lower respiratory tract (URT and LRT, respectively) infections, including coronaviruses, enteroviruses, and rhinoviruses, are frequently unscreened.
To investigate the involvement of HPeV in respiratory disease, referred diagnostic respiratory samples from 2007 in Scotland were screened for HPeV by nested reverse transcription-PCR (RT-PCR). The results of diagnostic testing for other viruses and referral and epidemiological information recorded for the samples were combined to enable a comparison of the epidemiologies and clinical associations. As a part of this study, a highly sensitive HPeV typing method, which can be applied directly to clinical specimens rather than to cultured virus, was developed. This assay provides the starting point for future investigations of potential epidemiological and clinical differences between HPeV types.
MATERIALS AND METHODS
Test specimens.
The study was based on a total of 4,173 respiratory samples referred to the Specialist Virology Centre (SVC), Royal Infirmary of Edinburgh, for respiratory virus testing from 1 January to 31 December 2007. The specimens comprised the following: 2,259 nasopharyngeal swabs or aspirates, 1,317 nose and/or throat swabs, 201 tracheal swabs or aspirates, 153 sputum samples, 152 bronchoalveolar lavage specimens, 41 bronchial washings, and 50 “other” types recorded. Routine respiratory virus detection was composed of nested PCR for RSV, FLUA, FLUB, PIV1 to PIV3, and AdV based on previously described assays (16, 29). HPeV-positive samples were additionally screened for HBoV as previously described (22) but using a “detuned” single-round PCR protocol capable of detecting only high-viral-load samples, as recently advocated (2), and for HMPV by nested PCR using primers for the F gene (E. Gaunt, K. Templeton, and P. Simmonds, unpublished data).
All samples were anonymized and deposited in the SVC respiratory sample archive prior to testing. Approval was obtained from the Lothian Regional Ethics Committee (08/S11/02/2) to retain information during the anonymization process for epidemiological purposes while strictly protecting patient confidentiality. The stored data included age band, partial postcode, any recorded symptoms or clinical information, referral source, month of sample collection, and results of other virological testing of the sample.
HPeV1 and -3 isolates cultured on Vero cells were used as assay controls. A proficiency panel was obtained from Quality Control on Molecular Diagnostics (QCMD, Glasgow, United Kingdom). In addition, 21 previously typed clinical isolates (13 HPeV1, four HPeV3, two HPeV4, one HPeV5, and one HPeV6 isolate) were investigated using a modification of a previously described VP1-based PCR method (8), along with 26 nontypeable samples positive by 5′ untranslated region (5′ UTR) screening but negative by VP1 PCR.
HPeV detection.
RNA was extracted from 100 μl of clinical specimens by using a MinElute virus spin kit according to the manufacturer's instructions (Qiagen, Hemel Hempstead, United Kingdom). Extracted RNA was stored at −70°C. A nested RT-PCR method was used for HPeV screening, initially carried out in pools of 10, with positive pools split into individual components and retested to identify positive samples.
RNA from pools or individual samples was reverse transcribed into cDNA by use of a reverse transcription system (Promega, United Kingdom). The RT step was performed in a reaction volume of 20 μl per the manufacturers' instructions but using 5 μl of the extracted RNA. The reaction mixtures were incubated at room temperature for 10 min and at 42°C for 60 min and then heated to 95°C for 5 min and placed on ice for 5 min. cDNA was either used directly for PCR or stored frozen at −20°C.
The primers which target the highly conserved 5′ UTR are as follows (with the positions of the HPeV1 prototype strain [L02971] in parentheses): outer sense and antisense primers 5′GGGTGGCAGATGGCGTGCCATAA (253) and 5′CCTRCGGGTACCTTCTGGGCATCC (583) and inner sense and antisense primers 5′YCACACAGCCATCCTCTAGTAAG (313) and 5′GTGGGCCTTACAACTAGTGTTTG (556). Amplification was performed using 0.4 U of GoTaq Taq polymerase (Promega, United Kingdom) and the manufacturers' amplification buffer containing a final concentration of 1.5 mM MgCl2, 0.5 μM primers, and 30 μM deoxynucleoside triphosphates in a 20-μl reaction volume for both rounds of PCR. In the first-round PCR, 2 μl of cDNA was amplified using the following parameters: 30 cycles of 18 s at 94°C, 21 s at 50°C, and 90 s at 72°C, combined with a final extension of 5 min. One microliter from the first-round PCR was used as the template in a second round of PCR and amplified using the same cycling parameters, except that the annealing step was performed at 55°C. PCR products were run on 2% agarose gels prestained with ethidium bromide to identify positive samples with a predicted size of 243 bp. Positive pools were split and the above-described nested RT-PCR was repeated to identify individual positive samples. To evaluate assay sensitivity, HPeV samples on enterovirus proficiency panel 2008 from QCMD were tested.
Parechovirus typing.
For parechovirus typing, samples positive by the 5′ UTR PCR were amplified using primers from the VP3/VP1 junction region and a SuperScript III one-step RT-PCR kit (Invitrogen, United Kingdom) according to the manufacturer's instructions. Primers, with the positions in relation to L02971 shown in parentheses, were outer sense and antisense primers 5′GAYAATGCYATMTAYACWATYTGTGA (2090) and 5′ACWGTRAARATRTCHACATTSATDG (2523) and inner sense and antisense primers 5′TTYTCMACHTGGATGMGGAARAC (2159) and 5′DGGYCCATCATCYTGWGCTGA (2458).
Each combined RT-PCR was carried out in a 20-μl reaction volume of the manufacturers' supplied buffer (containing 0.4 mM deoxynucleoside triphosphates and 2.4 mM MgSO4), 6 μl of extracted RNA, 0.8 μl of Superscript III RT/Platinum Taq polymerase, and 0.5 μM of outer VP3/VP1 primers. The RT and PCR cycling conditions for the first round were, sequentially, 43°C for 1 h and 20 cycles of 53°C (1 min) and 55°C (1 min), followed by 70°C for 15 min and 94°C for 2 min. PCR comprised 40 cycles of 94°C (30 s), 50°C (30 s), and 68°C (105 s) and a final extension at 68°C for 5 min. One microliter of the product was transferred for a second PCR, which was performed as described for the 5′ UTR PCR but with the VP3/VP1 inner primers. The final product of 304 bp was visualized by agarose gel electrophoresis as described above.
Amplified DNA was directly sequenced using a BigDye Terminator kit (Applied Biosystems, Warrington, United Kingdom) in both directions, from which a complete sequence between positions 2182 and 2437 was assembled. Type identification was achieved by phylogenetic analysis of nucleotide and inferred amino acid sequences using a data set containing sequences from published HPeV variants of previously assigned HPeV types.
Nucleotide sequence accession numbers.
Nucleotide sequences from the VP3/VP1 region have been assigned the GenBank accession numbers FJ009260 to FJ009290.
RESULTS
Study group.
A total of 3,844 respiratory samples (83% nasopharyngeal swabs or aspirates) from 2,200 different individuals (1,211 male, 989 female) were available from the archive for HPeV screening. These represent 92% of all samples referred to the SVC for respiratory virus screening between January 2007 and December 2007. These samples were referred equally from all age groups, with good representation of samples from very young children (9.5% under 3 months) and older patients (13.3% over 65 years). Over 95% of study subjects were treated in accident and emergency (A&E) departments or inpatient wards in referral hospitals in Edinburgh and surrounding areas.
All samples were screened for a range of respiratory viruses by multiplexed nested PCR for RSV, FLUA and FLUB, PIV1 to PIV3, and AdV. The following viruses were detected in the study samples: RSV in 584 samples, FLUA in 127 samples, FLUB in 0 samples, PIV1 in 44 samples, PIV2 in 27 samples, PIV3 in 109 samples, and AdV in 329 samples. The total number of samples in which respiratory viruses were detected was 1,220 (32%, from 930 patients), of which 101 were coinfections of two viruses. Most often, coinfections consisted of RSV and AdV (n = 54), but other viruses were also found in coinfected individuals, including PIV3/AdV (25 samples), PIV1/AdV (three samples), PIV2/AdV (two samples), FLUA/AdV (one sample), PIV3/RSV (five samples), FLUA/RSV (five samples), PIV2/RSV (four samples), and PIV1/RSV (two samples). In addition, two triple infections were recorded (RSV/AdV/PIV2 and RSV/AdV/PIV3; one each). None of the multiple infections were detected in consecutive samples from the same individual.
Frequency of HPeV infections.
The sensitivity of the screening PCR (using 5′ UTR primers) for HPeV RNA was assessed by using the QCMD 2008 panel for detection of enteroviruses, which contained two HPeV3 specimens (EV08-03 and EV08-08). Both samples were detected by our new HPeV PCR assay. Sample EV08-03 contained a low level of HPeV RNA (stock dilution of 1.0 × 10−7), and HPeV was detected in samples from less than one-third of the participants (30.7%).
The 3,844 study samples were screened for HPeV RNA sequences by nested RT-PCR, initially in pools of 10; positive pools were split to identify individual positive samples. For HPeV, a total of 34 individual positive samples were identified with the screening primers from the 5′ UTR; all except one were also positive by RT-PCR using primers from the VP3/VP1 gene region (0.8% sample prevalence). These 33 confirmed samples were taken from a total of 27 different individuals, yielding an overall study subject prevalence of infection of 1.2% in the study group. Interestingly, HPeV was detected more often in males than females (male-to-female ratio of 2.4:1), demonstrating a higher prevalence among males (1.5%, compared to 0.8% in females). Other respiratory viruses were frequently codetected in the HPeV-positive samples (25 of 33), of which 22 were coinfections of two viruses (with AdV [n = 10], RSV [n = 9], PIV3 [n = 2], or PIV1 [n = 1]) and three were triple infections (HPeV/AdV/RSV [n = 2] and HPeV/AdV/PIV3 [n = 1]).
RSV was the most frequently detected virus, amplified from 584 samples from 481 different individuals, followed by AdV (329 samples from 240 individuals). FLUA was detected in 127 samples from 94 individuals, whereas no FLUB was found in the study year 2007. PIV3 was the most often diagnosed parainfluenza virus, with 109 positive samples collected from 90 individuals (PIV1 was found in 44 samples from 32 individuals and PIV2 in 27 samples from 23 individuals).
HPeV typing assay.
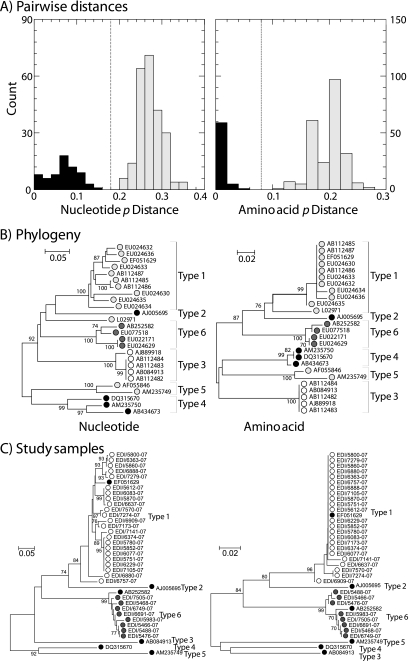
The region amplified by the VP3/VP1 primers was highly variable and allowed previously assigned HPeV types to be reliably differentiated. Pairwise distances between and within types of both nucleotide and translated amino acid sequences formed nonoverlapping distributions (Fig. 1A), with thresholds of 18% divergence for nucleotide and 8% divergence for amino acid dividing the intra- and intertype categories. This division is reflected in separate phylogenetic groupings of previously published and typed sequences (Fig. 1B). The only exception was the highly divergent Harris HPeV1 isolate, designated the type member of the parechoviruses but substantially divergent from other published HPeV1 sequences in the amplified region.
FIG. 1.
Identification of HPeV types by sequence comparisons of the VP3/VP1 regions. (A) Frequency histogram of pairwise distances between (gray bars) and within (black bars) HPeV types, determined using published assignments. Nucleotide p (uncorrected) distances and inferred translated amino acid sequence p distances are shown. (B) Phylogenetic analysis of the same data set, carried out using neighbor-joining trees based on maximum composite likelihood (nucleotide) distances and amino acid p distances. Bootstrap resampling was used to demonstrate the robustness of groupings; values of ≥70% are shown (percentages are shown at left). For clarity, symbols depicting sequences of HPeV2 are black, those depicting sequences of HPeV1 and HPeV4 to -6 are different shades of gray, and those depicting sequences of HPeV3 are white. GenBank accession numbers are indicated. (C) Phylogenetic analysis of sequences from clinical specimens (HPeV1, white circles; HPeV6, gray circles), determined by the same method using the single reference sequence of each HPeV type (black circles).
To investigate the sensitivity of the typing assay, we reassayed a series of 47 clinical specimens identified as positive by 5′ UTR screening, 21 of which had been typed by VP1 sequencing (8) and 26 of which were nontypeable by this method (Table 1). The VP3/VP1 PCR was followed by sequencing, and a much higher frequency of samples overall (including 21 of the 26 previously nontypeable samples) was successfully amplified and sequenced, including 15 type 1, three type 3, one type 4, and two type 5 HPeV variants. The method was applied to identify the HPeV types in the samples found to be positive by the respiratory screen (Fig. 1C). All but 1 of the 34 HPeV-positive samples could be amplified using the VP3/VP1 primers, and sequences from the amplicons grouped closely with either HPeV type 1 (EF051629; 25 specimens from 22 study subjects) or type 6 (AB252582; eight specimens from five individuals) sequences, providing unequivocal HPeV type assignments. Identical or closely similar sequences were obtained from the three study subjects providing multiple positive samples.
TABLE 1.
Sensitivity and specificity comparison of HPeV VP3/VP1 typing of previously typed specimens
| VP3/VP1 sequencing type | No. of specimens of preassigned typea:
|
No. of specimens not typeda | Total | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| 1 | 12 | 15 | 27 | |||||
| 2 | 0 | |||||||
| 3 | 4 | 3 | 7 | |||||
| 4 | 2 | 1 | 3 | |||||
| 5 | 1 | 2 | 3 | |||||
| 6 | 1 | 1 | ||||||
| Negative | 1 | 5 | 6 | |||||
| Total | 13 | 0 | 4 | 2 | 1 | 1 | 26 | 47 |
By VP1 sequencing (see reference 8).
Epidemiological and clinical associations of HPeV infections.
Sample and study subject information was recorded for each sample tested and was available in anonymized form for analysis of virus epidemiology and clinical associations. The analysis of seasonal variation was based on sample totals, whereas the age profiles were based on study subject totals to prevent overrepresentation of individuals from whom multiple samples were referred.
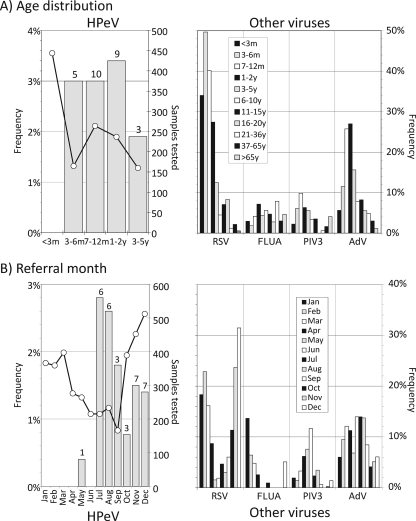
HPeV infections were restricted entirely to children aged between 6 months and 5 years (Fig. 2A). The five study subjects with HPeV6 infections were distributed throughout the age range (two were 7 to 12 months old, one was 1 to 2 years old, and two were 3 to 5 years old). Although RSV tended to infect neonates more frequently than HPeV, RSV infections showed a similar age profile, with infections confined almost completely to young children. Similarly, AdV showed the highest frequencies in the age group from 6 months to 5 years but lower frequencies in very young children. Greater numbers are needed to determine whether the incidences of HPeV1 and -6 (and of other types) show different age distributions.
FIG. 2.
Epidemiology of HPeV and other respiratory viruses. Comparison of (A) age distributions and (B) referral months of HPeV infections with those of other frequently detected viruses (RSV, FLUA, PIV3, and AdV). Numbers above bars show numbers of samples (A) or study subjects (B) positive for HPeV. m, months; y, years.
The frequencies of HPeV infection varied substantially over the study period (Fig. 2B), with a high rate of HPeV detection in late summer (July and August) and early winter (November and December). Incidences of RSV and FLUA infections also showed seasonal variation, with peaks during the midwinter from November to February, while AdV showed a relatively constant frequency of infection throughout the year. Infections with HPeV1 and -6 were found throughout the sampling period, with no evidence of differences in seasonal prevalence (from low numbers tested) (data not shown).
HPeV was detected more frequently in samples with other respiratory viruses present, with over two-thirds of HPeV-positive samples containing another virus (Table 2). These frequencies were substantially higher than those observed for other respiratory viruses, AdV being the highest, with codetected viruses in 86 of 201 study subjects under the age of 5 years (43%). For the HPeV-positive subjects, AdV and RSV were the most frequently codetected viruses (Table 3), although in the latter case this frequency (9 of 27; 33%) was similar to that observed for HPeV-negative individuals (415 of 1,243; 33%) (P = 1.00). It was only with AdV that a significantly higher frequency of codetection with HPeV was identified (37%, compared with 15% [P = 0.006]).
TABLE 2.
Frequencies of virus codetection in study subjects (<5 years) infected with HPeV and other viruses
| Virus | No. of coinfected subjects/total no. of subjects (%) |
|---|---|
| HPeV | 19/27 (70) |
| Other viruses | |
| RSV | 70/424 (17) |
| FLUA | 4/51 (8) |
| PIV1 | 5/24 (21) |
| PIV2 | 7/13 (54) |
| PIV3 | 30/70 (43) |
| AdV | 86/201 (43) |
| Total for other virusesa | 100/783 (13) |
Excluding HPeV.
TABLE 3.
Frequencies of detection of other viruses in HPeV-infected and uninfected subjects (<5 years)
| Virus | No. of HPeV-coinfected subjects/total no. of subjects (%) | No. of HPeV-negative subjects/total no. of subjects (%) | P valuea |
|---|---|---|---|
| Any virus | 19/27 (70) | 656/1,243 (53) | 0.08 |
| RSV | 9/27 (33) | 415/1,243 (33) | 1.00 |
| AdV | 10/27 (37) | 191/1,243 (15) | 0.006 |
| PIV3 | 2/27 (7) | 68/1,243 (5) | 0.66 |
Calculated by Fisher's exact test. The value below 0.05 appears in bold.
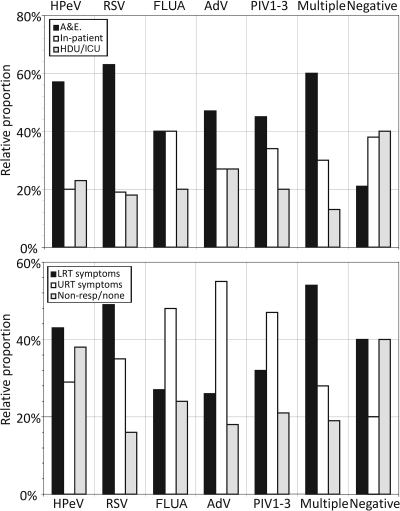
Finally, referral information, including the source of samples and reported symptoms of HPeV-positive samples, was compared with those of other commonly detected respiratory viruses (Fig. 3). Over half of the HPeV-positive samples originated from A&E departments, a proportion similar to that of RSV but higher than those of AdV and FLUA. The distribution was distinct from that of samples in which no viruses were detected, in which cases inpatient and critical care unit referral predominated. Although the referral source data suggest that HPeV may present with acute respiratory disease (as typically occurs for RSV), an analysis of clinical data indicated a much higher frequency of samples originating from subjects without respiratory symptoms (approximately 40% of cases, similar to that for samples in which no virus was isolated). In contrast, over 80% of RSV-positive samples originated from individuals with respiratory, particularly LRT, symptoms.
FIG. 3.
Referral sources and presenting symptoms of study subjects infected with HPeV and other respiratory viruses. Relative proportions of total study group infected with each virus originating from A&E departments, general hospital wards, and critical care wards (high-dependency unit [HDU]/intensive care unit [ICU]) (other sources [e.g., general practitioners] have been excluded because of low numbers) (top) and presenting symptoms broadly categorized into LRT, URT, and those unrelated to respiratory infections (Non-resp) (bottom).
DISCUSSION
This is the first systematic study of the incidence, epidemiology, and clinical associations of HPeV infections detected in respiratory samples. The study used a newly developed sensitive and specific nested RT-PCR amplification method with highly conserved primers from the 5′ UTR capable of detecting all HPeV types; the sense primers hybridized to conserved regions between positions 235 and 335 distinct from primer targets used in other published 5′ UTR-based assays for HPeV (5, 24, 26), while the antisense primers (positions 534 to 583) overlapped probe or primer sites used in these other assays. Its very high sensitivity (as demonstrated using the QCMD panel) also indicated its potential suitability for screening cerebrospinal fluid from meningitis cases, where viral loads are likely to be low.
The direct typing of HPeV by VP3/VP1 PCR and sequencing proved similarly highly sensitive, enabling its use directly on clinical specimens and providing an epidemiological tool much more useful than previously used methods based on serotyping or sequencing HPeV isolates (12, 30); parechoviruses often grow poorly in culture, and antibodies used for typing are not widely available for more recently discovered HPeV types. By use of the panel of previously typed and nontypeable clinical specimens, our new typing method identified HPeV in all but one of the typed samples (20/21) and most of the samples previously refractory to amplification with VP1 primers (8). The high sensitivity of this method demonstrates its potential value in future investigations of the molecular epidemiology of different HPeV types and possible differences in disease associations and tropism for the central nervous system.
HPeV incidence and epidemiology.
Despite the sensitivity of the screening method used in the current study, we found a relatively low frequency of HPeV infections in the study population (27 of 2,200 different study subjects investigated). Several features of seasonal and age distribution are, however, concordant with previous investigations of HPeV epidemiology, including the observed greater frequencies of infections in late summer and early winter months (13) and an age range of infection restricted to individuals under 5 years old (7, 13). The absence of any detectable HPeV infections in older children and adults (despite representing 37% of the total 3,844 samples in the archive) is consistent with previous seroepidemiological studies documenting 72% seroprevalence of antibodies to HPeV1 by the age of 2, reaching 99% in adulthood (28).
Although HPeV1 was the most common type detected in the study population in Scotland, the relatively frequent detection of type 6 represents the first report of this type from the United Kingdom and confirms previous data indicating its wide geographical distribution (4, 7, 24, 30). Recent studies have provided evidence for a biannual cycle of HPeV infections, particularly evident with HPeV3 infections, which occur most frequently in even-numbered years in The Netherlands (32; K. Benschop, X. Thomas, C. Serpenti, R. Molencamp, and K. Wolthers, unpublished data). If this pattern is shared throughout Europe, it might explain the absence of HPeV3 infections in the current study.
Clinical significance of HPeV infections.
It has been claimed that HPeV1 is a common cause of respiratory disease, including URT and LRT illnesses (13, 15). In addition, HPeV1 has been identified as a cause of nosocomial respiratory infections involving neonates and small children (9, 14). A retrospective investigation of 109 individuals with HPeV1 infection demonstrated respiratory symptoms in 13% of study subjects (13), similar to the 26% frequency in a larger series of 581 cases of HPeV1 and HPeV2 infections (15). Among a group of premature infants in a neonatal ward with respiratory disease, HPeV1 was isolated from 18/64 individuals, of which 11 had URT symptoms and seven had pneumonia, although a range of other respiratory viruses was detected in the study group (9). Recently, a few isolated cases of HPeV infections have been associated with respiratory tract infections (7, 18, 30), although as with the studies described above (13, 15), other potential viral or bacterial causes for the observed respiratory disease were not always excluded.
In contrast to these previously claimed disease associations, our study does not necessarily provide evidence confirming the proposed etiological role of HPeV in childhood respiratory disease. The observed incidence of HPeV infections in respiratory samples (27 of 1,299 study subjects less than 5 years of age; 2.1%) is arguably not different from the expected frequency of HPeV infections occurring in the general population. There are little data on the duration of HPeV shedding from the respiratory tract during primary infections of immunocompetent individuals. However, a ballpark estimate of 1 to 3 weeks of virus shedding detectable by a highly sensitive PCR such as that used in the current study, based on durations of detection of other respiratory viruses and combined with the documented 70% seroprevalence for HPeV1 antibodies by the age of 2 years, would predict HPeV detection frequencies of 0.7% to 2.1% (assuming they occur at constant frequency between the ages of 6 and 24 months). The observed frequency of 1.9% HPeV-positive study subjects from children aged less than 2 years old thus lies within the range of the expected detection frequency in the nonhospitalized population. HPeV-infected individuals are not overrepresented among individuals presenting predominantly with respiratory symptoms or diagnosed respiratory disease, and thus it might be reasoned that the detection of HPeV is incidental to the respiratory disease and/or other clinical presentation that led to hospital admission and sampling.
Some (but not all) aspects of the data collected in the current study were consistent with this interpretation. First, over two-thirds of children in whom HPeV was detected were coinfected with another respiratory virus that might account for the observed respiratory disease (Table 3; Fig. 3B). An alternative interpretation of the observed high rate of coinfection might be that HPeV plays an exacerbating role in other respiratory virus infections, as has been suggested for HBoV (23). Second, although a direct etiological role in respiratory disease might be considered in the remaining third of cases where HPeV was the only virus detected, this frequency was actually lower than that of samples in the archive negative for all routinely screened respiratory viruses (70%), indicating that a substantial proportion of viruses or bacteria causing respiratory disease are undetected by current diagnostic screening. However, it was interesting that AdV infections were specifically associated with HPeV; 10 of 27 HPeV cases were coinfections with AdV, significantly greater than codetection frequencies for HPeV-negative samples (15%) (Table 3) and comparable to those with other respiratory viruses (43%) (Table 2). One possibility here is that primary HPeV infections may reactivate AdV, leading to respiratory disease.
In the current anonymized study design, the only clinical information available for the study subjects was that recorded on the specimen referral forms. Nevertheless, the available data did show the expected association of LRT symptoms with RSV infections and of URT symptoms with PIVs and AdV and a higher frequency of absent or nonrespiratory symptoms from study subjects whose samples were virus negative on screening (Fig. 3). The data collection procedure and study design used in the Edinburgh respiratory sample archive was previously able to demonstrate the association of HBoV infections with LRT disease at frequencies approaching that of RSV (22) and a lack of respiratory disease associations with WU and KI polyomaviruses (25). Based on the available data, HPeV-positive samples showed a clinical profile with approximately equal frequencies of LRT, URT, and nonrespiratory symptoms that most closely matched that of screen-negative samples. It was distinct from those of RSV and other routinely screened respiratory viruses and is therefore consistent with a potentially incidental role of HPeV in respiratory disease in the study subjects.
In the future, the screening and typing methods developed in the current study will clearly be of value in more-focused testing of individuals with specific illnesses of possible viral etiology, such as neonatal sepsis and meningitis. With evidence for the involvement of HPeV in viral meningitis and possible type-associated differences in viral tropism and disease (7), the ability to detect and type HPeV directly from cerebrospinal fluid would greatly enhance diagnostic testing for these diseases.
Acknowledgments
We are very grateful to Peter McCullough, Eleanor Leslie, Angus MacCaulay, and Carol Thomson for providing samples, data, and other virus testing results from the respiratory sample archive and to Elly Gaunt and Kathryn O'Neill for unpublished results from HMPV and HBoV testing.
Footnotes
Published ahead of print on 27 August 2008.
REFERENCES
- 1.Abed, Y., and G. Boivin. 2005. Molecular characterization of a Canadian human parechovirus (HPeV)-3 isolate and its relationship to other HPeVs. J. Med. Virol. 77566-570. [DOI] [PubMed] [Google Scholar]
- 2.Allander, T., T. Jartti, S. Gupta, H. G. Niesters, P. Lehtinen, R. Osterback, T. Vuorinen, M. Waris, A. Bjerkner, A. Tiveljung-Lindell, B. G. van den Hoogen, T. Hyypiä, and O. Ruuskanen. 2007. Human bocavirus and acute wheezing in children. Clin. Infect. Dis. 44904-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Sunaidi, M., C. H. Williams, P. J. Hughes, D. P. Schnurr, and G. Stanway. 2007. Analysis of a new human parechovirus allows the definition of parechovirus types and the identification of RNA structural domains. J. Virol. 811013-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgarte, S., L. K. Souza Luna, K. Grywna, M. Panning, J. F. Drexler, C. Karsten, H. I. Huppertz, and C. Drosten. 2008. Prevalence, types, and RNA concentrations of human parechoviruses, including a sixth parechovirus type, in stool samples from patients with acute enteritis. J. Clin. Microbiol. 46242-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benschop, K., R. Molenkamp, A. van der Ham, K. Wolthers, and M. Beld. 2008. Rapid detection of human parechoviruses in clinical samples by real-time PCR. J. Clin. Virol. 4169-74. [DOI] [PubMed] [Google Scholar]
- 6.Benschop, K. S., J. Schinkel, M. E. Luken, P. J. van den Broek, M. F. Beersma, N. Menelik, H. W. van Eijk, H. L. Zaaijer, C. M. VandenBroucke-Grauls, M. G. Beld, and K. C. Wolthers. 2006. Fourth human parechovirus serotype. Emerg. Infect. Dis. 121572-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benschop, K. S., J. Schinkel, R. P. Minnaar, D. Pajkrt, L. Spanjerberg, H. C. Kraakman, B. Berkhout, H. L. Zaaijer, M. G. Beld, and K. C. Wolthers. 2006. Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin. Infect. Dis. 42204-210. [DOI] [PubMed] [Google Scholar]
- 8.Benschop, K. S., C. H. Williams, K. C. Wolthers, G. Stanway, and P. Simmonds. 2008. Widespread recombination within human parechoviruses: analysis of temporal dynamics and constraints. J. Gen. Virol. 891030-1035. [DOI] [PubMed] [Google Scholar]
- 9.Berkovich, S., and J. Pangan. 1968. Recoveries of virus from premature infants during outbreaks of respiratory disease: the relation of ECHO virus type 22 to disease of the upper and lower respiratory tract in the premature infant. Bull. N. Y. Acad. Med. 44377-387. [PMC free article] [PubMed] [Google Scholar]
- 10.Birenbaum, E., R. Handsher, J. Kuint, R. Dagan, B. Raichman, E. Mendelson, and N. Linder. 1997. Echovirus type 22 outbreak associated with gastro-intestinal disease in a neonatal intensive care unit. Am. J. Perinatol. 14469-473. [DOI] [PubMed] [Google Scholar]
- 11.Boivin, G., Y. Abed, and F. D. Boucher. 2005. Human parechovirus 3 and neonatal infections. Emerg. Infect. Dis. 11103-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries, M., K. Pyrc, R. Berkhout, W. Vermeulen-Oost, R. Dijkman, M. F. Jebbink, S. Bruisten, B. Berkhout, and L. van der Hoek. 2008. Human parechovirus type 1, 3, 4, 5, and 6 detection in picornavirus cultures. J. Clin. Microbiol. 46759-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrnst, A., and M. Eriksson. 1993. Epidemiological features of type 22 echovirus infection. Scand. J. Infect. Dis. 25275-281. [DOI] [PubMed] [Google Scholar]
- 14.Ehrnst, A., and M. Eriksson. 1996. Echovirus type 23 observed as a nosocomial infection in infants. Scand. J. Infect. Dis. 28205-206. [DOI] [PubMed] [Google Scholar]
- 15.Grist, N. R., E. J. Bell, and F. Assaad. 1978. Enteroviruses in human disease. Prog. Med. Virol. 24114-157. [PubMed] [Google Scholar]
- 16.Heim, A., C. Ebnet, G. Harste, and P. Pring-Akerblom. 2003. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 70228-239. [DOI] [PubMed] [Google Scholar]
- 17.Hyypiä, T., C. Horsnell, M. Maaronen, M. Khan, N. Kalkkinen, P. Auvinen, L. Kinnunen, and G. Stanway. 1992. A distinct picornavirus group identified by sequence analysis. Proc. Natl. Acad. Sci. USA 898847-8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito, M., T. Yamashita, H. Tsuzuki, N. Takeda, and K. Sakae. 2004. Isolation and identification of a novel human parechovirus. J. Gen. Virol. 85391-398. [DOI] [PubMed] [Google Scholar]
- 19.Jartti, T., P. Lehtinen, T. Vuorinen, R. Osterback, B. van den Hoogen, A. D. Osterhaus, and O. Ruuskanen. 2004. Respiratory picornaviruses and respiratory syncytial virus as causative agents of acute expiratory wheezing in children. Emerg. Infect. Dis. 101095-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joki-Korpela, P., and T. Hyypiä. 1998. Diagnosis and epidemiology of echovirus 22 infections. Clin. Infect. Dis. 27129-136. [DOI] [PubMed] [Google Scholar]
- 21.Koskiniemi, M., R. Paetau, and K. Linnavuori. 1989. Severe encephalitis associated with disseminated echovirus 22 infection. Scand. J. Infect. Dis. 21463-466. [DOI] [PubMed] [Google Scholar]
- 22.Manning, A., V. Russell, K. L. G. H. Eastick, N. Hallam, K. E. Templeton, and P. Simmonds. 2006. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J. Infect. Dis. 1941283-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIntosh, K. 2006. Human bocavirus: developing evidence for pathogenicity. J. Infect. Dis. 1941197-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nix, W. A., K. Maher, E. S. Johansson, B. Niklasson, A. M. Lindberg, M. A. Pallansch, and M. S. Oberste. 4 June 2008. Detection of all known parechoviruses by real-time PCR. J. Clin. Microbiol. doi: 10.1128/JCM.00277-08. [DOI] [PMC free article] [PubMed]
- 25.Norja, P., I. Ubillos, K. Templeton, and P. Simmonds. 2007. No evidence for an association between infections with WU and KI polyomaviruses and respiratory disease. J. Clin. Virol. 40307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberste, M. S., K. Maher, and M. A. Pallansch. 1999. Specific detection of echoviruses 22 and 23 in cell culture supernatants by RT-PCR. J. Med. Virol. 58178-181. [DOI] [PubMed] [Google Scholar]
- 27.Stanway, G., and T. Hyypiä. 1999. Parechoviruses. J. Virol. 735249-5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tauriainen, S., M. Martiskainen, S. Oikarinen, M. Lonnrot, H. Viskari, J. Ilonen, O. Simell, M. Knip, and H. Hyoty. 2007. Human parechovirus 1 infections in young children—no association with type 1 diabetes. J. Med. Virol. 79457-462. [DOI] [PubMed] [Google Scholar]
- 29.Templeton, K. E., S. A. Scheltinga, M. F. Beersma, A. C. Kroes, and E. C. Claas. 2004. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J. Clin. Microbiol. 421564-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe, K., M. Oie, M. Higuchi, M. Nishikawa, and M. Fujii. 2007. Isolation and characterization of novel human parechovirus from clinical samples. Emerg. Infect. Dis. 13889-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wigand, R., and A. B. Sabin. 1961. Properties of ECHO types 22, 23 and 24 viruses. Arch. Gesamte Virusforsch. 11224-247. [DOI] [PubMed] [Google Scholar]
- 32.Wolthers, K. C., K. Benschop, J. Schinkel, R. Molenkamp, R. M. Bergevoet, I. J. B. Spijkerman, H. C. Kraakman, and D. Pajkrt. 2008. Human parechoviruses as an important viral cause for sepsis-like illness and meningitis in young children. Clin. Infect. Dis. 47358-363. [DOI] [PubMed] [Google Scholar]