Abstract
Campylobacter jejuni lipooligosaccharide (LOS) can trigger Guillain-Barré syndrome (GBS) due to its similarity to human gangliosides. Rapid and accurate structural elucidation of the LOS glycan of a strain isolated from a GBS patient could help physicians determine the spectrum of anti-ganglioside antibodies likely to be found and therefore provide valuable assistance in establishing an appropriate course of treatment. The ability of implemented mass spectrometry-based approaches in a clinical setting has been limited by the laborious and time-consuming nature of the protocols, typically 3 to 4 days, used to prepare LOS. In order to improve the analytical throughput, microwave-assisted enzymatic digestion was investigated. In this study, the bacterial cells were suspended in 50 μl of 20 mM ammonium acetate buffer containing DNase and RNase and treated by direct microwave irradiation for 3 min. Then, proteinase K was added and the samples were again microwaved. The intact LOS samples were analyzed using electrophoresis-assisted open-tubular liquid chromatography-mass spectrometry. The reliability of the rapid, high-throughput technique was demonstrated through analysis of LOS glycans from 73 C. jejuni strains. The structure was elucidated using material from a single colony. The total time for sample preparation and MS analysis is less than 60 min.
Guillain-Barré syndrome (GBS) is a postinfection autoimmune-mediated neuropathy that can be triggered by the display of lipooligosaccharide (LOS)-bound ganglioside mimics by the bacterium Campylobacter jejuni (6, 22). Most patients who develop GBS following C. jejuni enteritis have elevated levels of circulating immunoglobulin Gs, which are reactive toward the gangliosides GM1, GD1a, and GQ1b (11, 12). Several studies have linked the onset of GBS with exposure to a surface-bound ganglioside mimic, including animal models where GBS-like symptoms have been triggered following inoculation with C. jejuni LOS bearing a ganglioside mimic (11, 12, 22). Even with prompt medical attention, GBS-associated mortality and disability are highly significant (8), and development of novel therapeutic strategies is an ongoing goal. One attractive treatment option is immunoadsorption therapy, which could be tailored to remove only disease-specific antibodies while returning other serum components to the patient (21). In instances where a C. jejuni strain has been isolated from a GBS patient, rapid determination of its LOS glycan could help establish the adsorption protocol needed for effective treatment.
In recent years, considerable progress has been made toward the elucidation of the molecular determinants of pathogen-associated human diseases. A number of studies have demonstrated that there is a high level of variability in the LOS biosynthesis loci carried by C. jejuni; however, only select strains have the ability to synthesize ganglioside mimics and have been linked to autoimmune-mediated neuropathies (6). One complication that limits our ability to link a specific strain with an antibody response in a patient is that bacteria carrying the same genetic complement can express a large repertoire of glycan structures as a result of phase-variable gene expression (4). Furthermore, GBS patients may also be coinfected with multiple strains, with only one involved in triggering the autoimmune response (5, 7). Mass spectrometry (MS) is one of the few techniques which can provide a comprehensive view of the spectrum of glycans displayed by a given isolate and help to characterize multiple strains present in some fecal samples (14).
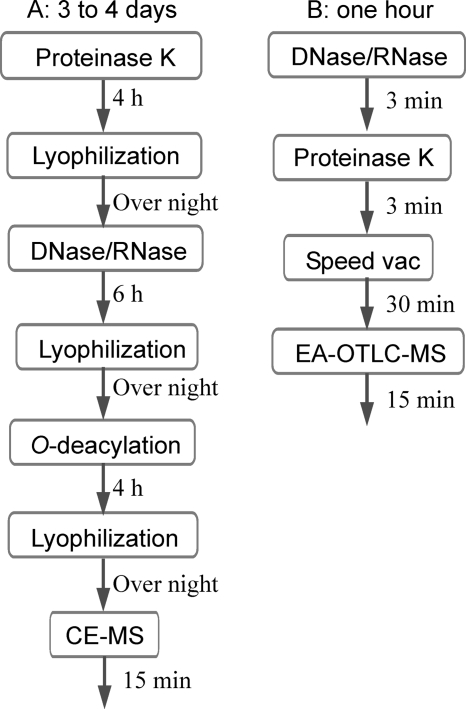
We have elucidated the LOS glycan structures in several strains associated with GBS and Miller Fisher syndromes (MFS) by using capillary electrophoresis MS (7, 10, 11). In our initial studies, the LOS was typically O deacylated prior to capillary electrophoresis-MS analysis in order to remove the O-linked fatty acid, which improved solubility and reduced aggregation in aqueous solutions. Nevertheless, C. jejuni can still contain up to four N-linked fatty acids, resulting in unwanted association with the capillary tube, which led to the implementation of electrophoresis-assisted open-tubular liquid chromatography-electrospray MS (EA-OTLC-MS) to characterize C. jejuni LOS (13). Moreover, because O deacylation causes the unwanted removal of biologically important O-linked glycan modifications and is a time-consuming process, we have recently applied the EA-OTLC-MS technique for the sensitive analysis of small quantities of fully intact LOS (3). For this method, the sample preparation includes 4 hours of proteinase K digestion and 6 hours of DNase/RNase digestion in combination with overnight lyophilization between the steps, which together take 2 days, a time period which would severely limit the usefulness of this method in a clinical setting, where treatment courses must be established as rapidly as possible.
In an effort to develop a more rapid and sensitive means to analyze C. jejuni LOS, we investigated the feasibility of microwave-assisted enzymatic digestions for LOS sample preparation. Microwave irradiation can accelerate enzymatic digestion of proteins, where reactions requiring several hours under conventional conditions can be reduced to only a few minutes with very high yields and reaction selectivity (9, 15, 17, 18, 20, 23, 24). Using this strategy, we have determined that C. jejuni LOS can be prepared for MS analysis in as few as 6 min following bacterial harvesting. We tested the general applicability of the technique by analyzing the LOS-bound glycan in 73 different C. jejuni strains, including many GBS-associated isolates. This rapid and sensitive MS approach could provide timely information to physicians considering treatment options for GBS patients.
MATERIALS AND METHODS
Bacterial cell culture.
C. jejuni strains were cultured for 24 to 48 h on Mueller-Hinton agar plates in a microaerobic atmosphere at 37°C. LOS was isolated by washing the colonies from the plates and dispersing them in 1.5-ml tubes, each containing 300 μl of phosphate (P)-buffered saline (pH 7.4). To this, 700 μl of 100% ethanol was added, and bacterial cells were incubated at room temperature for 1 h. The cells were pelleted (16,000 × g, 2 min), washed twice with 1 ml of ethanol, washed twice with 1 ml of acetone, and air dried.
Conventional LOS preparation.
As illustrated in the left panel of Fig. 1, the sample preparation could take as long as 4 days if O deacylation were required. In this protocol, proteinase K was typically used as the first digestion enzyme to help break down cells, followed by the application of a DNase/RNase cocktail. The dried cells were dissolved in 200 μl of deionized water, and a 60-μl aliquot of a 2-mg/ml solution of proteinase K was added to each vial. The suspended cell solutions were incubated at 37°C for 4 h, and the digestion was stopped by raising the temperature to 75°C for 10 min. The solutions were allowed to cool to room temperature and were subsequently freeze-dried. The cells were resuspended in 200 μl of 20 mM ammonium acetate (Ac) buffer (pH 7.5) containing DNase (100 μg/ml) and RNase (200 μg/ml) and incubated at 37°C for 6 h before being lyophilized. The digested cells were resuspended in 200 μl of deionized water. Following ultracentrifugation (436,000 × g, 4°C, 1 h), LOS pellets were redissolved in water and lyophilized.
FIG. 1.
Illustration of sample preparation procedures. CE-MS, capillary electrophoresis-MS.
Microwave-assisted LOS preparation.
Under microwave irradiation, the enzymatic digestion was carried out for only 3 minutes (right panel of Fig. 1). Since proteinase K can also digest DNase and RNase, we added proteinase K after DNase/RNase digestion without a denaturing step. We found that for microscale sample preparation, there were no significant differences when the order of reagent additions was changed. The bacterial cells were suspended in 50 μl of 20 mM ammonium Ac buffer (pH 7.5) containing DNase (100 μg/ml) and RNase (200 μg/ml). A container with 100 ml of water was placed beside the sample vials to absorb the excessive microwave energy. The samples were heated by direct microwave irradiation using a domestic 1,200-W microwave oven, with the power level being set at “level 2,” for 3 min (Panasonic, Ontario, Canada). Then, proteinase K was added to give a final concentration of 60 μg/ml and heated under the same conditions. The solutions were allowed to cool at room temperature and subsequently dried using a Speed Vac (vacuum centrifuge concentrator; Savant). Overall, with this protocol the sample preparation time was shortened from between 3 and 4 days to less than 1 hour. LOS samples were washed three times with methanol (100 μl) with vigorous stirring, and the insoluble residues were collected by centrifugation and suspended in 30 μl water for EA-OTLC-MS analysis.
EA-OTLC-MS analysis.
The detailed experiment procedures of EA-OTLC-MS have been reported previously (13). Briefly, 1.0 μl of a LOS sample was injected into a capillary column, followed by washing with 1.0 μl 100% methanol. A small plug (60 nl) of 1.0 M ammonium Ac in deionized water was injected to elute the adsorbed intact LOS from the capillary surface. The separation was performed using 30 mM morpholine in deionized water, pH 9.0. A separation voltage of 20 kV, together with a pressure of 50 kPa, was applied for the EA-OTLC-MS analysis. The electrospray ionization voltage applied on the sprayer was set at −5.2 kV. Data acquisition was performed for an m/z range of 600 to 1,800 at a 2-s/spectrum scan rate.
For all the MS experiments, nitrogen was used as both a curtain and a collision gas. In the tandem MS (MS/MS) (enhanced product ion scan) and MS/MS/MS experiments, the scan speed was set to 4,000 Da/s, with Q0 trapping. In MS/MS and MS/MS/MS experiments, the trap fill time was set as “dynamic” and the resolution of Q1 was set as “unit.” For MS/MS/MS experiments, the excitation coefficient was set to excite only the first isotope for a singly charged precursor, and the excitation time was set at 100 ms.
RESULTS
Analysis of intact LOS samples prepared using conventional protocols.
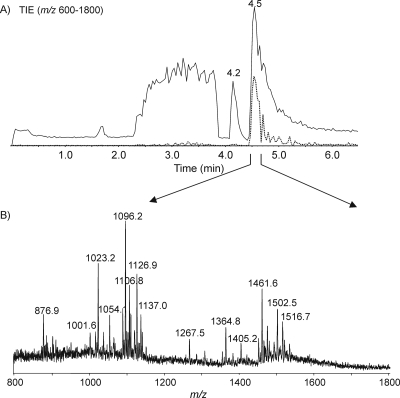
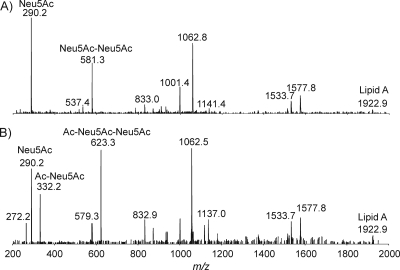
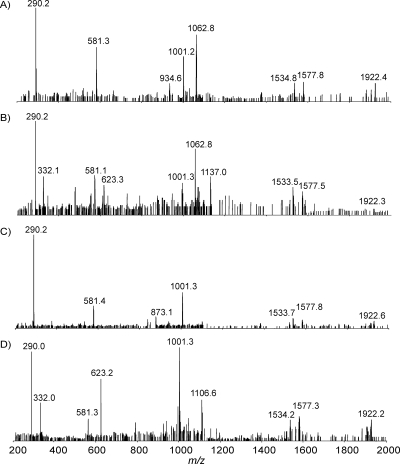
Initially, the intact LOS from C. jejuni MF6 was prepared using a traditional method, and the mass spectrum showed abundant quadruply charged ions with m/z values of 1,096.2 and 1,126.9, together with their corresponding triply charged ions with m/z values of 1,461.6 and 1,502.5 (Fig. 2). In addition, a quadruply charged ion and its triply charged counterpart ion were detected at m/z values of 1,137.5 and 1,516.7, corresponding to the presence of an acetyl group. The MS spectrum can be used to derive the compositions of each glycoform and confirmed by performing MS/MS. The fragment ions with m/z values of 290.2 and 581.2 for both precursor ions with m/z values of 1,126.9 and 1,137.5 give evidence for the existence of monosialic acid (N-acetylneuraminic acid [Neu5Ac]) and disialic acid (Neu5Ac-Neu5Ac), respectively (Fig. 3). The fragment ion with an m/z of 332.2, corresponding to O-acetylated Neu5Ac, and the fragment ion with an m/z of 623.3, corresponding to the additional attachment of Neu5Ac, were observed only in the tandem mass spectrum of the ion with an m/z of 1,137.5. This observation clearly indicated that the O-acetyl group was linked to the Neu5Ac residue. Indeed, the identification of O-acetylated sialic acids is an analytical challenge (16). Mild acid treatment results in Neu5Ac cleavage due to the acid lability of ketosidic linkage, while hydrazinolysis leads to removal of the ester-linked acyl groups. Therefore, direct analysis of intact LOS offers a powerful tool for sialome studies (1, 2, 16, 19).
FIG. 2.
EA-OTLC-MS analysis of intact LOS from C. jejuni MF6 prepared using a traditional method. A separation voltage of 20 kV, together with a pressure of 50 kPa, was employed. Samples (1.0 μl) were injected. (A) Total ion chromatogram (m/z values of 600 to 1,800) and extracted ion chromatogram (m/z of 1,096.2); (B) extracted mass spectrum at 4.5 min.
FIG. 3.
MS and MS/MS analysis of intact LOS from C. jejuni MF6. A separation voltage of 20 kV, together with a pressure of 50 kPa, was employed. Samples (1.0 μl) were injected for all experiments. For MS/MS analysis, N2 was used as a collision gas and −40 V was set as the collision energy level. (A) Extracted MS/MS spectrum of the ion with an m/z of 1,126.9 ([M-4H]4−); (B) extracted MS/MS spectrum of the ion with an m/z of 1,137.0 ([M-4H]4−).
The lipid A portion of the molecule consisted of a hybrid backbone of β-d-2,3-diamino-2,3-dideoxy-d-glucose (β-d-GlcN3N)-(1→6)-α-d-GlcN3N carrying P or pyrophosphoethanolamine (PPEtn) at positions O-1 and O-4′ and substituted by six fatty acid chains. Two major acylation patterns of lipid A were detected as type 1 (four N-linked 3-OH-C14:0 and two O-linked C16:0 fatty acids) or type 2 (four N-linked 3-OH-C14:0 fatty acids, one O-linked C14:0 fatty acid, and one O-linked C16:0 fatty acid). The MS/MS data also provided the information on lipid A composition, as summarized in Table 1. To locate the glycine residue in the inner core oligosaccharide, MS/MS experiments were performed on core oligosaccharide samples (3). The results revealed that glycine was located on the second heptose residue (HepII). Structural information on the linkage of glycine residue is under investigation.
TABLE 1.
MS data and proposed compositions for intact LOS of C. jejuni strain MF6 based on MS and MS/MS analyses
|
m/z of observed ion
|
Molecular mass (Da)
|
Proposed compositions
|
||||
|---|---|---|---|---|---|---|
| [M-4H]4− | [M-3H]3− | Observed | Calculateda | Sialylation and O modification in coreb | Phosphorylation in lipid A | Acylation in lipid A |
| 1,015.9 | 1,354.8 | 4,067.5 | 4,067.3 | Neu5Ac1 | PPEtn, P | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 1O-(C14:0), 1O-(C16:0) |
| 1,023.1 | 1,364.8 | 4,096.9 | 4,095.3 | Neu5Ac1 | PPEtn, P | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 2O-(C16:0) |
| 1,037.4 | 1,383.3 | 4,153.3 | 4,152.3 | Gly·Neu5Ac1 | PPEtn, P | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 2O-(C16:0) |
| 1,054.0 | 1,405.6 | 4,219.9 | 4,218.3 | Neu5Ac1 | PPEtn, PPEtn | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 2O-(C16:0) |
| 1,088.9 | 1,451.9 | 4,359.2 | 4,358.5 | Neu5Ac2 | PPEtn, P | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 1O-(C14:0), 1O-(C16:0) |
| 1,096.0 | 1,461.4 | 4,387.9 | 4,386.5 | Neu5Ac2 | PPEtn, P | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 2O-(C16:0) |
| 1,103.3 | 1,471.4 | 4,417.2 | 4,415.5 | Gly·Neu5Ac2 | PPEtn, P | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 1O-(C14:0), 1O-(C16:0) |
| 1,107.0 | 1,476.0 | 4,431.8 | 4,428.5 | Ac·Neu5Ac2 | PPEtn, P | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 2O-(C16:0) |
| 1,110.3 | 1,480.6 | 4,445.2 | 4,443.5 | Gly·Neu5Ac2 | PPEtn, P | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 2O-(C16:0) |
| 1,119.6 | 1,493.2 | 4,482.4 | 4,481.5 | Neu5Ac2 | PPEtn, PPEtn | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 1O-(C14:0), 1O-(C16:0) |
| 1,121.5 | 1,495.0 | 4,489.3 | 4,485.5 | Ac·Gly·Neu5Ac2 | PPEtn, P | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 2O-(C16:0) |
| 1,126.8 | 1,502.5 | 4,511.1 | 4,509.5 | Neu5Ac2 | PPEtn, PPEtn | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 2O-(C16:0) |
| 1,133.7 | 1,512.0 | 4,538.9 | 4,538.6 | Gly·Neu5Ac2 | PPEtn, PPEtn | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 1O-(C14:0), 1O-(C16:0) |
| 1,137.5 | 1,517.0 | 4,554.3 | 4,551.5 | Ac·Neu5Ac2 | PPEtn, PPEtn | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 2O-(C16:0) |
| 1,141.0 | 1,521.5 | 4,568.0 | 4,566.6 | Gly·Neu5Ac2 | PPEtn, PPEtn | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 2O-(C16:0) |
| 1,152.0 | 1,536.0 | 4,611.8 | 4,609.7 | Ac·Gly·Neu5Ac2 | PPEtn, PPEtn | 3 N-(3-OH-C14:0), 1 O-(3-OH-C14:0), 2O-(C16:0) |
Isotope-averaged mass units were used for calculation of molecular mass values based on the following proposed compositions: for hexose (Hex), 162.14; for HexNAc, 203.19; for Hep, 192.17; for 3-deoxy-d-manno-oct-2-ulosonic acid (KDO), 220.18; for P, 79.98; for PEtn, 123.05; for Neu5Ac, 291.26; for glycine (Gly), 57.05; for Ac, 42.03; for HexN, 161.07; for HexN3N, 160.18; for 3-OH-C14:0, 226.36; and for C16:0, 238.41; H2O, 18.01.
All the glycoforms contain a conserved structure: Hex4·HexNAc1·Hep2·PEtn1·KDO2.
Analysis of intact LOS prepared using microwave-assisted digestion.
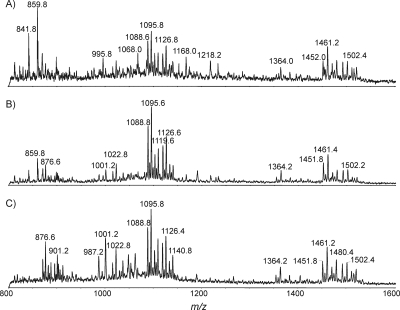
For LOS sample preparation, proteinase K was typically used as the first digestion enzyme to help break down cells, followed by the application of a DNase/RNase cocktail. However, for small colony samples, we found that there were no significant differences based on the order of reagent additions. Since proteinase K can also digest DNase and RNase, we added proteinase K right after DNase/RNase digestion without a denaturing step. To optimize the reaction conditions, microwave-assisted DNase/RNase digestion for five samples was initially carried out for 3 minutes. The five samples were digested with proteinase K for 1, 2, 3, 4, and 5 min. The extracted spectra for the samples with digestion times of 1, 3, and 5 min are presented in Fig. 4; for these samples, the optimal reaction time for proteinase K digestion was 3 minutes. When the reaction time was too long, the sample overheated and LOS degradation occurred, which was evident from the higher peaks associated with the lipid A moiety (e.g., m/z values of 987.2, 1,001.2, and 1,022.8) in Fig. 4C. Similarly, the time course of DNase/RNase digestion was investigated with a fixed reaction time of 3 min for proteinase K, and 3 min was also found to be the optimal time period for DNase/RNase digestion (data not shown). In all subsequent experiments, enzymatic digestion was carried out for only 3 minutes. Overall, with this protocol the sample preparation time was shortened from between 3 and 4 days to less than 1 hour (Fig. 1).
FIG. 4.
MS analysis of intact LOS after microwave-assisted proteinase K digestion for 1 min (A), 3 min (B), and 5 min (C). The analysis conditions were the same as those for Fig. 2.
Characterization of trace amounts of LOS.
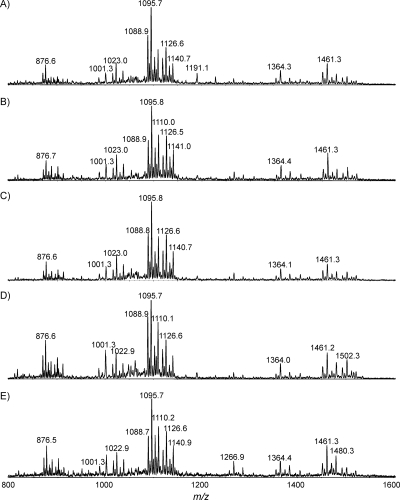
The reproducibility of the proposed protocol was evaluated with five replicate samples, each of them containing the cells from five colonies of C. jejuni MF6. The results for EA-OTLC-MS analysis are displayed in Fig. 5, and each spectrum demonstrated an excellent signal-to-noise ratio. The observed ions in the replicate samples were essentially identical to each other and to those detected from the whole-plate cell cultures or from dilutions thereof.
FIG. 5.
EA-OTLC-MS analysis of five intact LOS samples (A to E), each from five C. jejuni colonies, using the microwave-assisted digestion protocol. The analysis conditions were the same as those for Fig. 2.
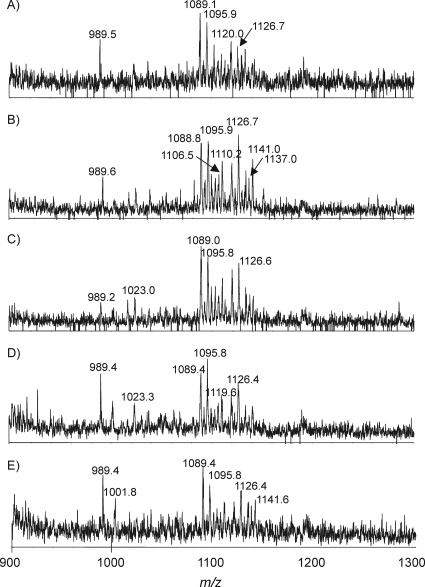
The sensitivity of the method was demonstrated through successful and reproducible characterization of single-colony samples, including that with MS and MS/MS. Figure 6 clearly indicates that the detection limit of the technique is at least at the single-colony level. The major glycoforms in all single-colony samples were the same as those in the five-colony samples. The speed and sensitivity of this technique demonstrate its potential for future diagnostic and clinical applications.
FIG. 6.
EA-OTLC-MS analysis of five intact LOS samples (A to E), each from a single C. jejuni colony. The analysis conditions were the same as those for Fig. 2.
MS/MS experiments were also carried out for intact LOS from single bacterial colonies. Figure 7 illustrates the product ion spectra of selected ions with m/z values of 1,126.7 (Fig. 7A), 1,137.0 (Fig. 7B), 1,096.0 (Fig. 7C), and 1,106.5 (Fig. 7D). All spectra contained an ion with an m/z of 290.2, indicating the presence of a sialic acid residue, and a fragment ion with an m/z of 581.3, confirming the presence of disialic acid residues. The observation of fragment ions with m/z values of 332.1 and 623.3 in the tandem mass spectra of ions with m/z values of 1,137.0 and 1,106.5 suggested the presence of Ac-Neu5Ac and Ac-Neu5Ac-Neu5Ac, respectively. An absence of fragment ions with m/z values of 332.1 and 623.3 for the product ion spectra of ions with m/z values of 1,126.7 and 1,096 suggested the absence of O-acetylated Neu5Ac in these glycoforms. The ion with an m/z of 1,577.8 was assigned to the core oligosaccharide fragment as expected (Hex4·HexNAc1·Hep2·PEtn1·KDO1). In addition, the fragment ion with an m/z of 1,062.5 (doubly charged ion) corresponded to lipid A, consisting of a disaccharide (GlcN3N or 2-amino-2-deoxy-d-glucose [GlcN]) to which two PPEtn, four N-linked 3-OH-C14:0, and two O-linked C16:0 fatty acids were attached. The fragment ion with an m/z of 1,001.4 (doubly charged ion) arose from the loss of phosphoethanolamine (PEtn), and that with an m/z of 1,922.9 (singly charged ion) was formed by the loss of the PPEtn residue from the lipid A moiety.
FIG. 7.
MS/MS analysis of intact LOS from C. jejuni MF6 (single colony). All experimental conditions were the same as those for Fig. 3. (A) Extracted MS/MS spectrum of the ion with an m/z of 1,126.7 ([M-4H]4−); (B) extracted MS/MS spectrum of the ion with an m/z of 1,137.0 ([M-4H]4−); (C) extracted MS/MS spectrum of the ion with an m/z of 1,096.0 ([M-4H]4−); (D) extracted MS/MS spectrum of the ion with an m/z of 1,106.5 ([M-4H]4−).
Analysis of intact LOS from GBS/MFS-associated isolates and enteritis controls.
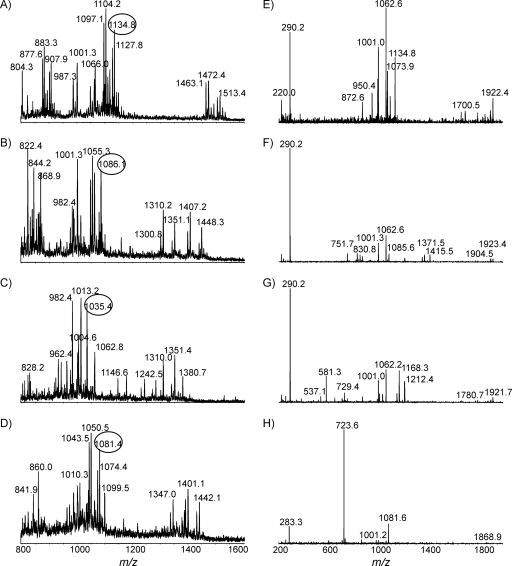
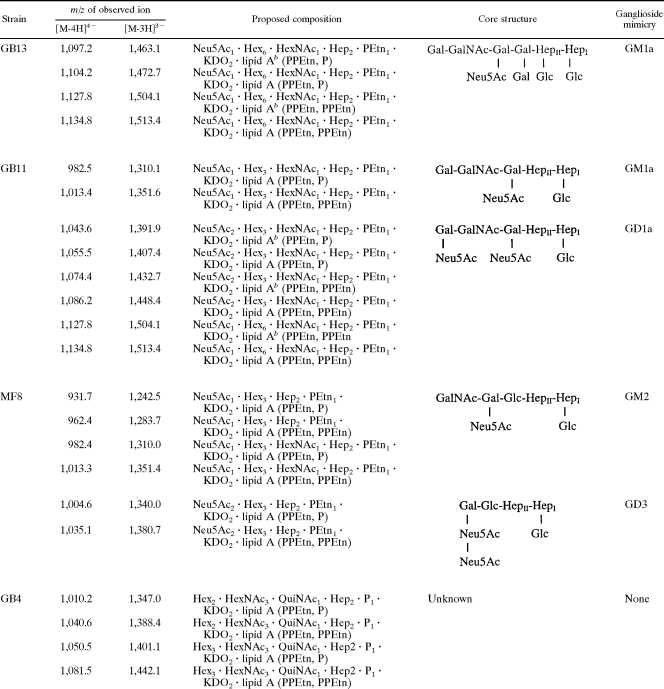
The MS spectra of intact LOS from four GBS-associated isolates, GB13, GB11, MF8, and GB4, are presented in Fig. 8A, B, C, and D, respectively. MS/MS results for each selected precursor are presented in Fig. 8E to H. For strains GB13, GB11, and MF8, detection of a fragment with an m/z of 290.2 suggested that the glycoforms were sialylated. The GM1a mimic was found in both GB13 and GB11, but the GD1a mimic was found only in GB11. The disialylation in strain MF8 was confirmed by the observation of a fragment ion with an m/z of 581.3 (Fig. 8G). It has been reported that MF8 expressed a mixture of mono- and disialylated LOS outer cores, whereas ganglioside mimics could not be detected in the LOS GBS-associated isolate GB4 (7). Detailed glycoform populations and chemical mimicry for each strain are summarized in Table 2, in which the variation in the lengths of the fatty acid chains is indicated as well.
FIG. 8.
EA-OTLC-MS and EA-OTLC-MS/MS of intact LOS. All experimental conditions were the same as those for Fig. 3. (A) Strain GB13; (B) strain GB11; (C) strain MF8; (D) strain GB4; (E) product ion spectrum with an m/z of 1,134.8 ([M-4H]4−); (F) product ion spectrum with an m/z of 1,086.1 ([M-4H]4−); (G) product ion spectrum with an m/z of 1,035.4 ([M-4H]4−); (H) product ion spectrum with an m/z of 1,081.4 ([M-4H]4−).
TABLE 2.
MS data and proposed compositions for intact LOS expressed by C. jejuni GB13, GB11, MF8, and GB4a
GalNAc, N-acetylgalactosamine; Gal, galactose; Glc, glucose; HexNAc, N-acetylhexosamine; KDO, 3-deoxy-d-manno-oct-2-ulosonic acid; QuiNAc, N-acetylquinovosamine.
bLipid A species containing fatty acid chains that are two methylene groups shorter than those in major species.
To further evaluate the application of the proposed strategy, a total of 73 clinical isolates were analyzed, and the data are summarized in Table S1 in the supplemental material. LOS outer core structures had already been proposed for the two MFS strains (MF6 and MF8) and 19 of the 21 GBS strains (the two exceptions being GB29 and GB30) by using O-deacylated LOS (7). We analyzed the intact LOSs of these strains by using the microwave preparation method and obtained mass species consistent with the structures proposed previously on the basis of the method using O-deacylated LOS. Moreover, we were able to determine if glycine was present in the LOSs of these strains. Intact LOS samples of the remaining GBS strains (GB29 and GB30) and enteritis controls (matched for age and sex) were also analyzed. We compare the occurrences of various sialic acid species in strains from patients who developed GBS/MFS and an enteritis control. As expected, sialic acid is clearly associated with GBS/MFS (78.3% versus 65.3% for enteritis controls). It is also interesting to note that glycine has a greater extent in GBS/MFS strains (91.3%) than in the enteritis control (69.4%). We do not consider the glycine residue a part of chemical mimicry; however, the presence of glycine on l-glycero-d-manno-heptose (Hep) may influence the conformation of LOS and consequently the epitope presentation. Further studies are under way to establish the genetic base for glycine expression and its biological function.
DISCUSSION
The combination of microwave-assisted digestion and EA-OTLC-MS analysis has allowed us to rapidly characterize C. jejuni LOS in order to accurately determine the glycan composition. Our method is not only rapid but also very sensitive and requires minimal material, as demonstrated by successful analysis using single-colony samples. The application of this technique has also enabled us to detect LOS-bound O-acetylated sialic acid and modification with O-linked glycine. The presence of glycine has previously been detected as a substituent in the second heptose of LOS (3), although the structural information on the linkage of the glycine residue could not be detected for C. jejuni LOS. This MS approach could complement conventional serological techniques used for the detection and identification of GBS-associated C. jejuni strains. Because this technique can identify the complete spectrum of ganglioside-like epitopes expressed by a given strain and also help to characterize strains in the case of infection by multiple strains, an MS approach may provide reliable indication as to the range of anti-ganglioside antibodies to be expected in a given patient. MS analysis of additional neuropathy-associated strains from various geographical regions may further expand our insight into the relationship between the structure of the LOS glycan and the clinical symptoms. Immunoassays can provide information about specific epitopes, but MS can provide a more complete picture of the LOS structures being expressed by a strain. It is our hope that accurate molecular fingerprinting of GBS strains provided by MS technology can be transferred from the academic world to a routine diagnostic laboratory. Further, we believe that the techniques outlined in this paper could also be implemented to study the LOSs and lipopolysaccharides from other disease-associated gram-negative bacterial species.
Supplementary Material
Footnotes
Published ahead of print on 27 August 2008.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Altheide, T. K., T. Hayakawa, T. S. Mikkelsen, S. Diaz, N. Varki, and A. Varki. 2006. System-wide genomic and biochemical comparisons of sialic acid biology among primates and rodents: evidence for two modes of rapid evolution. J. Biol. Chem. 28125689-25702. [DOI] [PubMed] [Google Scholar]
- 2.Corfield, A. P., S. R. Donapaty, S. D. Carrington, S. J. Hicks, R. Schauer, and G. Kohla. 2005. Identification of 9-O-acetyl-N-acetylneuraminic acid in normal canine pre-ocular tear film secreted mucins and its depletion in Keratoconjunctivitis sicca. Glycoconj. J. 22409-416. [DOI] [PubMed] [Google Scholar]
- 3.Dzieciatkowska, M., D. Brochu, H. P. Endtz, N. Yuki, R. S. Houliston, J. C. Richards, M. Gilbert, and J. Li. 2007. Mass spectrometric analysis of intact lipooligosaccharide from Campylobacter jejuni: the direct evidence for O-acetylated sialic acid and O-glycine. Biochemistry 4614704-14714. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert, M., M. F. Karwaski, S. Bernatchez, N. M. Young, E. Taboada, J. Michniewicz, A. M. Cunningham, and W. W. Wakarchuk. 2002. The genetic bases for the variation in the lipooligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 277327-337. [DOI] [PubMed] [Google Scholar]
- 5.Godschalk, P. C., M. Gilbert, B. C. Jacobs, T. Kramers, A. P. Tio-Gillen, C. W. Ang, N. Van den Braak, J. Li, H. A. Verbrugh, A. van Belkum, and H. P. Endtz. 2006. Coinfection with two different Campylobacter jejuni strains in a patient with the Guillain-Barré syndrome. Microbes Infect. 8248-253. [DOI] [PubMed] [Google Scholar]
- 6.Godschalk, P. C., A. P. Heikema, M. Gilbert, T. Komagamine, C. W. Ang, J. Glerum, D. Brochu, J. Li, N. Yuki, B. C. Jacobs, A. van Belkum, and H. P. Endtz. 2004. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barré syndrome. J. Clin. Investig. 1141659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godschalk, P. C., M. L. Kuijf, J. Li, F. St. Michael, C. W. Ang, B. C. Jacobs, M. F. Karwaski, D. Brochu, A. Moterassed, H. P. Endtz, A. van Belkum, and M. Gilbert. 2007. Structural characterization of Campylobacter jejuni lipooligosaccharide outer cores associated with Guillain-Barré and Miller Fisher syndromes. Infect. Immun. 751245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes, R. A., A. V. Swan, J. C. Raphael, D. Annane, R. van Koningsveld, and P. A. van Doorn. 2007. Immunotherapy for Guillain-Barré syndrome: a systematic review. Brain 1302245-2257. [DOI] [PubMed] [Google Scholar]
- 9.Juan, H. F., S. C. Chang, H. C. Huang, and S. T. Chen. 2005. A new application of microwave technology to proteomics. Proteomics 5840-842. [DOI] [PubMed] [Google Scholar]
- 10.Koga, M., M. Gilbert, J. Li, S. Koike, M. Takahashi, K. Furukawa, K. Hirata, and N. Yuki. 2005. Antecedent infections in Fisher syndrome: a common pathogenesis of molecular mimicry. Neurology 641605-1611. [DOI] [PubMed] [Google Scholar]
- 11.Koga, M., M. Gilbert, M. Takahashi, J. Li, S. Koike, K. Hirata, and N. Yuki. 2006. Comprehensive analysis of bacterial risk factors for the development of Guillain-Barré syndrome after Campylobacter jejuni enteritis. J. Infect. Dis. 193547-555. [DOI] [PubMed] [Google Scholar]
- 12.Koga, M., N. Yuki, T. Ariga, and K. Hirata. 1999. Antibodies to GD3, GT3, and O-acetylated species in Guillain-Barré and Fisher's syndromes: their association with cranial nerve dysfunction. J. Neurol. Sci. 16450-55. [DOI] [PubMed] [Google Scholar]
- 13.Li, J., M. Koga, D. Brochu, N. Yuki, K. Chan, and M. Gilbert. 2005. Electrophoresis-assisted open-tubular liquid chromatography/mass spectrometry for the analysis of lipooligosaccharide expressed by Campylobacter jejuni. Electrophoresis 263360-3368. [DOI] [PubMed] [Google Scholar]
- 14.Li, J., and J. C. Richards. 2007. Application of capillary electrophoresis mass spectrometry to the characterization of bacterial lipopolysaccharides. Mass Spectrom. Rev. 2635-50. [DOI] [PubMed] [Google Scholar]
- 15.Lill, J. R., E. S. Ingle, P. S. Liu, V. Pham, and W. N. Sandoval. 2007. Microwave-assisted proteomics. Mass Spectrom. Rev. 26657-671. [DOI] [PubMed] [Google Scholar]
- 16.Schauer, R., H. Schmid, J. Pommerencke, M. Iwersen, and G. Kohla. 2001. Metabolism and role of O-acetylated sialic acids. Adv. Exp. Med. Biol. 491325-342. [DOI] [PubMed] [Google Scholar]
- 17.Sun, W., S. Gao, L. Wang, Y. Chen, S. Wu, X. Wang, D. Zheng, and Y. Gao. 2006. Microwave-assisted protein preparation and enzymatic digestion in proteomics. Mol. Cell. Proteomics 5769-776. [DOI] [PubMed] [Google Scholar]
- 18.Swatkoski, S., S. Russell, N. Edwards, and C. Fenselau. 2007. Analysis of a model virus using residue-specific chemical cleavage and MALDI-TOF mass spectrometry. Anal. Chem. 79654-658. [DOI] [PubMed] [Google Scholar]
- 19.Varki, A. 2007. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 4461023-1029. [DOI] [PubMed] [Google Scholar]
- 20.Vesper, H. W., L. Mi, A. Enada, and G. L. Myers. 2005. Assessment of microwave-assisted enzymatic digestion by measuring glycated hemoglobin A1c by mass spectrometry. Rapid Commun. Mass Spectrom. 192865-2870. [DOI] [PubMed] [Google Scholar]
- 21.Willison, H. J., K. Townson, J. Veitch, J. Boffey, N. Isaacs, S. M. Andersen, P. Zhang, C. C. Ling, and D. R. Bundle. 2004. Synthetic disialylgalactose immunoadsorbents deplete anti-GQ1b antibodies from autoimmune neuropathy sera. Brain 127680-691. [DOI] [PubMed] [Google Scholar]
- 22.Yuki, N., K. Susuki, M. Koga, Y. Nishimoto, M. Odaka, K. Hirata, K. Taguchi, T. Miyatake, K. Furukawa, T. Kobata, and M. Yamada. 2004. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barré syndrome. Proc. Natl. Acad. Sci. USA 10111404-11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong, H., S. L. Marcus, and L. Li. 2005. Microwave-assisted acid hydrolysis of proteins combined with liquid chromatography MALDI MS/MS for protein identification. J. Am. Soc. Mass Spectrom. 16471-481. [DOI] [PubMed] [Google Scholar]
- 24.Zhong, H., Y. Zhang, Z. Wen, and L. Li. 2004. Protein sequencing by mass analysis of polypeptide ladders after controlled protein hydrolysis. Nat. Biotechnol. 221291-1296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.