Abstract
A new rapid immunochromatographic assay based on the signal amplification system (SAS) has been developed by Diagnostics for the Real World (Europe) Ltd. for the detection of hepatitis B virus surface antigen (HBsAg) in plasma or serum specimens. The SAS format features enhanced sensitivity as a result of an increased binding valence of the detector molecules. We have now evaluated the performance of the new HBsAg rapid test (DRW-HBsAg) in comparison with a well-established commercial rapid test (Determine HBsAg; previously from Abbott Laboratories; now from Inverness Medical Innovations) and with a CE-marked enzyme immunoassay (EIA) (Hepanostika HBsAg Ultra; BioMérieux) as the gold standard. Testing of serially diluted in-house HBsAg-positive samples, the World Health Organization standard, and sensitivity and reference panels yielded an analytical sensitivity for the DRW test of 0.2 to 0.8 IU/ml across HBsAg serotypes. Evaluation with eight commercially available seroconversion panels showed that the DRW-HBsAg test detected HBsAg an average of 6.1 days (range, 3 to 8 days) earlier than the Determine assay (P = 0.0078). Test sensitivity was also examined with two low-titer HBsAg EIA-positive panels in Beijing, China. Whereas 100% of these samples were detected by the DRW-HBsAg test, only 15.0% (P < 0.0001) and 87.3% (P < 0.0001), respectively, were detected by the Determine HBsAg test. The performance of the DRW-HBsAg test was further evaluated with samples determined to be HBsAg positive or negative by the EIA in Conakry, Guinea, and Beijing, China. No significant difference in sensitivity between the DRW and Determine tests was apparent with the HBsAg EIA-reactive samples from Guinea (96.7% versus 94.4%, respectively) or China (99.46 versus 98.92%, respectively). The specificity of the Determine HBsAg test was slightly higher than that of DRW-HBsAg test (100 versus 99.2%, respectively) with samples from EIA-negative blood donors in China. In conclusion, the new DRW HBsAg rapid test is more sensitive than the Determine HBsAg test and is suitable for diagnostic and blood screening in resource-limited settings.
In the past decade, rapid tests have become widely accepted for use in the diagnosis of and screening for infectious diseases in both developed and developing countries. Several rapid tests for antibodies to human immunodeficiency virus (HIV) have demonstrated performance levels similar to those of enzyme immunoassays (EIAs) and are approved by the U.S. Food and Drug Administration (FDA) and for the CE mark by the European Union (5, 9, 10, 11, 17). However, no approved rapid test for hepatitis B virus surface antigen (HBsAg) is currently available in the United States or Europe, because the sensitivity level required by the regulatory authorities has not been met.
In developing countries, rapid tests provide a flexible, technically undemanding, and relatively inexpensive approach to diagnostic testing. Rapid tests, in general, are simple to perform, do not require equipment, and are easy to interpret. Furthermore, their reagents can generally be stored at room temperature. In developing countries, where most blood is collected in small hospital-based facilities with a turnover of fewer than 10,000 units per year (70% of blood banks in sub-Saharan Africa), rapid tests for antibodies to HIV, for HBsAg, or for antibodies to hepatitis C virus contribute substantially to the safety of the blood supply (3).
Most rapid tests for the detection of antigens have a relatively low level of analytical sensitivity compared with those of EIAs (16, 18). Rapid tests for HBsAg have not performed as well as standard EIAs (3); none of the 33 HBsAg rapid tests currently available commercially (15) have met the performance requirements for FDA approval or CE marking (7, 8). The major challenge for HBsAg rapid tests is to detect the low levels of the target antigen that are present in a relatively high proportion of asymptomatic blood donors and thereby to achieve a clinical sensitivity similar to that of EIAs. One commercially available rapid assay (Determine HBsAg; previously from Abbott Laboratories; now from Inverness Biomedical Innovations) is widely used because of its higher sensitivity and excellent specificity compared with those of other available assays.
The aim of the present study was to evaluate a high-performance HBsAg rapid test (DRW-HBsAg) newly developed by Diagnostics for the Real World (Europe) Ltd. based on the signal amplification system (SAS) (12; H. H. Lee, H. Y. Hu, and L. Huang, November 2000, United Kingdom patent application PCT/GB01/05325). SAS technology enhances the sensitivity of rapid tests by amplifying the visual signal, an effect that is achieved as a result of an increased valence and size of the colored immune complex. The analytical and clinical sensitivities, as well as the specificity, of the DRW-HBsAg test were evaluated in comparison with those of the Determine HBsAg test. The performance of both assays was assessed by using a CE-marked HBsAg EIA (Hepanostika HBsAg Ultra; BioMérieux) as the gold standard. Field studies were performed to evaluate the performance of the new rapid test in settings with a high endemicity of hepatitis B virus (HBV) infection.
MATERIALS AND METHODS
Specimens and panels.
The following specimens and panels were tested to evaluate the analytical sensitivity or specificity of the DRW-HBsAg test.
(i) WHO standard.
The World Health Organization (WHO) HBsAg standard (subtype adw2, genotype A; NIBSC code number 03/262) was obtained from the National Institute for Biological Standard Control (NIBSC; Potters Bar, United Kingdom) and was tested in a series of fourfold dilutions (8.25, 2.06, 0.52, and 0.13 IU/ml) prepared from a stock of 33 IU per vial.
(ii) Serial dilutions of spiked panels.
The second WHO-designated international standard for HBsAg (subtype adw2, genotype A; NIBSC code number 00/588; 33 IU per vial) was used to estimate the concentration of HBsAg for in-house sensitivity panels. Serial dilutions of plasma specimens positive for ad or ay subtypes of HBsAg were prepared with a pool of citrate-treated HBsAg-negative plasma (Life Therapeutics, Clarkston, GA) as the diluent. The HBsAg concentrations (in international units per milliliter) of the in-house panels were determined by an EIA (Hepanostika HBsAg Ultra; BioMérieux) and standardized relative to the second WHO-designated international standard.
(iii) Sensitivity panels and seroconversion panels.
The HBsAg sensitivity panels PHA206 and PHA808 (Boston Biomedica Inc. [BBI], West Bridgewater, MA) were used to estimate the analytical sensitivity of the DRW-HBsAg assay. The HBsAg mixed-titer performance panel PHA206 consists of a set of 23 specimens with reactivities in commercial screening tests ranging from weakly to strongly positive. The HBsAg sensitivity panel PHA808 consists of 10 ad and 10 ay subtype specimens with HBsAg concentrations ranging from 0.71 to 0.02 and from 0.67 to 0.02 IU/ml, respectively. Eight seroconversion panels (PHM907, PHM909, PHM910, PHM927, PHM928, PHM929, PHM932, and PHM935; BBI), consisting of serial specimens collected at known intervals from individual plasma donors during seroconversion, were also tested. In addition, an HBsAg panel consisting of 10 calibrated members representing all common serotypes was obtained for testing from the Institut National de Transfusion Sanguine (INTS; Paris, France) (14).
(iv) Clinical specimens.
The specificity of the DRW-HBsAg assay was evaluated with fresh surplus EDTA-treated plasma samples (n = 205) from healthy blood donors, obtained at the Blood Center of the Stanford University School of Medicine (Stanford, CA), as well as with frozen EDTA-treated plasma samples from blood donors (n = 54) or pregnant women (n = 50) in Ghana.
HBsAg assays.
The DRW-HBsAg assay is a rapid immunochromatographic test for the qualitative detection of HBsAg that is performed manually and has a visual readout. The test is underpinned by the proprietary SAS technology, which improves the sensitivity of antigen detection by increasing the valence of antigen-antibody complexes (12; Lee et al., United Kingdom patent application PCT/GB01/05325). The test consists of a test strip (nitrocellulose membrane) and a single-use tube containing two lyophilized reagent beads. One reagent bead contains a hapten-labeled mouse monoclonal antibody to HBsAg (primary antibody), and the other contains a mouse monoclonal antibody to hapten conjugated to colloidal gold (secondary antibody). Serum or plasma (50 μl) is added to the tube, which is then mixed gently before insertion of the test strip. The sample mixture flows up the test strip by capillary action, and the visual result is read after 30 min. In the presence of HBsAg, the primary and secondary antibodies form visible complexes and are immobilized at the test line, which is coated with mouse monoclonal antibodies to HBsAg. The development of a purple line in the test line region indicates the presence of HBsAg in the sample. The absence of a colored test line indicates the absence of HBsAg or its presence at a concentration below the detection limit of the test. Each test strip also incorporates a built-in procedural control for reagent stability and test functionality; if the control line is absent, the test is invalid.
The Determine HBsAg assay (previously from Abbott Laboratories; now from Inverness Medical Innovations, Waltham, MA) was performed and interpreted in accordance with the recommendations of the manufacturer. The Hepanostika HBsAg Ultra (BioMérieux, Lyon, France) EIA was used as the gold standard and was performed as suggested by the manufacturer.
External evaluations.
The performance of the DRW-HBsAg rapid test was evaluated at the Beijing Red Cross Blood Center (BRCBC) in China in February and November of 2007 and at the National Blood Transfusion Center (NBTC) in Conakry, Guinea, in February to March 2007. At both sites, the DRW HBsAg rapid test and the Determine HBsAg test were performed in parallel and compared with the gold standard EIA.
At the BRCBC, donors are routinely screened with the Wantai HBsAg rapid test (Beijing Wantai Biological Pharmacy Enterprise, Beijing, China) before donation. Blood collected from donors who are negative by the Wantai test is further tested by two EIAs (Hepanostika HBsAg Ultra [BioMérieux, Lyon, France] and a locally produced HBsAg EIA from InTec Products, Inc., Xiamen, China). The sensitivity of the DRW and Determine HBsAg rapid tests was evaluated with two panels of selected low-titer HBsAg-positive samples that tested negative by the Wantai HBsAg rapid test but positive by EIA: a quality control (QC) panel and a mixed-titer panel. The QC panel routinely used at the BRCBC for test performance evaluation consists of 20 citrate-treated plasma specimens with an HBsAg concentration ranging from 0.6 to 8.4 IU/ml as determined by EIA (BRCBC and DRW, unpublished data). The mixed-titer panel is a collection of 126 citrate-treated plasma specimens assembled by the BRCBC. The specificities of the DRW and Determine HBsAg rapid tests were evaluated with surplus random citrate-treated plasma specimens (n = 498) that had tested negative by the Wantai HBsAg rapid test and the two EIAs routinely used at the BRCBC.
A second BRCBC field study was performed with 500 random blood donor serum specimens (485 negative, 15 positive) that had not been prescreened by the Wantai HBsAg rapid test and 171 clinical specimens confirmed to be positive for HBsAg by the reference EIA (Hepanostika HBsAg Ultra). At the NBTC in Conakry, Guinea, 491 surplus EDTA-treated plasma samples (399 negative, 92 positive) were tested by the DRW and Determine HBsAg rapid tests and one reference EIA (Hepanostika HBsAg Ultra). In addition, 88 stored serum specimens positive for HBsAg by EIA were tested at this site.
Statistical analysis.
The analytical sensitivities of the DRW and Determine HBsAg tests were compared with dilutions of an HBsAg standard, HBsAg-positive plasma samples, and sensitivity panels. The detection limit was defined as the highest dilution (or lowest HBsAg concentration) that could be detected visually by testing the sample in triplicate, with at least two replicates testing positive. The sensitivities of the rapid tests were also determined from the first samples of eight seroconversion panels that tested positive. The Wilcoxon rank test was used to determine the significance of the difference between the number of days until the first positive results for the two tests (19). The clinical sensitivity of each rapid test was calculated as the number of positive test results divided by the total number of HBsAg-positive samples as determined by EIA. Specificity was calculated as the number of negative test results divided by the total number of HBsAg-negative samples as determined by EIA. The 95% confidence intervals (CI) for sensitivity and specificity were calculated. Differences in clinical sensitivity or specificity between the two rapid tests were evaluated with McNemar's test for correlated proportions. A P value of <0.05 was considered statistically significant.
RESULTS
In-house evaluation of the DRW-HBsAg rapid test.
The performance of the DRW-HBsAg test was first assessed in-house with an HBsAg standard, commercial panels, and stored samples from previous studies and was compared with that of the Determine HBsAg test, conducted in parallel. The detection limit of the DRW-HBsAg test was 0.2 to 0.5 IU of HBsAg/ml irrespective of the serotype (Table 1). In contrast, the Determine HBsAg test had a detection limit of 2.0 to 3.5 IU/ml when evaluated with two in-house HBsAg sensitivity panels, the WHO HBsAg standard, and the PHA206 mixed-titer performance panel, and it failed to detect ad or ay subtypes of HBsAg in the PHA808 sensitivity panel. Testing of a serotyped and genotyped sensitivity panel from INTS showed that the sensitivities of the DRW and Determine HBsAg rapid tests ranged from 0.15 to 0.8 IU/ml and from 1.6 to 8 IU/ml, respectively (Table 2).
TABLE 1.
Analytical sensitivities of the DRW and Determine HBsAg rapid tests with calibrated samples of different serotypes
| Panela | Subtypeb | HBsAg detection limit (IU/ml)c by the following test:
|
|
|---|---|---|---|
| DRW | Determine | ||
| DRW | |||
| In-house HBsAg sensitivity panel 1 | ay | 0.23 | 3.5 |
| In-house HBsAg sensitivity panel 2 | ad | 0.30 | >2.0 |
| WHO HBsAg reference panel | adw2 | 0.50 | 2.0 |
| BBI | |||
| PHA206 mixed-titer performance panel | ND | 0.30 | 2.7 |
| PHA808 sensitivity panel | ad | 0.22 | NR |
| ay | 0.24 | NR | |
The DRW in-house HBsAg sensitivity panels were calibrated against the second WHO-designated international HBsAg standard (genotype A, subtype adw2) after the HBsAg concentrations were determined by the Hepanostika HBsAg Ultra EIA (BioMerieux, Lyon, France). The WHO HBsAg reference panel was obtained from NIBSC (code number 03/262).
ND, not determined.
NR, no member of the PHA808 panel was detected by the Determine HBsAg test.
TABLE 2.
Comparison of the DRW and Determine HBsAg rapid tests for detection of different HBsAg serotypes with a panel obtained from INTS
| Sample | Genotype | Subtype | HBsAg detection limit (IU/ml)a by the following test:
|
|
|---|---|---|---|---|
| DRW | Determine | |||
| P1 | A | ayw1 | 0.5 | 8 |
| P2 | D | ayw2 | 0.2 | 3.2 |
| P3 | D | ayw3 | 0.4 | 3.2 |
| P4 | E | ayw4 | 0.8 | 1.6 |
| P5 | C | ayr | 0.3 | 2.4 |
| P6 | A | adw2 | 0.2 | 3.2 |
| P7 | E | adw4 | 0.4 | 3.2 |
| P8 | C | adrq+ | 0.25 | 2.0 |
| P9 | C | adrq−hma | 0.15 | 2.4 |
| P10 | D | ayw3 fer | 0.2 | 6.4 |
The mean detection limits of the DRW and Determine HBsAg rapid tests were 0.34 IU/ml (95% CI, 0.22 to 0.46 IU/ml) and 3.56 IU/ml (95% CI, 2.27 to 4.85 IU/ml), respectively (P = 0.002).
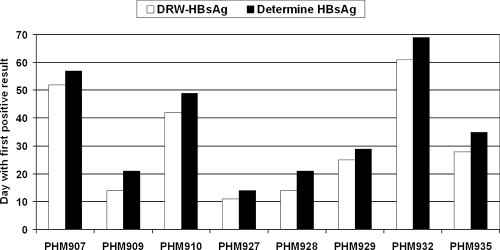
Testing of eight commercial seroconversion panels revealed that the DRW-HBsAg test detected HBsAg 6.1 ± 1.9 days earlier (mean ± standard error of the mean; range, 3 to 8 days; P = 0.0078) than the Determine HBsAg test (Fig. 1). In most instances, this difference corresponded to the detection of HBsAg one serial sample earlier by the DRW test than by the Determine HBsAg test.
FIG. 1.
Comparison of the sensitivities of two rapid tests, the DRW and Determine HBsAg tests, in detecting acute HBV infection during seroconversion. Seroconversion panels from BBI (West Bridgewater, MA) were used. The day during seroconversion on which HBsAg was first detected by each test is shown for eight panels. On average, the DRW-HBsAg test detected HBsAg 6.1 ± 1.9 days earlier (mean ± standard error of the mean; range, 3 to 8 days; P = 0.0078) than the Determine HBsAg test.
The specificity of the DRW-HBsAg test was evaluated with EDTA-treated HBsAg-negative plasma specimens from random blood donors in the United States (n = 205) and Ghana (n = 54) as well as from pregnant Ghanaian women (n = 50). The specificity of the DRW-HBsAg test ranged from 96 to 100% for these specimen sets, with an overall value of 98.7% (data not shown).
Field evaluation of the DRW-HBsAg rapid test.
The performance of the DRW-HBsAg rapid test was also assessed by independent collaborators using samples collected in developing countries with high prevalences of HBV infection. The test was performed with either fresh or frozen specimens.
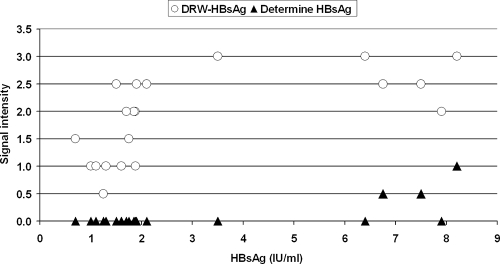
Test sensitivity was evaluated with two panels of selected low-titer HBsAg-positive samples from the BRCBC previously identified as nonreactive with the Wantai HBsAg rapid test but as reactive with two different EIAs: (i) an HBsAg mixed-titer panel consisting of 126 plasma samples and (ii) an HBsAg-positive panel consisting of 20 plasma specimens with HBsAg concentrations ranging from 0.6 to 8.4 IU/ml (BRCBC and DRW, unpublished), which is routinely used at the BRCBC as a QC panel. With the mixed-titer HBsAg-positive panel, the DRW-HBsAg test detected all 126 samples (sensitivity, 100%) whereas the Determine HBsAg test detected 110 samples (sensitivity, 87.3%) (P < 0.0001) (Table 3). The DRW-HBsAg rapid test detected all 20 members of the QC panel (sensitivity, 100%), whereas the Determine HBsAg test detected only 3 of these samples (sensitivity, 15%) (P < 0.0001) (Table 3). The relations between the signal intensity for the two rapid tests and the concentration of HBsAg in the 20-member QC panel are shown in Fig. 2. None of the samples containing HBsAg at <4 IU/ml was detected by the Determine HBsAg test.
TABLE 3.
Sensitivities and specificities of the DRW and Determine HBsAg rapid tests with selected low-titer HBsAg EIA-positive or HBsAg EIA-negative blood donor samples at the BRCBC
| HBsAg panel | Sensitivitya
|
Specificityb
|
||
|---|---|---|---|---|
| DRW | Determine | DRW | Determine | |
| Mixed-titer panel (n = 126) | 126/126 (100 [100-100]) | 110/126 (87.3 [81.5-93.1]) | ||
| EIA-positive QC panel (n = 20) | 20/20 (100 [100-100]) | 3/20 (15.0 [0-30.6]) | ||
| EIA-negative donor samples (n = 498) | 492/498 (98.8 [97.8-99.8]) | 498/498 (100 [100-100]) | ||
Expressed as the number of samples positive by the indicated test/number positive by EIA (percentage [95% CI]). The differences in sensitivity between the DRW and Determine tests were significant at P values (determined by McNemar's test) of <0.0001 for both the HBsAg mixed-titer panel and the HBsAg EIA-positive QC panel.
Expressed as the number of samples negative by the indicated test/number negative by EIA (percentage [95% CI]). The P value (determined by McNemar's test) for the difference in specificity between the DRW and Determine tests was 0.0313.
FIG. 2.
Comparison of dipstick signal intensity between two rapid tests, the DRW and Determine HBsAg tests, on a 20-member low-titer HBsAg EIA-positive QC panel from the BRCBC. The relation between reactivity, recorded as signal intensity, and HBsAg concentration is shown. The signal intensities for the DRW and Determine HBsAg rapid tests are expressed according to an arbitrary scale ranging from 0 to 5 (with a negative result graded as 0 and a saturated signal as 5). The HBsAg concentrations of the samples were determined by an EIA calibrated against the WHO HBsAg standard.
Another group of 186 HBsAg-positive specimens (171 from hospital patients and 15, showing EIA reactivity, from blood donors [out of 500 randomly selected blood donor specimens]) was tested at the BRCBC (Table 4). Compared with the EIA, the sensitivities of the DRW and Determine HBsAg rapid tests with these specimens were 99.46 and 98.92%, respectively.
TABLE 4.
Performance evaluation of the DRW and Determine HBsAg rapid tests in China and Guinea using EIA as the gold standarda
| Country | Test | Sensitivity
|
Specificity
|
||
|---|---|---|---|---|---|
| No. positive/true positivesb | % (95% CI) | No. negative/true negativesc | % (95% CI) | ||
| China | DRW | 185/186 | 99.46 (98.41-100) | 481/485 | 99.18 (98.37-99.98) |
| Determine | 184/186 | 98.92 (97.44-100) | 485/485 | 100 (100-100) | |
| Guinea | DRW | 174/180 | 96.66 (94.04-99.29) | 399/399 | 100 (100-100) |
| Determine | 170/180 | 94.44 (91.10-97.79) | 399/399 | 100 (100-100) | |
The sensitivities and specificities of both tests were calculated relative to the results of the Hepanostika HBsAg Ultra EIA (BioMerieux, Lyon, France).
Number of samples positive by the DRW or Determine HBsAg rapid test/number of samples positive by two EIAs. The 186 samples from China comprise 15 EIA-positive samples from 500 randomly selected blood donor samples and 171 HBsAg-positive clinical samples. The 180 samples from Guinea comprise 92 EIA-positive samples from 491 randomly selected blood donor samples and 88 stored HBsAg-positive samples.
Number of samples negative by the DRW or Determine HBsAg rapid test/number of samples negative by two EIAs.
At the NBTC in Conakry, Guinea, 88 stored HBsAg-positive samples and 92 blood donor samples positive for HBsAg by EIA (out of 491 random blood donor samples) were tested. Of the total of 180 HBsAg-positive samples detected by EIA, 174 and 170 were reactive with the DRW and Determine HBsAg rapid tests, respectively, giving sensitivities of 96.66 and 94.44%, respectively (Table 4). The signal/cutoff ratios determined by EIA for the 6 and 10 samples not detected by the DRW and Determine HBsAg tests, respectively, ranged between 1 and 2; two of the samples not detected by either test had HBsAg concentrations of <0.5 and 1.2 IU/ml.
For evaluation of test specificity, plasma samples from 498 blood donors at the BRCBC that were nonreactive with the Wantai HBsAg rapid test and two EIAs were tested. Compared with the EIA reference, the specificities of the DRW and Determine HBsAg rapid tests were 98.8 and 100%, respectively (Table 3). Testing of a group of 500 random blood donor specimens (485 negative, 15 positive), which were not prescreened by the Wantai rapid test at the BRCBC, revealed the specificities of the DRW and Determine HBsAg rapid tests to be 99.18 and 100%, respectively (Table 4). At the NBTC in Conakry, Guinea, testing of 491 random blood donor specimens revealed that all 399 samples that were negative by EIA were also negative by the two rapid tests, yielding a specificity of 100% for each test (Table 4).
DISCUSSION
The rapid tests that are currently available for the detection of HBsAg range in clinical sensitivity from 50 to 94% relative to EIA results depending on the technology on which they are based (2). Even those rapid tests based on lateral-flow technology, which appears to be the most sensitive format, do not achieve a sensitivity for HBsAg of 1 IU/ml (3, 18). The results of the present study now show that the newly developed DRW-HBsAg rapid test had an analytical detection limit between 0.2 and 0.8 IU/ml depending on the serotype, values that are similar to those for some HBsAg EIAs currently in use for diagnostic and blood screening purposes (13). This level of sensitivity was substantially greater than that observed with the Determine HBsAg rapid test, performed in parallel. The difference in sensitivity between these two rapid tests was also apparent from the results of testing of seroconversion panels, with the DRW test shortening latency to detection by 6.1 days.
Independent field studies performed in China and Guinea, two countries with high prevalences of chronic infection with HBV of different genotypes (genotypes B and C in China, genotype E in Guinea) (4), further highlighted the greater sensitivity of the DRW-HBsAg rapid test compared with the Determine HBsAg rapid test. Rapid tests and EIAs are routinely used for HBsAg screening in both countries. At the BRCBC, blood donors are routinely prescreened with a locally manufactured HBsAg rapid test (Wantai). Despite its apparent relatively low analytical sensitivity, the Wantai HBsAg rapid test identifies most HBsAg carriers with a clinical sensitivity of 98.39% (package insert, 2004). This relatively low level of performance is overcome after blood is collected by retesting with EIAs. Potential donors whose samples are reactive with the Wantai HBsAg test are rejected for donation, and there are no EIA data for these samples. Donor samples negative by the Wantai HBsAg test but positive by EIA constitute a selected population of two panels of low-titer HBsAg-positive specimens (a total of 146 samples), which were tested by the DRW and Determine HBsAg rapid tests for comparison of test sensitivity. The sensitivity of the DRW-HBsAg test was significantly greater than that of the Determine HBsAg test with these two panels. Given the lack of EIA data for the rejected donors, the true total number of positive samples from which the two panels of 146 samples were derived was estimated to be 9,068, with 8,922 reactive to the Wantai HBsAg rapid test on the basis of the claimed sensitivity of 98.39% for the Wantai rapid test relative to EIA. The sensitivities of the DRW and Determine HBsAg tests would thus be 100 and 98.7%, respectively, relative to the estimated true total number of positive samples, assuming that the estimated 8,922 positive samples identified by the Wantai HBsAg rapid test would also be reactive with both the DRW and the Determine HBsAg rapid test.
The difference in sensitivity between the DRW and Determine HBsAg rapid tests was no longer apparent when a group of unselected HBsAg-positive samples from random blood donors and hospital patients was tested at the BRCBC, likely because the frequency of low-level HBsAg positivity is relatively low in China (6). In Guinea, where HBV genotype E is prevalent, six samples that were reactive with the reference EIA were negative by both the DRW and Determine HBsAg tests, but an additional four EIA-positive samples were detected by the DRW but not by the Determine HBsAg test. Populations of HBsAg-positive blood donors in Guinea and Ghana were previously found to be infected almost exclusively with HBV genotype E, and 65% of these individuals had a viral (HBV DNA) load of <10,000 IU/ml (2; J.-P. Allain, unpublished data). In contrast, in China, where HBV genotypes B and C are prevalent, viral loads are generally high (Allain, unpublished). Although the level of HBV DNA is not necessarily correlated with that of HBsAg (1), lower HBsAg concentrations in the Guinean blood donors than in the Chinese blood donors and hospital patients likely explain why the difference in sensitivity between the two rapid tests was apparent in Guinea but not in China. The lack of sufficient sample volume precluded further testing of these specimens. In areas of high HBV endemicity, even a small gain in test sensitivity may translate into a substantial improvement in the level of blood safety (2).
Although assay sensitivity is the main criterion for HBsAg testing of blood donors or donations in developing countries with high prevalences of HBV infection, test specificity is also important, since it determines the need for result confirmation, and resources for such confirmation may be limited. The specificity of the Determine HBsAg test was slightly higher than that of the DRW-HBsAg test in a field evaluation in China, although the 99.18% specificity level achieved by the DRW-HBsAg test is generally considered adequate for regulatory and practical purposes.
In conclusion, our results indicate that the simple, rapid, and highly sensitive DRW HBsAg rapid test is a potentially powerful tool for blood screening and diagnostic testing in resource-limited areas of developing countries as well as in inner-city clinics of developed countries.
Acknowledgments
This work was supported by a grant (1R43HL071434) from the NIH.
We thank the staff of the Beijing Red Cross Blood Center in China and the National Blood Transfusion Center in Conakry, Guinea, for assistance during the study; M. Cheang of the University of Manitoba for performing statistical analysis; L. Ramaswamy, J. Tuan, and R. So for technical assistance; L. Huang for her work in identifying monoclonal antibodies used as the capture reagent in the DRW HBsAg rapid test; and C.-E. Michel for help with the organization of the trial in Guinea.
The author from the Diagnostics Development Unit, University of Cambridge, holds equity in a spin-off company, Diagnostics for the Real World (Europe) Ltd. (DRW), based on the rapid test technologies developed at the university. The University of Cambridge is also an equity holder of DRW. The authors from DRW declare no conflict of interest other than their employment, and the remaining authors declare no such conflicts.
Footnotes
Published ahead of print on 13 August 2008.
REFERENCES
- 1.Allain, J. P., D. Candotti, F. Sarkodie, and O. Opare-Sem. 2005. Lack of correlation between hepatitis B surface antigen and hepatitis B virus DNA levels in blood donors. Transfusion 451039-1040. [DOI] [PubMed] [Google Scholar]
- 2.Allain, J. P., D. Candotti, K. Soldan, F. Sarkodie, B. Phelps, C. Giachetti, V. Shyamala, F. Yeboah, M. Anokwa, S. Owusu-Ofori, and O. Opare-Sem. 2003. The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood 1012419-2425. [DOI] [PubMed] [Google Scholar]
- 3.Allain, J. P., and H. H. Lee. 2005. Rapid tests for detection of viral markers in blood transfusion. Expert Rev. Mol. Diagn. 531-41. [DOI] [PubMed] [Google Scholar]
- 4.André, F. 2000. Hepatitis B epidemiology in Asia, the Middle East and Africa. Vaccine 18(Suppl. 1)S20-S22. [DOI] [PubMed] [Google Scholar]
- 5.Branson, B. M. 2003. Point-of-care rapid tests for HIV antibodies. J. Lab. Med. 27288-295. [Google Scholar]
- 6.Chu, C. J., M. Hussain, and A. S. Lok. 2002. Quantitative serum HBV DNA levels during different stages of chronic hepatitis B infection. Hepatology 361408-1415. [DOI] [PubMed] [Google Scholar]
- 7.European Union. 2002. Commission decision of 7 May 2002 on common technical specifications for in vitro- diagnostic medical device (text with EEA relevance) (notified under document number c(2002) 1344) (2002/364/EC). Off. J. Eur. Commun. L13117-30. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:131:0017:0030:EN:PDF. [Google Scholar]
- 8.Food and Drug Administration. July-November 2007. Guidance for industry. Adequate and appropriate donor screening tests for hepatitis B; hepatitis B surface antigen (HBsAg) assays used to test donors of whole blood and blood components, including source plasma and source leukocytes. Center for Biologics Evaluation and Research, Food and Drug Administration, Department of Health and Human Services, Rockville, MD. http://www.fda.gov/Cber/gdlns/hbslotrel.htm.
- 9.Greenwald, J. L., G. R. Burstein, J. Pincus, and B. Branson. 2006. A rapid review of rapid HIV antibody tests. Curr. Infect. Dis. Rep. 8125-131. [DOI] [PubMed] [Google Scholar]
- 10.Lampe, M., B. Branson, S. Paul, C. Burr, E. Gross, C. Eicher, R. Maupin, D. Averitt, B. Forsyth, and M. G. Fowler. 2004. Rapid HIV-1 antibody testing during labor and delivery for women of unknown HIV status: a practical guide and model protocol. Centers for Disease Control and Prevention, Atlanta, GA.
- 11.Machado, A. A., R. Martinez, A. A. Haikal, and M. C. Rodrigues da Silva. 2001. Advantages of the rapid HIV-1 test in occupational accidents with potentially contaminated material among health workers. Rev. Inst. Med. Trop. Sao Paulo 43199-201. [DOI] [PubMed] [Google Scholar]
- 12.Michel, C.-E. E., A. W. Solomon, J. P. V. Magbanua, P. A. Massae, L. Huang, J. Mosha, S. K. West, E. C. B. Nadala, R. Bailey, C. Wisniewski, D. C. W. Mabey, and H. H. Lee. 2006. Field evaluation of a rapid point-of-care assay for targeting antibiotic treatment for trachoma control: a comparative study. Lancet 3671585-1590. [DOI] [PubMed] [Google Scholar]
- 13.Mizuochi, T., Y. Okada, K. Umemori, S. Mizusawa, and K. Yamaguchi. 2006. Evaluation of 10 commercial diagnostic kits for in vitro expressed hepatitis B virus (HBV) surface antigens encoded by HBV of genotypes A to H. J. Virol. Methods 136254-256. [DOI] [PubMed] [Google Scholar]
- 14.Norder, H., B. Hammas, S. Löfdahl, A.-M. Couroucé, and L. O. Magnius. 1992. Comparison of the amino acid sequences of nine different serotypes of hepatitis B surface antigen and genomic classification of the corresponding hepatitis B virus strains. J. Gen. Virol. 731201-1208. [DOI] [PubMed] [Google Scholar]
- 15.PATH. 2002. Rapid tests for hepatitis B: commercially available hepatitis B tests. www.rapid-diagnostics.org/rti-hepb-com.htm.
- 16.PATH. June 2005. HealthTech historical profile: lateral-flow, point-of-care diagnostic tests for infectious disease. http://www.path.org/publications/details.php?i=1134.
- 17.Stetler, H. C., T. C. Granade, C. A. Nunez, R. Meza, S. Terrell, L. Amador, and J. R. George. 1997. Field evaluation of rapid HIV serologic tests for screening and confirming HIV-1 infection in Honduras. AIDS 11369-375. [DOI] [PubMed] [Google Scholar]
- 18.WHO. May 2001. Hepatitis B surface antigen assays: operational characteristics (phase I). Report 1. WHO/BCT/BTS/01.4. Blood Safety and Clinical Technology, WHO, Geneva, Switzerland. www.who.int/diagnostics_laboratory/evaluations/en/hep_B_rep1.pdf.
- 19.Wilcoxon, F. 1945. Individual comparisons by ranking methods. Biometrics 180-83. [PubMed] [Google Scholar]