Abstract
For rapid and low-cost detection of multidrug-resistant (MDR) Mycobacterium tuberculosis, we applied the nitrate reductase assay (NRA) using a liquid medium directly to sputum samples. A total of 179 sputum samples were analyzed by the NRA, and results were compared to those obtained by the indirect proportion method (IPM) as a standard reference. Out of 144 specimens for which comparable results were available, only one discrepant result was obtained: MDR by NRA but susceptible by the IPM. In total 56% of the results were obtained in 10 days by the NRA. NRA performed in liquid medium is rapid and inexpensive and can be easily implemented in low-income countries.
Tuberculosis (TB) remains the greatest cause of mortality due to a single infectious agent and represents a major public health problem, especially in developing countries, now compounded by the rising incidence of multidrug-resistant (MDR) TB (5, 23, 24) and the human immunodeficiency virus/AIDS pandemic (12). Timely detection of MDR TB patients is therefore important to avoid the spread of MDR M. tuberculosis strains in the community.
Implementation of current standard tests for determining MDR TB in developing countries is limited by their cost or the time necessary to achieve results (7, 10, 17); several rapid and inexpensive tests have therefore been developed (1, 4, 8, 15). Among them, a low-cost colorimetric nitrate reductase assay (NRA), based on the ability of M. tuberculosis to reduce nitrate to nitrite, has been successfully applied on solid medium either indirectly using strains (4) or directly on sputum samples, resulting in a dramatic reduction of the time needed to obtain results (2, 14, 18).
Using a liquid medium, NRA has been applied to M. tuberculosis strains, with a significant improvement in the time to obtain results compared to NRA performed on solid media (19, 20). However, to our knowledge, NRA using liquid medium has not, so far, been applied directly to sputum samples.
In the present study we evaluated the application of NRA directly to sputum samples using Middlebrook 7H9 liquid medium for the rapid detection of M. tuberculosis resistance to rifampin (RMP) and isoniazid (INH). The results were compared to those obtained with the indirect proportion method (IPM) as a standard reference.
MATERIALS AND METHODS
Specimen processing.
From May to September 2007, a total of 179 consecutive smear-positive sputum samples from new and retreatment patients, with a positivity score of 1+ or more (21), were collected at the National Reference Mycobacteriology Laboratory in Cotonou, Benin. The samples (one per patient) were processed using the modified Petroff digestion decontamination method (22). After centrifugation the sediment was resuspended in 2 ml of sterile distilled water and portions were inoculated into NRA media and on Löwenstein-Jensen (LJ) medium, which was used later for the IPM.
Culture medium.
Culture medium 7H9-N (N for nitrate) consisted of Middlebrook 7H9 broth supplemented with 0.1% Casitone, 0.5% glycerol, 10% oleic acid-albumin-glucose-catalase (Becton Dickinson, Erembodegem, Belgium), and 2% PANTA antibiotic mixture (polymyxin B, amphotericin B, nalidixic acid, trimethoprim, azlocillin; Becton Dickinson) and 1,000 μg of sodium nitrate (NaNO3)/ml.
Inoculation.
For each specimen, 0.5 ml of the undiluted decontaminated suspension was inoculated into 4.6 ml of 7H9-N medium with RMP (2.0 μg/ml) and 4.6 ml of 7H9-N medium with INH (0.2 μg/ml) and 0.5 ml of the 1:10 dilution was inoculated into 4.6 ml of drug-free 7H9-N medium. The tubes were incubated at 37°C.
NRA.
The NRA was performed as described by Syre et al. (19) with a few modifications. Briefly, after 5 days of incubation, 0.2 ml of freshly prepared reagent mixture (1 part 50% concentrated hydrochloric acid, 2 parts 0.2% sulfanilamide, and 2 parts 0.1% N-1-naphtylethylenediamine dihydrochloride) was added to 1 ml of drug-free medium. The results were classified as negative (no color change) or positive (color change) and, depending on the color change, from 1+ (pink) to 4+ (deep red to violet).
If the color change in the drug-free medium was at least 2+ positive, the tubes with antibiotics were developed with the reagent mixture. Otherwise, the tubes were reincubated and the procedure was repeated at days 7, 10, 14, and 18.
To rule out bacterial contamination, 2 drops of each positive suspension were inoculated onto a blood agar plate, incubated at 37°C, and examined after 48 h.
For INH, results were interpreted as described by Angeby et al. (4): an isolate was considered resistant if the color change in the INH tube was equal to or greater than that in the 1:10-diluted growth control and was considered susceptible if there was no color change or if the color change was less than that in the 1:10-diluted growth control.
For RMP, the interpretation was modified: an isolate was considered resistant if there was any color change in the RMP tube and was considered susceptible if there was no color change in the RMP tube.
With the critical concentrations used, the criterion to define resistance to RMP was different from that for defining resistance to INH since, if the INH criterion was used, almost all RMP-resistant isolates would have falsely been considered susceptible.
IPM.
One LJ tube was inoculated with 0.2 ml of undiluted decontaminated suspension and incubated for up to 42 days. Isolates from this tube were used for IPM blindly performed on LJ medium according to the standard procedure with critical concentrations of 40 μg/ml for RMP and 0.2 μg/ml for INH (7).
Quality control.
Internal quality control was done using the fully susceptible M. tuberculosis H37Rv and a known MDR M. tuberculosis isolate.
Sequencing.
For the isolate with discordant results, rpoB, katG, and inhA gene sequencing was performed at the Institute of Tropical Medicine as described previously (11, 16).
MGIT test.
After the retreatment failure of the patient with discordant results, a manual mycobacterial growth indicator tube (MGIT) test was performed directly on the retreatment failure patient's sputum, as previously described (9).
Data analysis.
The performance of the NRA in comparison with the IPM was evaluated in terms of sensitivity and specificity. The agreement between the two methods, estimated on the basis of the kappa value, was interpreted as follows: <0.2, poor; 0.21 to 0.4, fair; 0.41 to 0.6, moderate; 0.61 to 0.8, good; ≥0.81, excellent (3).
RESULTS
Of the 179 specimens processed, the NRA was completed for 144 (80%). The remaining 35 specimens were culture negative at day 18 (24 specimens) or contaminated (11 specimens). Acid-fast bacillus test results for culture-negative specimens were 1+ for 10 specimens, 2+ for 9, and 3+ for 5.
Acid-fast bacillus test results for the 144 specimens were 1+ for 27 specimens (19%), 2+ for 61 specimens (42%), and 3+ for 56 specimens (39%).
NRA results were available for 1 specimen at day 5, 24 specimens (17%) at day 7, 56 specimens (39%) at day 10, 41 specimens (28%) at day 14, and 22 specimens at day 18. Thus, 56% of results were available in 10 days.
The comparison of NRA and IPM (Table 1) showed a discrepancy for one specimen for both INH and RMP. This specimen was found susceptible to RMP and INH by IPM but MDR by NRA. For this specimen, a repeated IPM showed the same result and the sequencing analysis found no mutations in rpoB, katG, and inhA genes. However, the specimen was taken from a patient with treatment failure, and after 5 months of retreatment regimen, his treatment failed again. Direct drug susceptibility testing (DST) using a MGIT test performed on the specimen from this patient confirmed that it was MDR.
TABLE 1.
Results of NRA compared to IPM
| Drug and NRA result | No. of specimens with IPM result of:
|
Sensitivity (%) | Specificity (%) | Agreement (kappa) | |
|---|---|---|---|---|---|
| Resistant | Susceptible | ||||
| INH | 100 | 99.2 | 0.91 | ||
| Resistant | 14 | 1 | |||
| Susceptible | 0 | 129 | |||
| RIF | 100 | 99.3 | 0.96 | ||
| Resistant | 6 | 1 | |||
| Susceptible | 0 | 137 | |||
Considering, however, the specimen for which the NRA result was discordant with that of IPM, the sensitivity and specificity of NRA for RMP were 100% and 99.3%, respectively, while for INH sensitivity and specificity were 100% and 99.2%, respectively. There was an excellent agreement between the two tests for both RMP and INH, with kappa values of 0.96 for RMP and 0.91 for INH.
Figure 1 shows NRA results for a susceptible isolate and an MDR isolate.
FIG. 1.
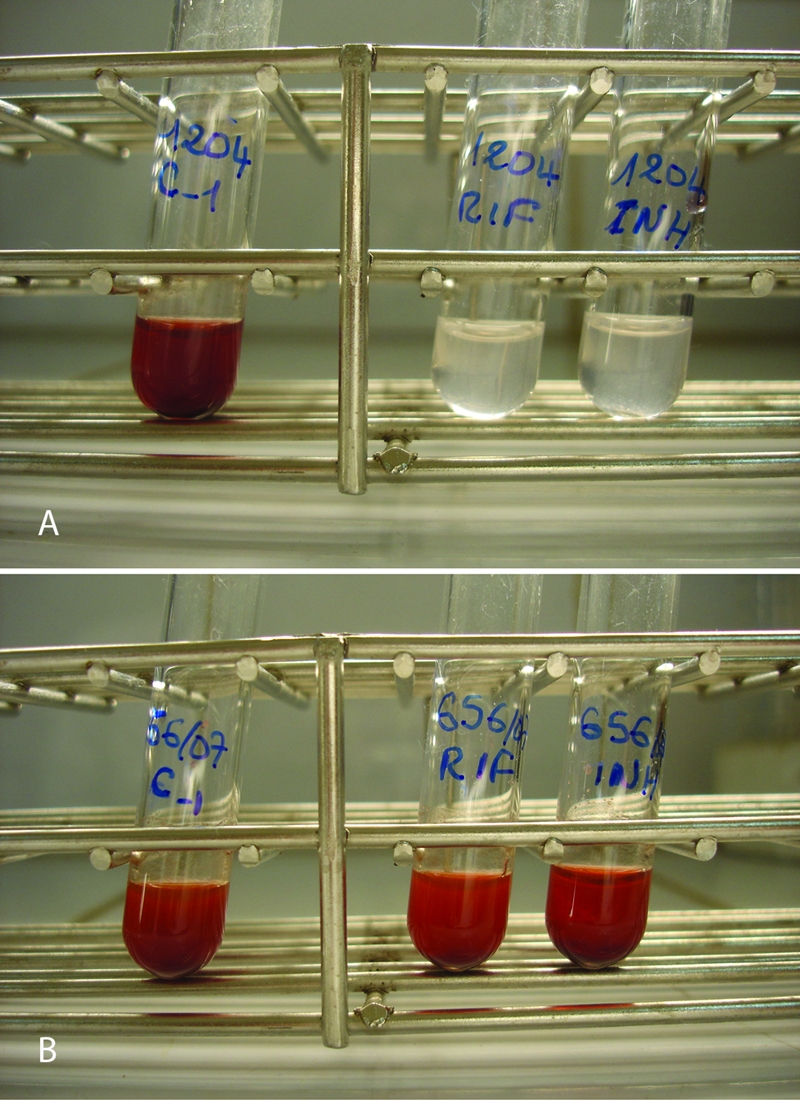
NRA results for susceptible (A) and MDR (B) isolates. C, control.
DISCUSSION
The ability of liquid medium to support faster growth of M. tuberculosis is in contrast to its susceptibility to contamination when applied for primary isolation from sputum specimens; the incorporation of antibiotics in the medium, however, can reduce or prevent contamination, as has been shown in mycobacterial isolation systems (9, 17). Liquid media have also been successfully used for rapid DST. Among the tests currently available, the MGIT test and the (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay have been applied directly to sputum samples to detect drug resistance, with a substantial reduction in the time to obtain results (1, 9). However, unlike the MGIT and MTT assays, the NRA described in the present study is simple and needs no additional equipment such as an MGIT machine, UV transilluminator, or optical density reader.
Moreover, the direct NRA performed in liquid medium could be used for all smear-positive sputum specimens, not only highly positive smear specimens; indeed the positivity grade for 61% of the specimens tested was 1+ or 2+.
The contamination rate is often of great concern when liquid media are used; the rate of contamination was, however, relatively limited in our study (7.6%) due to the use of the PANTA antimicrobial mixture. We also observed that almost all contaminated INH-containing tubes, but not the RMP-containing tube, still yielded nitrate reductase positivity, indicating that the majority of contaminants were susceptible to RMP. It is therefore recommended to carefully check for contamination in INH-monoresistant specimens.
In this study we used the commercial oleic acid-albumin-glucose-catalase supplement to stimulate the growth of mycobacteria and the PANTA antibiotic mixture to reduce the contamination rate; however, in order to further reduce the cost of the test, these solutions could also be prepared in a laboratory with adequate quality control.
In our setting in Benin, we have recently evaluated NRA on LJ medium applied directly to sputum samples and found that only 9% of results were available in 10 days (2); in the present study 56% of results were obtained in 10 days, representing thus a significant improvement (P < 0.01).
Using the IPM as the reference method, one false-resistant result was recorded, which was confirmed by sequencing analysis showing no mutations in the rpoB, katG, and inhA genes. The history of the patient was, however, consistent with MDR TB. It is possible that the sputum contained a mixture of susceptible and MDR bacilli, and, since MDR bacilli are sometimes more difficult to isolate in primary culture on LJ than susceptible bacilli (6), we may have selected only susceptible bacilli for IPM on LJ. A DST applied directly to sputum samples is indeed more representative of the population of bacteria present in the sputum (13). However, a mistake occurring at the inoculation of the different media could not be excluded.
Even when this result was considered as falsely resistant, the overall performance of NRA (sensitivity, specificity, and agreement with the standard) was excellent.
The number of resistant strains included in this study, mainly RMP resistant, is rather low. Further studies are ongoing to include a higher number of specimens with resistant strains and to adjust antibiotic concentrations used so that the same criterion to define resistance could be used for either RMP or INH. Further optimization of the technique is also needed to reduce the number of negative cultures by using undiluted sputa in the control tube.
Acknowledgments
We thank Gladys Anyo for the critical review of the manuscript.
Dissou Affolabi is supported by a grant from the Directorate General for the Development Cooperation (DGDC, Brussels, Belgium).
Footnotes
Published ahead of print on 27 August 2008.
REFERENCES
- 1.Abate, G., A. Aseffa, A. Selassie, S. Goshu, B. Fekade, D. WoldeMeskal, and H. Miörner. 2004. Direct colorimetric assay for rapid detection of rifampin-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 42871-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Affolabi, D., M. Odoun, A. Martin, J. C. Palomino, S. Anagonou, and F. Portaels. 2007. Evaluation of direct detection of Mycobacterium tuberculosis rifampin resistance by a nitrate reductase assay applied to sputum samples in Cotonou, Benin. J. Clin. Microbiol. 452123-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman, D. G. 1999. Practical statistics for medical research. Chapman & Hall/CRC, London, United Kingdom.
- 4.Angeby, K. A., L. Klintz, and S. E. Hoffner. 2002. Rapid and inexpensive drug susceptibility testing of Mycobacterium tuberculosis with a nitrate reductase assay. J. Clin. Microbiol. 40553-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz, M. A., A. Wright, A. Laszlo, A. De Muynck, F. Portaels, A. Van Deun, C. Wells, P. Nunn, L. Blanc, M. Raviglione, and WHO/International Union Against Tuberculosis And Lung Disease Global Project on Anti-Tuberculosis Drug Resistance Surveillance. 2006. Epidemiology of antituberculosis drug resistance (the Global Project on Anti-Tuberculosis Drug Resistance Surveillance): an updated analysis. Lancet 3682142-2154. [DOI] [PubMed] [Google Scholar]
- 6.Bastian, I., and F. Portaels. 2000. Multidrug-resistant tuberculosis: past, present and future, p. 1-15. In I. Bastian and F. Portaels (ed.), Multidrug-resistant tuberculosis, 1st ed. Kluwer Academic Publishers, London, United Kingdom.
- 7.Canetti, G., W. Fox, A. Khomenko, H. T. Malher, N. K. Menon, D. A. Mitchison, N. Rist, and N. A. Smelev. 1969. Advances in techniques of testing mycobacterial drug sensitivity and the use of sensitivity tests in tuberculosis control programmes. Bull. W. H. O. 4121-43. [PMC free article] [PubMed] [Google Scholar]
- 8.Caviedes, L., T. S. Lee, R. H. Gilman, P. Sheen, E. Spellman, E. H. Lee, D. E. Berg, S. Montenegro-James, and the Tuberculosis Working Group in Peru. 2000. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J. Clin. Microbiol. 381203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goloubeva, V., M. Lecocq, P. Lassowsky, F. Matthys, F. Portaels, and I. Bastian. 2001. Evaluation of mycobacteria growth indicator tube for direct and indirect drug susceptibility testing of Mycobacterium tuberculosis from respiratory specimens in a Siberian prison hospital. J. Clin. Microbiol. 391501-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology. A guide for the level III laboratory. Centers for Disease Control and Prevention, Atlanta, GA.
- 11.Kiepiela, P., K. S. Bishop, A. N. Smith, L. Roux, and D. F. York. 2000. Genomic mutations in the katG, inhA and aphC genes are useful for the prediction of isoniazid resistance in Mycobacterium tuberculosis isolates from Kwazulu Natal, South Africa. Tuber. Lung Dis. 8047-56. [DOI] [PubMed] [Google Scholar]
- 12.Laserson, K. F., and C. D. Wells. 2007. Reaching the targets for tuberculosis control: the impact of HIV. Bull. W. H. O. 85377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathew, S., C. N. Paramasivan, F. Rehman, R. Balambal, K. Rajaram, and R. Prabhakar. 1995. A direct rifampicin sensitivity test for tubercle bacilli. Indian J. Med. Res. 10299-103. [PubMed] [Google Scholar]
- 14.Musa, H. R., M. Ambroggi, A. Souto, and K. A. Angeby. 2005. Drug susceptibility testing of Mycobacterium tuberculosis by a nitrate reductase assay applied directly on microscopy-positive sputum samples. J. Clin. Microbiol. 433159-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palomino, J. C., A. Martin, M. Camacho, H. Guerra, J. Swings, and F. Portaels. 2002. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 462720-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rigouts, L., O. Nolasco, P. de Rijk, E. Nduwamahoro, A. Van Deun, A. Ramsay, J. Arevalo, and F. Portaels. 2007. Newly developed primers for comprehensive amplification of the rpoB gene and detection of rifampin resistance in Mycobacterium tuberculosis. J. Clin. Microbiol. 45252-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts, G. D., N. L. Goodman, L. Heifets, H. W. Larsh, T. H. Lindner, J. K. McClatchy, M. R. McGinnis, S. H. Siddiqi, and P. Wright. 1983. Evaluation of the BACTEC radiometric method for recovery of mycobacteria and drug susceptibility testing of Mycobacterium tuberculosis from acid-fast smear-positive specimens. J. Clin. Microbiol. 18689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solis, L. A., S. S. Shin, L. L. Han, F. Llanos, M. Stowell, and A. Sloutsky. 2005. Validation of a rapid method for detection of M. tuberculosis resistance to isoniazid and rifampin in Lima, Peru. Int. J. Tuber. Lung Dis. 9760-764. [PMC free article] [PubMed] [Google Scholar]
- 19.Syre, H., S. Phyu, P. Sandven, B. Bjorvatn, and H. M. Grewal. 2003. Rapid colorimetric method for testing susceptibility of Mycobacterium tuberculosis to isoniazid and rifampin in liquid cultures. J. Clin. Microbiol. 415173-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syre, H., H. Valvatne, P. Sandven, and H. M. Grewal. 2006. Evaluation of the nitrate-based colorimetric method for testing the susceptibility of Mycobacterium tuberculosis to streptomycin and ethambutol in liquid cultures. J. Antimicrob. Chemother. 57987-991. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. 1998. Laboratory services in tuberculosis control. Part II. Microscopy. WHO, Geneva, Switzerland.
- 22.World Health Organization. 1998. Laboratory services in tuberculosis control. Part III. Culture. WHO, Geneva, Switzerland.
- 23.World Health Organization. 2004. The WHO/IUATLD global project on antituberculosis drug resistance surveillance. Antituberculosis drug resistance in the world, report no. 3. WHO/HTM/TB/2005.349. WHO, Geneva, Switzerland.
- 24.World Health Organization. 2007. Global tuberculosis control: surveillance, planning, financing. WHO report 2007. WHO/HTM/TB/2007.376. WHO, Geneva, Switzerland.


