Abstract
Borrelial infection may manifest with a wide range of clinical signs, and in many cases, microbiological findings are essential for a proper diagnosis. This study included 48 patients with a working clinical diagnosis of Lyme neuroborreliosis, 45 patients with a working clinical diagnosis of suspected Lyme neuroborreliosis, and a control group comprising 42 patients with tick-borne encephalitis and 21 neurosurgical patients. The aim of the study was to analyze and compare findings of two PCR methods and Borrelia burgdorferi sensu lato culture results by examination of prospectively collected cerebrospinal fluid (CSF) and blood specimens from patients with clinical features of Lyme neuroborreliosis. Borrelial DNA was detected with at least one of the PCR approaches in 16/135 (11.9%) blood samples and 24/156 (15.4%) CSF samples. Using MseI restriction of PCR products of the amplified rrf-rrl region, we identified the majority of strains as Borrelia afzelii. Borreliae were isolated from 1/135 (0.7%) blood samples and from 5/156 (3.2%) CSF specimens. Using MluI restriction for characterization of isolated strains, Borrelia garinii was identified in all CSF isolates. Our study revealed that different approaches for direct demonstration of borrelial infection give distinct results, that there is an urgent need for standardization of the methods for direct detection of borrelial infection, and that the design of studies evaluating the validation of such methods should include appropriate control group(s) to enable assessment of both sensitivity and specificity.
Borrelia burgdorferi sensu lato is the causative agent of Lyme borreliosis, the most common tick-transmitted disease in Slovenia (22). In 2006, the incidence of the disease was 222.9 cases/100,000 inhabitants (http://www.ivz.si/javne_datoteke/datoteke/1420-03_Nalezljive_bolezni_2006.pdf). Borrelial infection may manifest with a wide range of clinical signs. The initial sign is most often erythema migrans, and from this skin lesion, bacteria can disseminate to various organs and may result in clinical manifestations such as multiple erythema migrans skin lesions, nervous system involvement, and arthritis. Early nervous system involvement (early Lyme neuroborreliosis) appears weeks to months after infection and is usually manifested by the involvement of cranial and/or peripheral nerves associated with meningitis (6, 18). In Europe, Lyme neuroborreliosis is mostly caused by Borrelia garinii, rarely by Borrelia afzelii, and only exceptionally by B. burgdorferi sensu stricto (3, 16, 23).
Microbiological findings are essential for the diagnosis of all clinical manifestations of Lyme borreliosis, with the exception of typical erythema migrans (19, 20). Several microbiological approaches have been utilized for demonstration of borrelial infection, such as isolation of borreliae from clinical specimens, detection of borrelial DNA by PCR in tissue or tissue fluids, and detection of specific antibodies (24). Isolation of borreliae enables reliable confirmation of the etiology of the infection, but the procedure is slow, labor intensive, and expensive and has low sensitivity (10). In comparison with culture, PCR is faster and appears to be more sensitive (24). Although PCR has been accepted as being of high diagnostic value for the detection of borreliae in synovial fluid and in synovia, its diagnostic significance for other tissue and tissue fluids has been equivocal (1, 20).
The aim of the present study was to analyze and compare the findings of two PCR methods and B. burgdorferi sensu lato culture results by examination of prospectively collected cerebrospinal fluid (CSF) and blood specimens obtained from patients with clinical features of Lyme neuroborreliosis.
MATERIALS AND METHODS
Definitions and basic characteristics of patients with Lyme neuroborreliosis and patients in the control groups.
The group of patients with Lyme neuroborreliosis comprised 48 adults with a working clinical diagnosis of Lyme neuroborreliosis (erythema migrans within 4 months before the appearance of neurological symptoms and signs, including radiculoneuritis and/or peripheral facial palsy, and pleocytosis) and 45 adults with a working clinical diagnosis of suspected Lyme neuroborreliosis (erythema migrans within 4 months before the appearance of neurological symptoms, but no pleocytosis). At inclusion in the study (time of the initial examination), the median duration of neurological signs/symptoms was 10 (median, 2 to 90) days in patients with Lyme neuroborreliosis and 14 (1 to 120) days in those with suspected Lyme neuroborreliosis. Erythema migrans was still present in 13/48 patients with a working clinical diagnosis of Lyme neuroborreliosis and in 40/45 patients with suspected Lyme neuroborreliosis (P > 0.0001 [chi-square test]). The control group comprised 42 adult patients with tick-borne encephalitis (TBE; clinical signs/symptoms of meningoencephalitis, pleocytosis, and serological confirmation of TBE virus infection demonstrated by the presence of specific serum immunoglobulin M and immunoglobulin G antibodies) and 21 adult patients with no signs of borrelial or TBE virus infection from whom CSF samples were collected during surgery for brain tumors at the Department of Neurosurgery, University Medical Center Ljubljana. Patients with (suspected) Lyme neuroborreliosis and those with TBE presented between 2005 and 2007 at the Department of Infectious Diseases, University Medical Center Ljubljana; patients treated at the Department of Neurosurgery presented in 2007.
The study approach was approved by the Medical Ethics Committee at the Ministry of Health of the Republic of Slovenia.
Material for PCR testing and culture.
Blood (5 ml) and CSF (1 ml) samples were obtained at initial examination from all patients with a working clinical diagnosis of Lyme neuroborreliosis, suspected Lyme neuroborreliosis, and TBE. In the control group of neurosurgical patients, only CSF samples (1 ml) were acquired.
Methods for confirmation of borrelial infection.
To establish borrelial infection we cultivated specimens in modified Kelly-Pettenkofer (MKP) medium and amplified two DNA targets—the intergenic rrf-rrl region and the gene ospA.
Isolation of Borrelia strains from blood and CSF.
One milliliter of CSF, obtained by lumbar puncture, was immediately inoculated into a tube with 5 ml of MKP medium and promptly transported to the laboratory (16). Five milliliters of blood obtained by venipuncture was placed in a tube containing sodium citrate and centrifuged at 100 × g for 5 min, and the supernatant was inoculated into two or more tubes of MKP medium (16, 21, 23). Samples were cultivated at the Institute of Microbiology and Immunology of the Faculty of Medicine Ljubljana at 33°C as described previously (16) and were examined once a week for the presence of spirochetes by using dark-field microscopy. Samples were considered negative if no growth was detected after 9 weeks of incubation for CSF and 12 weeks for blood (16).
Genotypic characterization of isolated strains.
Borrelial DNA from positive cultures was isolated by the gel-insert method as previously described (3, 14, 16). For species identification, borrelial DNA was digested overnight at 37°C with the restriction endonuclease MluI, and fragments were separated by pulsed-field gel electrophoresis as described elsewhere (3, 14, 16).
Nucleic acid extraction from blood and CSF.
Blood with EDTA was centrifuged at 100 × g for 10 min. Separated plasma and approximately 1 ml of CSF were centrifuged at 14,000 × g for 1 h. Nucleic acids from plasma and CSF pellets were extracted with a QIAamp DNA mini kit (Qiagen, Santa Clara, CA). Initially, 180 μl lysing buffer and 20 μl proteinase K were added to the pellets and incubated for 1 h at 56°C; the rest of extraction was performed according to the manufacturer's instructions.
Nested PCR of the intergenic rrf-rrl region and restriction fragment length polymorphism (RFLP) analysis.
PCR amplification was performed using primers described by Postic et al. (15). Briefly, 10 μl of isolated DNA was amplified with external primers SPA1 and SPA2 and internal primers P1 and P2 by using 60 cycles of 3 min at 94°C, followed by 20 cycles of 3 min at 93°C, 2 min at 70°C, and 2 min at 72°C, and then 40 cycles of 1 min at 93°C, 2 min at 50°C, and 2 min at 72°C, followed by a final 7-min hold at 72°C. PCR amplification resulted in a 250-bp product. Amplicons were visualized on a 1% agarose gel stained with ethidium bromide (15).
A panel of positive and negative control samples was included in each experiment to control for amplification and contamination. We also took precautions to avoid PCR contamination and amplicon carry-over; samples were processed in separate rooms, and the use of plugged pipette tips was obligatory (18). The quality of each DNA sample (verification of DNA extraction and inhibition control) isolated from blood was verified with amplification of a 268-bp fragment of the gene for human β-globin by using PC04 and GH20 primers (8). The quality of DNA extraction and the presence of inhibitors in CSF were monitored by spiking 45 μl of CSF with 5 μl of B. afzelii culture before the PCR protocol was performed.
To determine Borrelia species, 20 μl of the nested-PCR product of the amplified rrf-rrl region were subjected to RFLP analysis by digestion with 5 U of the MseI restriction enzyme (Biolabs, New England) as described by Postic et al. (15). Electrophoresis was carried out in a 16% acrylamide-0.8% bisacrylamide gel for 2 h at 110 V. RFLP of samples was compared with RFLP of particular B. burgdorferi sensu lato species (15).
Nested PCR of ospA.
Primers for amplification of ospA described by Guy and Stanek (9) were used in nested PCR under the conditions described by Zore et al. (25). The reaction was carried out in 30 cycles of 95°C for 45 s, 50°C for 45 s, and 72°C for 60 s. Each sample was transferred to a second reaction and amplified under the same conditions for another 30 cycles. A panel of positive and negative control samples was included in each experiment to control for amplification and contamination. PCR amplification resulted in a 351-bp product. Amplification products were analyzed on ethidium bromide-stained 1% agarose gels.
Statistical analysis.
Yates's corrected χ2 test or two-tailed Fisher′s exact test with the level of significance set at P < 0.05 was used for statistical comparison of descriptive data.
RESULTS
Borrelial DNA in blood and CSF samples was detected using amplification of two target DNA sequences, the intergenic rrf-rrl region and the gene ospA. The results of the two approaches and a comparison of the results are shown in Table 1. Concordant findings of the two PCR methods were established in 131/135 (97%) blood samples and in 146/156 (93.6%) CSF specimens.
TABLE 1.
Comparison of results of two PCR methodsa
| Findings | Sample type | No. (%) of samples from patients with:
|
Total no. (%) of samples | |||
|---|---|---|---|---|---|---|
| NB | sNB | TBE | NsP | |||
| ospA pos, rrf-rrl pos | Blood | 5 (10.4%) | 5 (11.1%) | 2 (4.8%) | ND | 12 (8.9%) |
| CSF | 7 (14.5%) | 6 (13.4%) | 1 (2.4%) | 0 | 14 (9%) | |
| ospA neg, rrf-rrl neg | Blood | 41 (85.4%) | 38 (84.5%) | 40 (95.2%) | ND | 119 (88.1%) |
| CSF | 35 (72.9%) | 36 (80.0%) | 40 (95.2%) | 21 (100%) | 132 (84.6%) | |
| ospA neg, rrf-rrl pos | Blood | 0 | 2 (4.4%) | 0 | ND | 2 (1.5%) |
| CSF | 3 (6.3%) | 2 (4.4%) | 0 | 0 | 5 (3.2%) | |
| ospA pos, rrf-rrl neg | Blood | 2 (4.2%) | 0 | 0 | ND | 2 (1.5%) |
| CSF | 3 (6.3%) | 1 (2.2%) | 1 (2.4%) | 0 | 5 (3.2%) | |
| Total | Blood | 48 (100.0%) | 45 (100.0%) | 42 (100.0%) | ND | 135 (100.0%) |
| CSF | 48 (100.0%) | 45 (100.0%) | 42 (100.0%) | 21 (100%) | 156 (100.0%) | |
PCR methods include amplification of the gene ospA and the intergenic rrf-rrl region in blood and CSF samples of patients with Lyme neuroborreliosis (NB), suspected Lyme neuroborreliosis (sNB), TBE and neurosurgical patients (NsP). ND, not done; pos, positive; neg, negative.
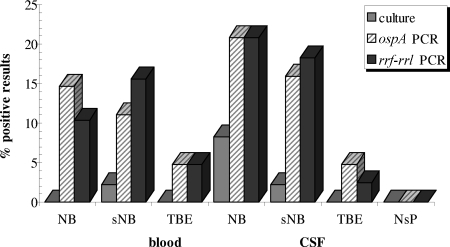
PCR targeting ospA detected the presence of borrelial DNA in 14/135 (10.4%) blood samples and in 19/156 (12.2%) CSF specimens. Blood samples were positive in 7/48 (14.6%) patients with a working clinical diagnosis of Lyme neuroborreliosis, in 5/45 (11.1%) patients with suspected Lyme neuroborreliosis, and in 2/42 (4.8%) of patients with TBE (nonsignificant differences). The corresponding results for CSF samples were 10/48 (20.8%), 7/45 (15.6%), and 2/42 (4.8%), respectively. PCR targeting ospA found no borrelial DNA in the CSF samples of the 21 patients in the neurosurgical control group (Table 1 and Fig. 1). Significant differences were found when comparing the Lyme neuroborreliosis group with the neurosurgical group (P = 0.0259) and with the control group overall (TBE and neurosurgical group together) (P = 0.0078), but not with the TBE group.
FIG. 1.
Comparison of results of cultivation and PCRs targeting the gene ospA and the rrf-rrl intergenic region in blood and CSF specimens of patients with Lyme neuroborreliosis (NB), suspected Lyme neuroborreliosis (sNB), tick-borne encephalitis (TBE), and neurosurgical patients (NsP). Blood samples of NsP were not tested.
For the group with suspected Lyme neuroborreliosis, the difference was significant only in comparison with the whole control group (P = 0.0324).
Using PCR targeting the rrf-rrl region, 14/135 (10.4%) blood samples and 19/156 (12.2%) CSF samples tested positive. Blood samples were positive in 5/48 (10.4%) patients with a working clinical diagnosis of Lyme neuroborreliosis, in 7/45 (15.6%) patients with suspected Lyme neuroborreliosis and in 2/42 (4.8%) patients with TBE (nonsignificant differences). The corresponding findings for CSF were 10/48 (20.8%), 8/45 (17.8%) and 1/42 (2.4%), respectively (Table 1). Absence of borrelial DNA in the CSF of the neurosurgical patients was established with PCR targeting the rrf-rrl region. Significant differences were found when comparing the Lyme neuroborreliosis group with the TBE group (P = 0.0191), the neurosurgical group (P = 0.0259), and the control group overall (P = 0.0009). For the group with suspected Lyme neuroborreliosis, the corresponding P values were 0.0306 for comparison with the TBE group, 0.0478 for comparison with the neurosurgical group, and 0.0037 for comparison with the control group overall. The simultaneous presence of borrelial DNA in both blood and CSF was established in only three patients—in two with working clinical diagnosis of Lyme neuroborreliosis and in one with suspected Lyme neuroborreliosis.
With MseI restriction of the PCR product of the amplified intergenic rrf-rrl region, we were able to characterize 13/14 (92.9%) DNA-positive blood samples and 18/19 (94.7%) DNA-positive CSF samples; the majority of strains were found to be B. afzelii (Table 2).
TABLE 2.
Identification of Borrelia species in CSF and blood samples by using RFLP analysis after MseI restriction of the rrf-rrl PCR amplicon
| Species | No. of samples from patients with:
|
|||||
|---|---|---|---|---|---|---|
| Lyme neuroborreliosis
|
Suspected Lyme neuroborreliosis
|
TBE
|
||||
| CSF | Blood | CSF | Blood | CSF | Blood | |
| B. afzelii | 3 | 2 | 5 | 4 | 0 | 2 |
| B. garinii | 3 | 1 | 2 | 2 | 1 | 0 |
| B. burgdorferi sensu stricto | 1 | 1 | 0 | 0 | 0 | 0 |
| B. afzelii + B. garinii | 2 | 0 | 1 | 1 | 0 | 0 |
| Nontypeable | 1 | 1 | 0 | 0 | 0 | 0 |
Borreliae were isolated from 1/135 (0.7%) blood samples and from 5/156 (3.2%) CSF specimens. Four out of the five CSF isolates were from patients with a clinical diagnosis of Lyme neuroborreliosis; the fifth was from a patient with suspected Lyme neuroborreliosis. Borreliae were not isolated from CSF samples of patients without signs of borrelial infection (control group of TBE and neurosurgical patients). Although the isolation rate was low overall, it was higher in patients with a clinical diagnosis of Lyme neuroborreliosis than in those in the control group (4/48 versus 0/63; P = 0.0235). The findings are depicted in Fig. 1.
We were able to identify to the species level all five strains isolated from CSF and the single strain isolated from blood (Table 3). Using pulsed-field gel electrophoresis after MluI restriction, four of the CSF isolates (all from patients with a working clinical diagnosis of Lyme neuroborreliosis) were found to be B. garinii (three typed as Mlg2, one as Mlg3); the fifth CSF isolate (from a patient with suspected Lyme neuroborreliosis) was B. afzelii (typed as Mla1). The strain isolated from the blood of a patient with suspected Lyme neuroborreliosis was also found to be B. afzelii (typed Mla1).
TABLE 3.
Genotypic characterization of all strains isolated from CSF and blood samples in comparison with results obtained in two PCR approachesa
| Diagnosis (sample type) | MluI genotyping | PCR targeting the rrf-rrl gene (species) | PCR targeting the ospA gene |
|---|---|---|---|
| NB (CSF) | B. garinii Mlg2 | Neg | Pos |
| NB (CSF) | B. garinii Mlg2 | Neg | Neg |
| NB (CSF) | B. garinii Mlg3 | Neg | Pos |
| NB (CSF) | B. garinii Mlg2 | Neg | Neg |
| sNB (CSF) | B. afzelii Mla1 | Pos (B. afzelii) | Pos |
| sNB (blood) | B. afzelii Mla1 | Neg | Neg |
NB, Lyme neuroborreliosis; sNB, suspected Lyme neuroborreliosis; Pos, positive PCR result; Neg, negative PCR result.
In only half of the culture-positive CSF and blood specimens were we able to demonstrate the presence of borrelial DNA directly in the corresponding samples with at least one of the two PCR approaches (Table 3).
DISCUSSION
The diagnosis of early Lyme neuroborreliosis is usually based on clinical characteristics and laboratory findings. Lymphocytic pleocytosis and demonstration of borrelial infection of the central nervous system (in Europe usually by demonstration of specific intrathecal antibody production) are essential for the diagnosis (1, 2, 19). In the present study, we focused our attention on the direct demonstration of borrelial infection by analyzing two differing approaches—isolation of the etiological agent from patient samples (blood and CSF) and detection of borrelial DNA in CSF and blood. For the latter approach we amplified two target DNAs, the gene ospA and the intergenic ribosomal rrf-rrl region. Specimens were obtained from a group of patients with a working clinical diagnosis of Lyme neuroborreliosis and a group with suspected Lyme neuroborreliosis. To determine the specificity of cultivation and the PCR methods, CSF and blood samples of patients with TBE and CSF samples of neurosurgical patients with no signs of borrelial infection were also tested. Our main intention was to evaluate these methods for the routine diagnosis of Lyme neuroborreliosis.
Borrelial DNA in blood was detected in 16/135 (11.9%) specimens with at least one of the PCR approaches; the corresponding result for CSF was 24/156 (15.4%) (Table 1). It has been postulated that in patients with Lyme neuroborreliosis, borreliae disseminate from skin through the blood into the central nervous system (19, 20). We expected to detect borreliae during transit through the blood in some of the patients with Lyme neuroborreliosis and to establish the presence of borrelial DNA in a larger proportion of CSF than blood. However, there was no significant difference in positivity between blood and CSF within the individual PCR target approaches or when the findings of the two PCR approaches were compared. The main advantage of targeting the rrf-rrl region rather than ospA is the possibility of identifying the borrelial species. In this way, we were able to characterize all but two (31/33 [94%]) PCR-positive blood and CSF samples (Table 2). The two specimens that were not characterized had an unusual restriction pattern that will be investigated further using sequence analysis. In the group of patients with Lyme neuroborreliosis, all the main borrelial species that cause disease in humans were found, whereas in the group with suspected Lyme neuroborreliosis and the group with TBE, the only species present were B. afzelii and B. garinii (Table 2). Overall, B. afzelii was the species most frequently identified, although it is not the principal causative agent of Lyme neuroborreliosis in Europe (23).
Low sensitivity of borrelia detection in the blood of patients with (suspected) Lyme neuroborreliosis (Table 1) could have been the consequence of a transitional spirochetemia, which in the majority of patients most probably ended at the time of central nervous system involvement, and/or the result of low numbers of spirochetes in the blood. With regard to the method itself, possible inhibitors in host blood can diminish positive findings (1). Although we predicted a low proportion of PCR-positive blood samples, we were surprised to find an almost equal CSF-positive rate (Table 1). According to literature reports, the sensitivity of PCR in CSF ranges from 12% to 100% (median, 23%) and depends upon numerous factors, including method, origin of samples, and the differing approaches used in individual studies (1).
Several significant differences were established when comparing PCR results for blood and CSF samples from patients in the Lyme neuroborreliosis group (and to a lesser extent in those constituting the suspected Lyme neuroborreliosis group) with findings for the control group(s), and it was somewhat surprising that borrelial DNA was detected in some of the patients with TBE. We emphasize that all contamination precautions were strictly followed, that all routine negative controls gave negative results, and that the quality of the samples was monitored. To determine the specificity of the methods, we also used CSF samples of neurosurgical patients who had no signs of borrelial infection and no known (recent) exposure to ticks; the results of all tests were negative, indicating high specificity of the utilized methods. Positive PCR findings in the group of patients with TBE can be explained by the facts that Slovenia is a region where TBE as well as Lyme borreliosis are endemic and that both pathogens are transmitted by the tick Ixodes ricinus. The ticks may often be coinfected with several borrelia strains of different species and may also be simultaneously infected with borreliae and TBE virus (11). In Slovenia, approximately 30% of Ixodes ricinus ticks are infected with Borrelia burgdorferi sensu lato (data based on tick culture) and 0.3% to 1.2% with TBE virus (15; N. Knap, unpublished results); cases of concomitant Lyme borreliosis and TBE have also been reported (5). In addition, concerning borrelia infection, a person may be simultaneously infected with more than one borrelia species (7, 17). It is also quite possible that not all borrelia infections, even those that can be demonstrated in CSF and/or blood by PCR, result in an illness.
Our findings emphasize the importance of appropriate design of studies evaluating the validity of PCR approaches for the detection of borrelia infection; the design should include not only the assessment of sensitivity but also an appropriate control group for determination of specificity. Interpretation of the results of published studies that have used differing PCR approaches for the demonstration of borrelial DNA would be much safer with the inclusion of an appropriate control group that would enable assessment of both specificity and sensitivity.
Cultivation of blood samples gave an extremely low yield; we were able to isolate borreliae from only 1 out of 135 blood samples. We had expected to isolate more borrelia strains, although, in Europe, the recovery rate has generally been low (<10% in patients with early Lyme borreliosis manifested by erythema migrans) using volumes of blood similar to those in the present study (1, 19). The explanations for the low recovery rate could be the same as those for the low proportion of positive PCR findings in blood (4, 12, 13).
The isolation of the etiological agent from CSF samples is the most reliable method for diagnosis of borrelial central nervous system infection in patients with suspected Lyme neuroborreliosis and also provides live organisms that can be further characterized (23). However, this is a low-yield procedure that takes several weeks. In patients with proven Lyme neuroborreliosis, the reported recovery rate from CSF is <10% (23). In the present study, isolation from CSF was successful in 3.7% (5/135) of samples, but the isolation rate in the group of patients with a working clinical diagnosis of Lyme neuroborreliosis was 8.3% (4/48). We were able to characterize all the isolated borrelia strains (Table 3). Using MluI restriction for borrelia characterization, B. garinii was identified in all CSF isolates of patients with Lyme neuroborreliosis, whereas in a patient with suspected Lyme neuroborreliosis who did not have pleocytosis, the isolated species was B. afzelii. These findings corroborate the results of previous studies on the predominance of B. garinii strains isolated from CSF samples of patients with Lyme neuroborreliosis (3, 16, 23) and on the uncertain value of B. afzelii as the causative agent of Lyme neuroborreliosis (23). However, PCR performed directly on CSF samples revealed a predominance of B. afzelii (Table 2).
Comparison of the results of cultivation and the two PCR methods revealed that several culture-positive samples were PCR negative and vice versa. An explanation for the finding that an individual CSF sample may be positive only with cultivation but not with PCR could be the presence of a low number of borrelia cells in CSF; this may not allow the detection of borrelial DNA by PCR but would still allow proliferation of borrelia during cultivation. In patients with CSF samples positive with PCR only, negative culture results could be explained by the influence of the immune response and also by the fact that the borreliae must adapt to an artificial culture medium, which probably limits the isolation rate, whereas their DNA, not only from living but also from dead cells, can be detected with PCR.
In conclusion, our study has shown that different approaches for direct demonstration of borrelial infection give distinct results, that there is an urgent need for standardization or at least homogenization of the methods for direct detection of borrelial infection, and that the design of studies evaluating such methods should include appropriate control group(s) to enable assessment of both sensitivity and specificity.
Footnotes
Published ahead of print on 20 August 2008.
REFERENCES
- 1.Aguero-Rosenfeld, M. E., G. Wang, I. Schwartz, and G. P. Wormser. 2005. Diagnosis of Lyme borreliosis. Clin. Microbiol. Rev. 18484-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brouqui, P., F. Bacellar, G. Baranton, R. J. Birtles, A. Bjoërsdorff, J. R. Blanco, G. Caruso, M. Cinco, P. E. Fournier, E. Francavilla, M. Jensenius, J. Kazar, H. Laferl, A. Lakos, S. Lotric Furlan, M. Maurin, J. A. Oteo, P. Parola, C. Perez-Eid, O. Peter, D. Postic, D. Raoult, A. Tellez, Y. Tselentis, and B. Wilske. 2004. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 101108-1132. [DOI] [PubMed] [Google Scholar]
- 3.Busch, U., C. Hizo-Teufel, V. Fingerle, H. Nitschko, B. Wilske, and V. Preac-Mursic. 1996. Three species of Borrelia burgdorferi sensu lato (B. burgdorferi sensu stricto, B. afzelii and B. garinii) identified from cerebrospinal fluid isolates by pulse-field gel electrophoresis and PCR. J. Clin. Microbiol. 341072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cimperman, J., V. Maraspin, S. Lotrič-Furlan, E. Ružić-Sabljić, and F. Strle. 1999. Lyme meningitis: a one-year follow up controlled study. Wien. Klin. Wochenschr. 111961-963. [PubMed] [Google Scholar]
- 5.Cimperman, J., V. Maraspin, S. Lotrič-Furlan, E. Ružić-Sabljić, T. Avšič-Županc, and F. Strle. 2002. Double infection with tick borne encephalitis virus and Borrelia burgdorferi sensu lato. Wien. Klin. Wochenschr. 114620-622. [PubMed] [Google Scholar]
- 6.Coyle, P. K., and S. E. Schutzer. 2002. Neurologic aspects of Lyme disease. Med. Clin. N. Am. 86261-284. [DOI] [PubMed] [Google Scholar]
- 7.Demaerschalck, I., A. Ben Messaoud, M. De Kesel, B. Hoyois, Y. Lobet, P. Hoet, G. Bigaignon, A. Bollen, and E. Godfroid. 1995. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J. Clin. Microbiol. 33602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greer, C. E., S. L. Peterson, N. B. Kiviat, and M. M. Manos. 1991. PCR amplification from paraffin-embedded tissues: effects of fixative and fixation time. Am. J. Clin. Pathol. 95117-124. [DOI] [PubMed] [Google Scholar]
- 9.Guy, E. C., and G. Stanek. 1991. Detection of Borrelia burgdorferi in patients with Lyme disease by polymerase chain reaction. J. Clin. Pathol. 44610-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsson, M., K. Hovind-Hougen, B. Svenungsson, and G. Stiernstedt. 1990. Cultivation and characterization of spirochetes from cerebrospinal fluid of patients with Lyme borreliosis. J. Clin. Microbiol. 28473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korenberg, E. I., Y. V. Kovalevskii, A. S. Karanov, and G. G. Moskvitina. 1999. Mixed infections by tick-borne encephalitis virus and borrelia in ticks. Med. Vet. Entomol. 13204-208. [DOI] [PubMed] [Google Scholar]
- 12.Maraspin, V., E. Ružić-Sabljić, J. Cimperman, S. Lotrič-Furlan, T. Jurca, R. N. Picken, and F. Strle. 2001. Isolation of Borrelia burgdorferi sensu lato from blood of patients with erythema migrans. Infection 2965-70. [DOI] [PubMed] [Google Scholar]
- 13.Ornstein, K., J. Berglund, I. Nilsson, R. Norrby, and S. Bergström. 2001. Characterization of Lyme borreliosis isolates from patients with erythema migrans and neuroborreliosis in southern Sweden. J. Clin. Microbiol. 391294-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picken, R. N., Y. Cheng, F. Strle, J. Cimperman, V. Maraspin, S. Lotrič-Furlan, E. Ružić-Sabljić, D. Han, J. A. Nelson, M. M. Picken, and G. M. Trenholme. 1996. Molecular characterization of Borrelia burgdorferi sensu lato from Slovenia revealing significant differences between tick and human isolates. Eur. J. Clin. Microbiol. Infect. Dis. 15313-323. [DOI] [PubMed] [Google Scholar]
- 15.Postic, D., M. V. Assous, P. A. D. Grimont, and G. Baranton. 1994. Diversity of Borrelia burgdorferi sensu lato evidenced by restriction fragment length polymorphism of rrf (5S)-rrl (23S) intergenic spacer amplicons. Int. J. Syst. Bacteriol. 44743-752. [DOI] [PubMed] [Google Scholar]
- 16.Ružić-Sabljić, E., V. Maraspin, S. Lotrić-Furlan, T. Jurca, M. Logar, A. Pikelj-Pečnik, and F. Strle. 2002. Characterization of Borrelia burgdorferi sensu lato strains isolated from human material in Slovenia. Wien. Klin. Wochenschr. 114544-550. [PubMed] [Google Scholar]
- 17.Ružić-Sabljić, E., M. Arnež, M. Logar, V. Maraspin, S. Lotrič-Furlan, J. Cimperman, and F. Strle. 2005. Comparison of Borrelia burgdorferi sensu lato strains isolated from specimens obtained simultaneously from two different sites of infection in individual patients. J. Clin. Microbiol. 432194-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt, B. L. 1997. PCR in laboratory diagnosis of human Borrelia burgdorferi infections. Clin. Microbiol. Rev. 10185-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanek, G., and F. Strle. 2003. Lyme borreliosis. Lancet 3621639-1647. [DOI] [PubMed] [Google Scholar]
- 20.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345115-125. [DOI] [PubMed] [Google Scholar]
- 21.Strle, F., J. A. Nelson, E. Ružić-Sabljić, J. Cimperman, V. Maraspin, S. Lotric-Furlan, Y. Cheng, M. M. Picken, G. M. Trenholme, and R. N. Picken. 1996. European Lyme borreliosis: 231 culture-confirmed cases involving patients with erythema migrans. Clin. Infect. Dis. 2361-65. [DOI] [PubMed] [Google Scholar]
- 22.Strle, F. 1999. Lyme borreliosis in Slovenia. Zentralbl. Bakteriol. 289643-652. [DOI] [PubMed] [Google Scholar]
- 23.Strle, F., E. Ružić-Sabljić, J. Cimperman, S. Lotrič-Furlan, and V. Maraspin. 2006. Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin. Infect. Dis. 43704-710. [DOI] [PubMed] [Google Scholar]
- 24.Wilske, B., V. Fingerle, and U. Schulte-Spechtel. 2007. Microbiological diagnosis of Lyme borreliosis. FEMS Immunol. Med. Microbiol. 4913-21. [DOI] [PubMed] [Google Scholar]
- 25.Zore, A., E. Ružić-Sabljić, V. Maraspin, J. Cimperman, S. Lotrič-Furlan, A. Pikelj, T. Jurca, M. Logar, and F. Strle. 2002. Sensitivity of culture and PCR for the etiologic diagnosis of erythema migrans. Wien. Klin. Wochenschr. 114606-609. [PubMed] [Google Scholar]