Abstract
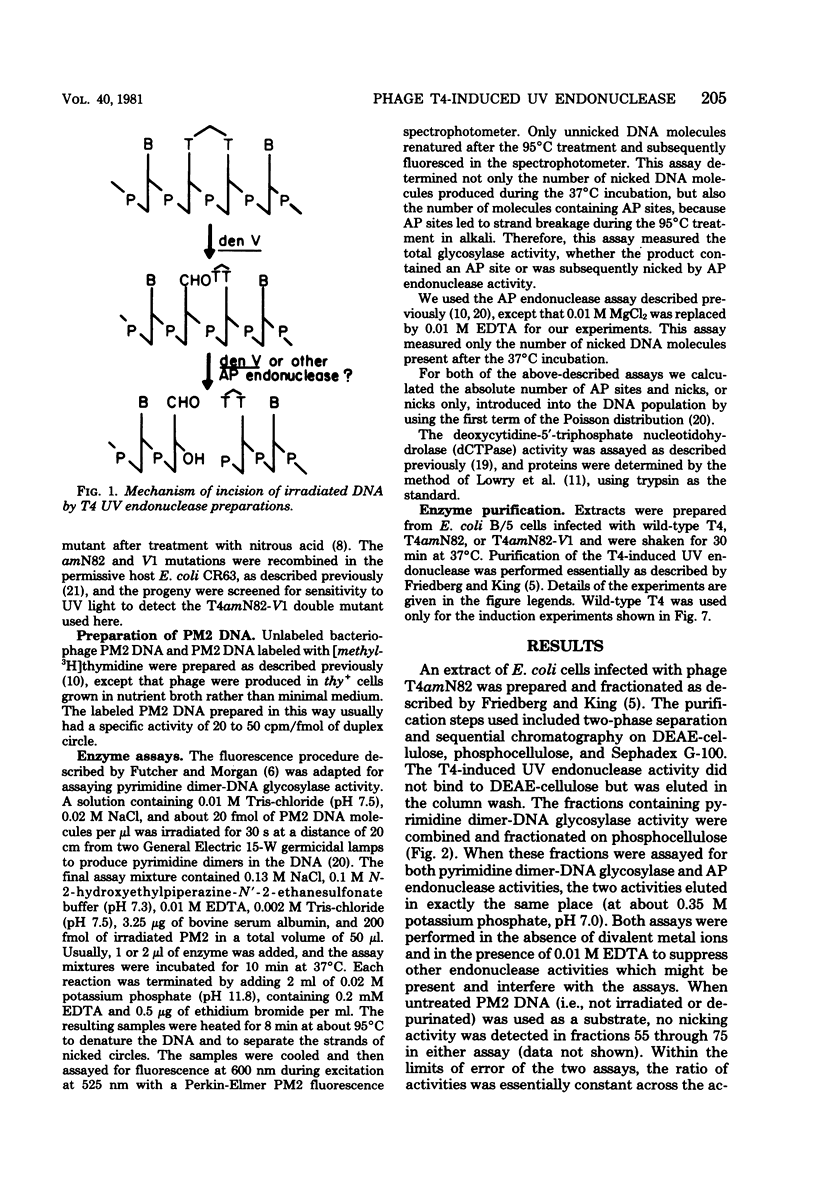
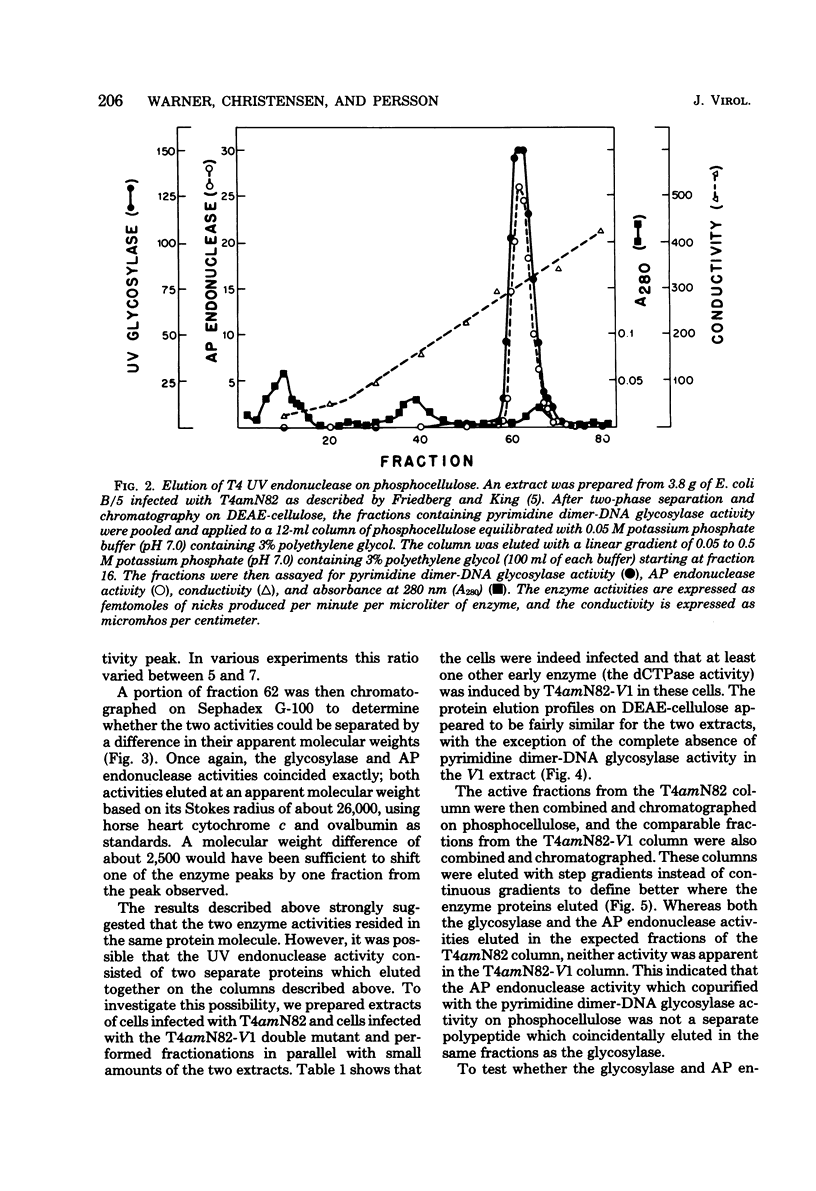
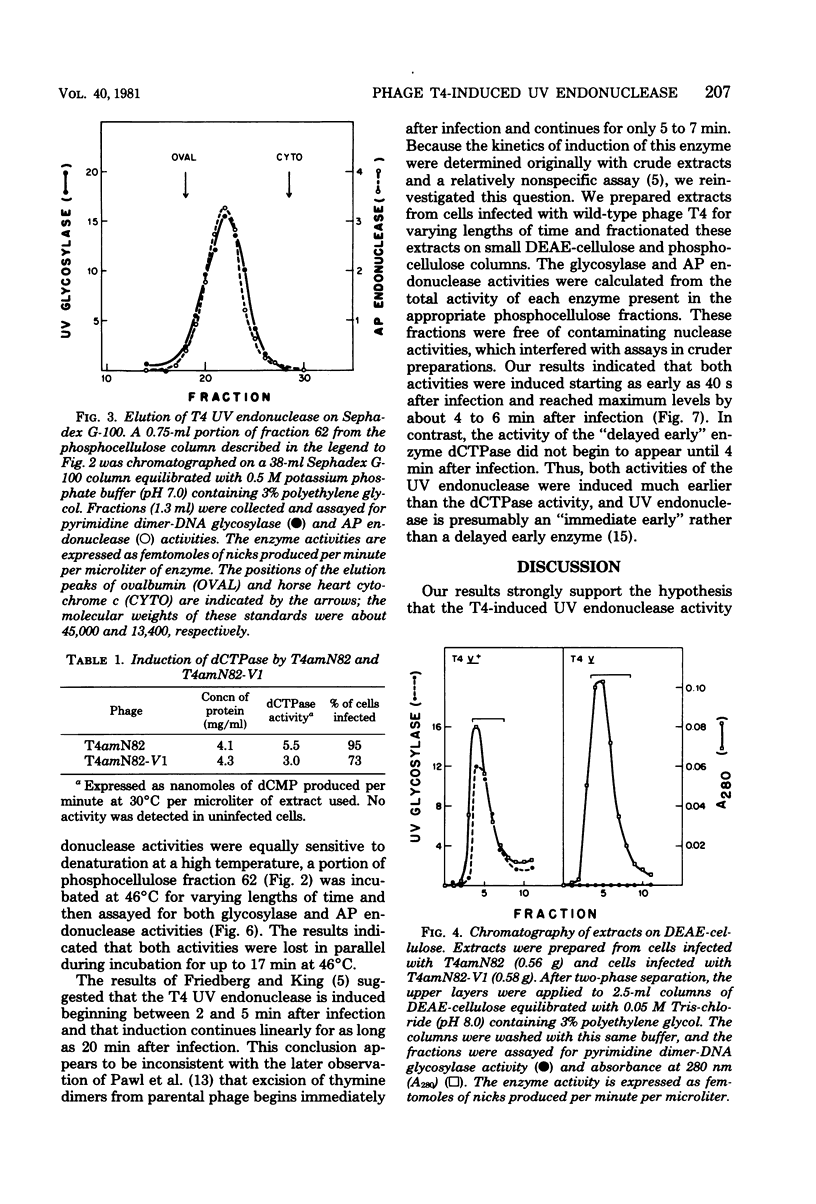
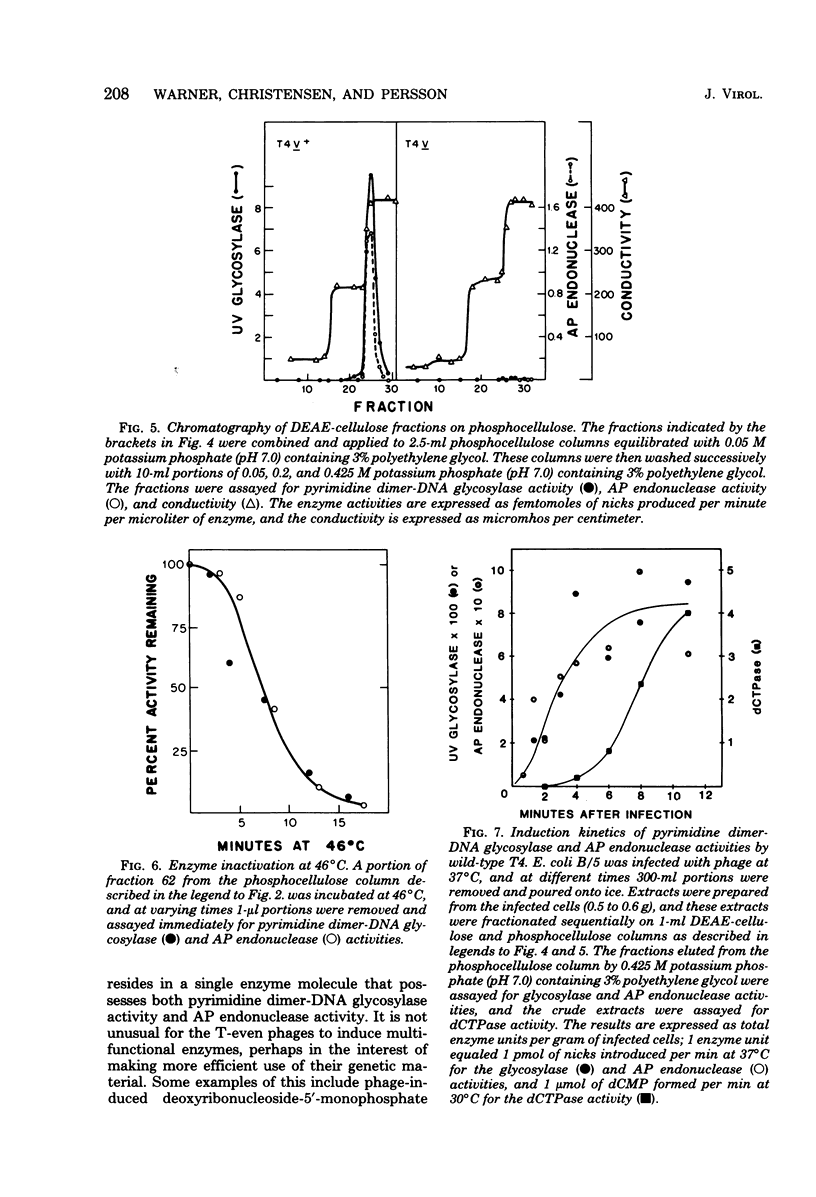
We performed experiments to determine whether the phage T4-induced UV endonuclease activity is a single protein containing both pyrimidine dimer-DNA glycosylase and apyrimidinic endonuclease activities. The UV endonuclease activity is induced by the denV gene and codes for the glycosylase activity. We obtained several kinds of evidence that the protein containing the glycosylase activity also contains the apyrimidinic endonuclease activity. After chromatography on DEAE-cellulose, the two activities copurified during phosphocellulose chromatography and Sephadex G-100 chromatography, with a constant ratio of activities across the activity peaks. On Sephadex G-100 columns the molecular weights of the two activities agreed within 2,500 or less. When an extract of cells infected with the T4 V1 mutant was purified in exactly the same way as an extract of cells infected with T4 V1+, neither glycosylase nor apyrimidinic endonuclease activity was detected in the normal elution position of the T4 UV endonuclease activity. The glycosylase and apyrimidinic endonuclease activities were induced with similar kinetics, which were characteristic of immediate early rather than delayed early enzymes. This correlated well with the presumed major role of these activities in repairing thymine dimers in parental DNA before DNA replication begins. Finally, glycosylase and apyrimidinic endonuclease activities were lost in parallel during incubation of the enzyme at 46 degree C. Our results indicated that both of these enzyme activities are contained in the same enzyme molecule and, probably, in the same polypeptide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron V., Uhlenbeck O. C. 3'-Phosphatase activity in T4 polynucleotide kinase. Biochemistry. 1977 Nov 15;16(23):5120–5126. doi: 10.1021/bi00642a027. [DOI] [PubMed] [Google Scholar]
- Demple B., Linn S. DNA N-glycosylases and UV repair. Nature. 1980 Sep 18;287(5779):203–208. doi: 10.1038/287203a0. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Bessman M. J. The enzymology of virus-infected bacteria. X. A biochemical-genetic study of the deoxynucleotide kinase induced by wild type and amber mutants of phage T4. J Biol Chem. 1967 Jun 25;242(12):2877–2885. [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Endonucleolytic cleavage of UV-irradiated DNA controlled by the V+ gene in phage T4. Biochem Biophys Res Commun. 1969 Nov 6;37(4):646–651. doi: 10.1016/0006-291x(69)90859-6. [DOI] [PubMed] [Google Scholar]
- Futcher A. B., Morgan A. R. A novel assay for endonucleases acting at apurinic sites and its use in measuring AP endonuclease activity in repair-deficient mutants of Saccharomyces cerevisiae. Can J Biochem. 1979 Jun;57(6):932–937. doi: 10.1139/o79-113. [DOI] [PubMed] [Google Scholar]
- Gates F. T., Linn S. Endonuclease from Escherichia coli that acts specifically upon duplex DNA damaged by ultraviolet light, osmium tetroxide, acid, or x-rays. J Biol Chem. 1977 May 10;252(9):2802–2807. [PubMed] [Google Scholar]
- HARM W. Mutants of phage T4 with increased sensitivity to ultraviolet. Virology. 1963 Jan;19:66–71. doi: 10.1016/0042-6822(63)90025-4. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Gordon L. K., Lindan C. P., Grafstrom R. H., Shaper N. L., Grossman L. Cleavage of pyrimidine dimers in specific DNA sequences by a pyrimidine dimer DNA-glycosylase of M. luteus. Nature. 1980 Jun 26;285(5767):634–641. doi: 10.1038/285634a0. [DOI] [PubMed] [Google Scholar]
- Kuhnlein U., Penhoet E. E., Linn S. An altered apurinic DNA endonuclease activity in group A and group D xeroderma pigmentosum fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1169–1173. doi: 10.1073/pnas.73.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McMillan S., Edenberg H. J., Radany E. H., Friedberg R. C., Friedberg E. C. den V gene of bacteriophage T4 codes for both pyrimidine dimer-DNA glycosylase and apyrimidinic endonuclease activities. J Virol. 1981 Oct;40(1):211–223. doi: 10.1128/jvi.40.1.211-223.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y., Sekiguchi M. Physical association of pyrimidine dimer DNA glycosylase and apurinic/apyrimidinic DNA endonuclease essential for repair of ultraviolet-damaged DNA. Proc Natl Acad Sci U S A. 1981 May;78(5):2742–2746. doi: 10.1073/pnas.78.5.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawl G., Taylor R., Minton K., Friedberg E. C. Enzymes involved in thymine dimer excision in bacteriophage T4-infected Escherichia coli. J Mol Biol. 1976 Nov;108(1):99–109. doi: 10.1016/s0022-2836(76)80097-6. [DOI] [PubMed] [Google Scholar]
- Radany E. H., Friedberg E. C. A pyrimidine dimer-DNA glycosylase activity associated with the v gene product of bacterophage T4. Nature. 1980 Jul 10;286(5769):182–185. doi: 10.1038/286182a0. [DOI] [PubMed] [Google Scholar]
- Salser W., Bolle A., Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the "early" RNA appear at different times and that some are "turned off" at late times. J Mol Biol. 1970 Apr 28;49(2):271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Seawell P. C., Smith C. A., Ganesan A. K. den V gene of bacteriophage T4 determines a DNA glycosylase specific for pyrimidine dimers in DNA. J Virol. 1980 Sep;35(3):790–796. doi: 10.1128/jvi.35.3.790-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snopek T. J., Wood W. B., Conley M. P., Chen P., Cozzarelli N. R. Bacteriophage T4 RNA ligase is gene 63 product, the protein that promotes tail fiber attachment to the baseplate. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3355–3359. doi: 10.1073/pnas.74.8.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomilin N. V., Paveltchuk E. B., Mosevitskaya T. V. Substrate specificity of the ultraviolet-endonuclease from Micrococcus luteus. Endonucleolytic cleavage of depurinated DNA. Eur J Biochem. 1976 Oct 1;69(1):265–272. doi: 10.1111/j.1432-1033.1976.tb10882.x. [DOI] [PubMed] [Google Scholar]
- WIBERG J. S., DIRKSEN M. L., EPSTEIN R. H., LURIA S. E., BUCHANAN J. M. Early enzyme synthesis and its control in E. coli infected with some amber mutants of bacteriophage T4. Proc Natl Acad Sci U S A. 1962 Feb;48:293–302. doi: 10.1073/pnas.48.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Barnes J. E. Evidence for a dual role for the bacteriophage T4-induced deoxycytidine triphosphate nucleotidohydrolase. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1233–1240. doi: 10.1073/pnas.56.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Demple B. F., Deutsch W. A., Kane C. M., Linn S. Apurinic/apyrimidinic endonucleases in repair of pyrimidine dimers and other lesions in DNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4602–4606. doi: 10.1073/pnas.77.8.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner H. R., Snustad D. P., Koerner J. F., Childs J. D. Identification and genetic characterization of mutants of bacteriophage T4 defective in the ability to induce exonuclease A. J Virol. 1972 Mar;9(3):399–407. doi: 10.1128/jvi.9.3.399-407.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


