Abstract
The OSOM Trichomonas rapid test (OSOM Trich) was compared to the wet preparation examination (WP) for the detection of Trichomonas vaginalis vaginitis in women with a low prevalence of infection. A total of 19/1,009 (2%) women had T. vaginalis infection. OSOM Trich had very good performance, with sensitivity, specificity, efficiency, positive predictive value, and negative predictive value of 94.7, 100, 99.9, 100, and 99.9%, respectively. The implementation of OSOM Trich would decrease labor costs.
Trichomoniasis is the most common nonviral sexually transmitted disease (STD) worldwide, although data are limited for women with a low prevalence of infection (7, 9, 11). Vaginitis due to Trichomonas vaginalis clinically manifests with symptoms of vaginal itching, odor, and discharge. Recent studies also show that T. vaginalis is an important cause of the premature rupture of membranes, premature delivery, pelvic inflammatory disease, urethritis, and chronic prostatitis (4, 9, 11, 14). Trichomoniasis infection also enhances the transmission of human immunodeficiency virus infection (8, 9, 11-14).
Our regional microbiology laboratory tests a high volume of vaginal swabs each day (i.e., >150) for vaginitis pathogens, and ∼30% are positive for bacterial vaginosis or candidiasis, although there is a low prevalence of T. vaginalis vaginitis (i.e., <0.5%) based on the detection of motile trichomonads by wet preparation examination (WP) (data not shown). Due to the poor sensitivity of WP (35 to 70%), T. vaginalis cases likely were being missed because of lost flagellate viability during specimen transport (10, 14, 15). New diagnostic methods for trichomoniasis recently have become commercially available that do not require the presence of viable flagellate, including rapid antigen immunocapillary and nucleic acid amplification tests (2, 3, 5, 6). It was therefore of interest to compare the performance of the OSOM Trichomonas rapid test (OSOM Trich) (Genzyme Diagnostics, Cambridge, MA) to that of WP for the routine detection of T. vaginalis vaginitis in our laboratory setting.
For a 3-month period in 2007, the laboratory selected simultaneously collected genital specimens from symptomatic women whose physician had submitted a vaginal swab for the detection of vaginitis pathogens (bacterial vaginosis, T. vaginalis, and Candida) and an endocervical swab to test for Chlamydia trachomatis and Neisseria gonorrhoeae. This laboratory selection procedure was designed to rule out other STDs as a cause of symptoms. A vaginal swab submitted in Copan liquid Amies transport medium (Copan Diagnostics Inc., Corona, CA) (1) was used to test for the presence of T. vaginalis using both OSOM Trich (Genzyme Diagnostic, Cambridge, MA; the Canadian distributor is Biopacifica Diagnostics Inc., North Vancouver, British Columbia) and WP. Vaginal swabs that were delayed in transport (i.e., >36 h) were rejected. Gram smears were read to detect other vaginitis pathogens, but the presence of either bacterial vaginosis or Candida infection was not recorded. Endocervical swabs were collected using the manufacturer's kits and were tested using the Aptima Combo2 C. trachomatis/N. gonorrhoeae assay (Gen-Probe, San Diego, CA).
OSOM Trich is an immunochromatographic capillary-flow enzyme immunoassay dipstick test that was performed according to the manufacturer's instructions. Vaginal swabs were tested in batches of 10, and analysis required 10 to 15 min. A positive result has both a red internal control and a blue positive test line, while the negative result has only a red internal control line. Invalid tests had an absent internal control line. Discrepant results between OSOM Trich and WP were resolved using the Aptima Trichomonas transcription-mediated amplification assay performed according to the manufacturer's instructions (Gen-Probe, San Diego, CA). Specimens were tested in a Gen-Probe DTS 400 instrumentation system using T. vaginalis-specific reagents and Aptima general-purpose reagents (target capture, transcription-mediated amplification, and hybridization protection using primers and probes that specifically target T. vaginalis rRNA). A T. vaginalis patient culture in Diamonds medium was used to inoculate a Gen-Probe Aptima swab specimen transport tube and an uninoculated Gen-Probe Aptima swab specimen transport tube, which were used as positive and negative controls, respectively. Endocervical samples with an Aptima result of >50,000 relative light units were considered positive.
Resource utilization costs from the study period were extrapolated to estimate the projected costs of the routine utilization of both methods during a routine testing month in order to project the annual resource impact of implementing OSOM Trich. All costs were calculated in Canadian dollars. Labor costs were calculated based on the current hourly rates paid to medical laboratory assistants (MLAs) and medical laboratory technologists (MLTs). Supply costs included the Canadian goods and services tax. The performance of OSOM Trich was compared to that of a composite reference standard (CPS) (i.e., a positive Aptima test plus either a positive OSOM Trich or WP test) for calculating sensitivity, specificity, efficiency, and positive and negative predictive values. Data were entered into a Microsoft Excel (version 3.0) spreadsheet and analyzed using Analyze-it software using standard statistical methods (Microsoft Corporation, Seattle, WA).
The mean age of the 1,009 enrolled women was 31.7; the standard deviation was ±11 years. Only 19 (2%) women had T. vaginalis vaginitis, and 3 of these women also had either C. trachomatis or N. gonorrhoeae cervicitis. Of the 39 (3.9%) patients who had C. trachomatis cervicitis, only 2 of them also had T. vaginalis vaginitis. Of the four (0.4%) patients who had N. gonorrhoeae cervicitis, only one patient also had T. vaginalis infection. No women had dual infection with C. trachomatis and N. gonorrhoeae.
Table 1 shows the performance data for OSOM Trich. WP missed two T. vaginalis cases that were detected by both Aptima and OSOM Trich. One sample had a falsely negative OSOM Trich result (i.e., the internal control was positive) but was positive by both of the other methods. OSOM Trich had very good performance compared to that of the CPS, with sensitivity, specificity, efficiency, positive predictive value, and negative predictive value of 94.7, 100, 99.9, 100, and 99.9%, respectively.
TABLE 1.
Performance of OSOM Trich for the detection of T. vaginalis
| Result by OSOM Tricha | Result by CPSb
|
Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 18 | 0 | 18 |
| Negative | 1 | 990 | 991 |
| Total | 19 | 990 | 1,009 |
Sensitivity, 18/19 (94.7%); specificity, 990/990 (100%); positive predictive value, 19/19 (100%); negative predictive value, 990/991 (99.9%); and efficiency, 1,008/1,009 (99.9%).
For the CPS, true positives were defined as those that were positive by Aptima and either WP slide exam or the OSOM Trich test.
Table 2 shows the component costs of performing T. vaginalis vaginitis testing using WP or OSOM Trich during a typical testing month. The implementation of OSOM Trich would increase material costs but decrease monthly labor costs by 46.2% (0.21 full-time equivalent [FTE]).
TABLE 2.
Comparison of Calgary Laboratory Services study costs for using OSOM Trich and the WP slide exam for T. vaginalis detection
| Test | Cost ($) of total materialsa | Cost ($) of total MLA/MLT time | Total FTE/mo | No. of tests/mo | Total monthly costb ($) | Total annual costc ($) |
|---|---|---|---|---|---|---|
| Wet Prep Exam | 426.00 | 4,767.21 | 0.55 | 1700 | 5,193.21 | 62,318.52 |
| OSOM Trich | 9,360.54 | 2,564.73 | 0.34 | 1700 | 11,925.27 | 143,103.24 |
| Immediate effects of change to OSOM Trich | +8,934.54 | −2,202.48 | −0.21 | No change | +6,732.06 | +80,784.72 |
Total material costs included the cost of all supplies and reagents.
Total costs include the Canadian goods and services tax, but not shipping and handling; costs were calculated based on a volume of 1,700 specimens per month and a positivity rate of 2%.
The projected annual resources used were extrapolated from the monthly labor/materials costs.
Our study is the first to evaluate the performance and feasibility of the implementation of OSOM Trich instead of WP in a high-volume laboratory providing service to a population of women with a low prevalence of T. vaginalis and other STD infections. Our study confirms that OSOM Trich had improved sensitivity (94.7%) compared to that of WP (89.4%) in this context. OSOM Trich also had the excellent specificity required for screening patients with a low prevalence of infection. Huppert et al. (5, 6) recently evaluated OSOM Trich in two separate studies in female populations with a high prevalence of T. vaginalis infection. The initial evaluation used OSOM Trich to rapidly detect T. vaginalis in vaginal specimens collected from sexually active women ≥18 years of age (n = 449) presenting with symptoms of vaginitis, exposure to T. vaginalis, or multiple sexual partners (5). Their study population had a high prevalence of T. vaginalis at 23.4%. OSOM Trich detected more T. vaginalis cases, with a sensitivity of 83.3%; that for WP was 71.4%. In a more recent study, sexually active adolescent women aged 14 to 21 years (n = 330) were recruited from a teen health center and the emergency department (6). Vaginal swabs were tested for T. vaginalis using WP, culture (In Pouch T. vaginalis; BioMed Diagnostics), OSOM Trich, and Aptima. Their study group also had a high prevalence of trichomoniasis at 18.5%. The sensitivity of each method was compared to that of a CPS (i.e., any test with positive results). WP had the lowest sensitivity (56%), while Aptima had the highest sensitivity (98.4%). Although OSOM Trich (83%) had lower sensitivity than that of culture (90%), test results were available the same day, whereas it takes a several days' delay for culture.
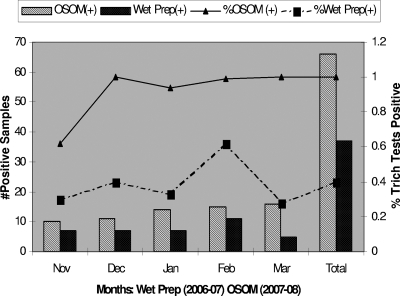
Our study is also the first to evaluate the resource outcomes of implementing OSOM Trich in a high-volume laboratory setting (>100 samples/day). The decreased hands-on time of performing OSOM Trich significantly decreased the amount of MLA/MLT time devoted to WP microscopy. The projected FTE savings can be utilized to perform other laboratory procedures. Although the overall resource costs increased, as projected, after the implementation of OSOM Trich (1 November 2007) as our routine testing method, this has been medically justified by the improved detection of T. vaginalis vaginitis cases. Figure 1 shows that the rate of detection of T. vaginalis vaginitis cases in the 5 months after the implementation of OSOM Trich has increased to ∼1%, up from <0.5% using WP. Proportional savings based on annual testing for vaginitis pathogens would occur in other laboratory setting, since the test volumes remain constant throughout the year with little seasonal variability. Further studies should confirm these findings in other low-prevalence populations and laboratory settings, particularly the performance of OSOM Trich compared to that of molecular T. vaginalis detection in this setting.
FIG. 1.
Performance of OSOM Trich and WP for the routine detection of T. vaginalis.
Acknowledgments
Genzyme Diagnostics (Cambridge, MA), through their Canadian distributor (Biopacifica Diagnostics Inc., North Vancouver, British Columbia, Canada), provided the OSOM Trich test kits for this study. Aptima Trichomonas assay kits were provided by Gen-Probe (San Diego, CA). The MLAs and MLTs that provided assistance to this study are gratefully acknowledged for their efforts.
Footnotes
Published ahead of print on 6 August 2008.
REFERENCES
- 1.Beverly, A. L., M. Venglarik, B. Cotton, and J. R. Schwebke. 1999. Viability of Trichomonas vaginalis in transport medium. J. Clin. Microbiol. 373749-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caliendo, A. M., J. A. Jordan, A. M. Green, J. Ingersoll, R. J. Diclemente, and G. M. Wingood. 2005. Real-time PCR improves detection of Trichomonas vaginalis infection compared with culture using self-collected vaginal swabs. Infect. Dis. Obstet. Gynecol. 13145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardick, A., J. Hardick, B. J. Wood, and C. Gaydos. 2006. Comparison between the Gen-Probe transcription-mediated amplification Trichomonas vaginalis research assay and real-time PCR for Trichomonas vaginalis detection using a Roche LightCycler instrument with female self-obtained vaginal swab samples and male urine samples. J. Clin. Microbiol. 444197-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobbs, M. M., D. M. Lapple, L. F. Lawing, J. R. Schwebke, M. S. Cohen, H. Swygard, J. Atashili, P. A. Leone, W. C. Miller, and A. C. Sena. 2006. Methods for detection of Trichomonas vaginalis in the male partners of infected women: implications for control of trichomoniasis. J. Clin. Microbiol. 443994-3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huppert, J. S., B. E. Batteiger, P. Braslins, J. A. Feldman, M. M. Hobbs, H. Z. Sankey, A. C. Sena, and K. A. Wendel. 2005. Use of an immunochromatographic assay for rapid detection of Trichomonas vaginalis in vaginal specimens. J. Clin. Microbiol. 43684-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huppert, J. S., J. E. Mortensen, J. L. Reed, J. A. Kahn, K. D. Rich, W. C. Miller, and M. M. Hobbs. 2007. Rapid antigen testing compares favorably with transcription-mediated amplification assay for the detection of Trichomonas vaginalis in young women. Clin. Infect. Dis. 45194-198. [DOI] [PubMed] [Google Scholar]
- 7.Mabey, D., J. Ackers, and Y. Adu-Sarkodie. 2006. Trichomonas vaginalis infection. Sex Transm Infect. 82(Suppl. 4)iv26-iv27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moodley, P., D. Wilkinson, C. Connolly, J. Moodley, and A. W. Sturm. 2002. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin. Infect. Dis. 34519-522. [DOI] [PubMed] [Google Scholar]
- 9.Nanda, N., R. G. Michel, G. Kurdgelashvili, and K. A. Wendel. 2006. Trichomoniasis and its treatment. Expert Rev. Anti. Infect. Ther. 4125-135. [DOI] [PubMed] [Google Scholar]
- 10.Patel, S. R., W. Wiese, S. C. Patel, C. Ohl, J. C. Byrd, and C. A. Estrada. 2000. Systematic review of diagnostic tests for vaginal trichomoniasis. Infect. Dis. Obstet. Gynecol. 8248-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwebke, J. R., and D. Burgess. 2004. Trichomoniasis. Clin. Microbiol. Rev. 17794-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorvillo, F., and P. Kerndt. 1998. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet 351213-214. [DOI] [PubMed] [Google Scholar]
- 13.Sorvillo, F., L. Smith, P. Kerndt, and L. Ash. 2001. Trichomonas vaginalis, HIV, and African-Americans. Emerg. Infect. Dis. 7927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swygard, H., A. C. Sena, M. M. Hobbs, and M. S. Cohen. 2004. Trichomoniasis: clinical manifestations, diagnosis and management. Sex Transm. Infect. 8091-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wendel, K. A., and K. A. Workowski. 2007. Trichomoniasis: challenges to appropriate management. Clin. Infect. Dis. 44(Suppl. 3)S123-S129. [DOI] [PubMed] [Google Scholar]