Abstract
Based on nucleotide sequence and phylogenetic analysis of the partial VP6 genes, group A rotaviruses can be mainly differentiated into two genogroups. In this study, a method employing reverse transcription-PCR (RT-PCR) and degenerate primers was established to assign the VP6 genogroup. VP6 genogroup I and genogroup II could be determined according to the sizes of the amplicons: 380 and 780 bp, respectively. The VP6 genogroup of human reference strains of G1 to G4 and G9 types and RotaTeq vaccine strains could be properly assigned by RT-PCR. Eighty rotavirus-positive fecal samples were subjected to enzyme-linked immunosorbent assay (ELISA), RT-PCR, and sequencing of the partial VP6 gene for subgroup and genogroup determination. The results correlated well among these three methods, except for seven samples whose subgroups could not be determined by ELISA. VP6 genogroups of another 150 rotavirus strains recovered between 1981 and 2005 were determined by RT-PCR and sequencing, and the same results were obtained by these two methods. Furthermore, an additional 524 rotavirus-positive fecal samples were tested by RT-PCR, and the VP6 genogroups could be easily determined. The RT-PCR assay developed here provided a reliable and convenient method for assigning the VP6 genogroups of human rotaviruses with a wide range of genetic variation.
Rotaviruses belong to the family of Reoviridae, and complete viral particles have a triple-layered icosahedral protein capsid that surrounds the genome of 11 segments of double-stranded RNA, which encode six structural proteins and six nonstructural proteins (5). VP7 and VP4, two outer capsid proteins, induce neutralizing antibodies and are responsible for the serotype specificity (7, 33). VP7 is a major glycoprotein encoded by gene 7, 8, or 9, and VP7-specific types are abbreviated as G serotypes or G genotypes (6, 7, 27). VP4 is a protease-sensitive protein encoded by gene 4, and VP4-specific types are abbreviated as P serotypes or P genotypes (6, 7, 27). Group A rotaviruses have been classified as 16 G serotypes and 27 P genotypes (12, 22). NSP4, a glycoprotein encoded by gene 10, serves as an intracellular receptor for double-layered rotavirus particles and interacts with viral capsid proteins during viral morphogenesis (1, 32), and it has been proposed as a possible viral enterotoxin capable of inducing diarrhea in young mice (2). According to NSP4 gene sequence analysis, five genotypes (A to E) have been identified among human and animal rotaviruses with different G and P types (3, 4, 13, 28).
VP6, a trimeric protein, encoded by gene 6, forms the middle-layer capsid and interacts with both outer capsid proteins VP4 and VP7 and the core protein VP2 (26). According to the antigenic epitopes present on VP6, rotaviruses can be classified into groups (A to G) and subgroups (5). Group A rotaviruses can be differentiated into subgroups I, II, I+II, and non-I non-II, depending on the presence or absence of two distinct epitopes which react with one, both, or neither of the monoclonal antibodies (MAbs) 255/60 and 631/9 (7, 11). Previous studies have mapped amino acid position 305 and the region from positions 296 to 299 to subgroup I specificity, and amino acid position 315 to subgroup II specificity (30), and showed that subgroup epitopes are conformational and appear to be located on the trimeric but not monomeric structures (9). At present, enzyme-linked immunosorbent assay (ELISA) incorporating subgroup-specific MAbs is widely used as an epidemiological tool to monitor rotavirus strains. However, antigenic drift through the accumulation of point mutations may result in poor reactivity in ELISA subgrouping. In the study by Iturriza-Gomara et al. (18), based on nucleotide sequencing and phylogenetic analysis, it was found that samples were clustered into two genogroups—genogroup I and genogroup II. Genogroup I comprised samples serologically determined as subgroup I, and genogroup II comprised samples serologically determined as subgroup II, subgroup I+II, and subgroup non-I non-II. These authors suggested that there were no true human subgroup I+II or subgroup non-I non-II strains, but this misclassification might have been due to the poor reactivity between subgroup II strains and the MAb used (18). In addition, subgroup-specific MAbs are not easily available for most laboratories. For these reasons, a convenient molecular genogrouping method is still needed.
In the present study, a molecular method, reverse transcription-PCR (RT-PCR), for the determination of the rotavirus VP6 genogroup was established. This method was evaluated by comparison with sequence analysis of the VP6 gene. The subgroups of some of the rotavirus strains were determined by ELISA. The associations of the VP6 genes with VP4, VP7, and NSP4 genes were also analyzed.
MATERIALS AND METHODS
Viruses and fecal samples.
A total of 754 fecal specimens containing rotavirus, determined by a commercial ELISA (Rotaclone; Meridian Diagnostics, Inc., Cincinnati, OH), were included in the present study. Of these, 604 samples were collected during the period 2000 to 2002, including 80 samples used for the comparison of three methods to determine VP6 subgroup or genogroup and 524 samples used for determination of VP6 genogroup only by RT-PCR, and 150 samples were collected from 1981 to 1999 and from 2003 to 2005 and used for nucleotide sequence analysis of partial VP6 genes to confirm the VP6 genogroup determined by RT-PCR. The samples were collected from patients with acute gastroenteritis who either were hospitalized or visited the pediatric clinics of National Taiwan University Hospital in Taipei, a city located in the northern part of Taiwan. Of these 754 patients with gastroenteritis, 41.0% were females and 59.0% were males, and 89.7% were younger than 5 years old. The fecal samples were suspended to approximately. 10% (wt/vol) in phosphate-buffered saline (PBS; pH 7.2) and clarified by low-speed centrifugation. The supernatant was collected and stored at −70°C. Reference human rotaviruses used in the present study were pretreated with 10 μg of trypsin/ml, propagated in MA104 cells in the presence of 0.5 μg of trypsin/ml, and harvested at 3 to 5 days postinfection.
Subgroup analysis by ELISA.
An ELISA with MAbs was carried out as described previously (31), with some modifications. The following MAbs (ascitic fluids) were used: group A-common YO-156 (directed to VP6), subgroup I-specific S2-37, and subgroup II-specific YO-5. The wells of a polystyrene microplate (Nunc, Denmark) were coated with a 1:10,000 dilution of S2-37 and YO-156 or a 1:3,000 dilution of YO-5 at 4°C overnight. Bovine serum albumin (3%) in PBS was added and kept at 4°C overnight. After a washing step with PBS-Tween 20, test samples were added, followed by incubation at 4°C overnight. After washing, rabbit anti-human rotavirus serum was added. The dilutions of anti-human rotavirus serum were 1:1,000 for the wells coated with YO-5 and YO-156 and 1:10,000 for the wells coated with S2-37. After 1 h of incubation at 37°C and washing, horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G was added, and the plate was incubated for 1 h at 37°C. After a final washing step, 3,3′,5,5′-tetramethylbenzidine was added, and the plate incubated for 5 min at room temperature, 1 N H2SO4 was added, and the absorbance was measured at 450 nm. The ratio, the absorbance obtained from reactivity with YO-5 divided by the absorbance obtained from reactivity with S2-37, was calculated. Samples with a ratio of <0.4 were considered indicative of subgroup I specificity, while samples with a ratio of >2.5 were indicative of subgroup II specificity (31).
Extraction and purification of viral RNA.
Rotavirus RNA was extracted and purified as follows. Rotavirus was concentrated and precipitated by adding ammonium sulfate (at a final concentration of 35% [wt/vol]) to clarified 10% stool suspension. After a thorough mixing, the precipitated rotavirus was spun down at 10,000 × g for 15 min. The supernatant was aspirated, and the pellet was suspended in TE buffer (10 mM Tris-HCl [pH 8.0] and 1 mM EDTA). Rotavirus particles were lysed with extraction buffer (at a final concentration of 0.02 M Tris-HCl [pH 7.4], 0.15 M NaCl, 0.01 M MgCl2, 1% sodium dodecyl sulfate, and 2% [wt/vol] Ficoll). The mixture was treated two or three times with phenol-chloroform. The RNA for RT-PCR was further purified with guanidine thiocyanate-silicon dioxide to remove inhibitors (25).
Design of the oligonucleotide primers for VP6 genogrouping.
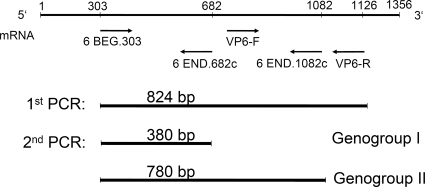
VP6 gene sequences of the subgroup I reference strains DS-1 (accession no. DQ870507), S2 (Y00437), IS2 (X94617), 1076 (D00325), SA11 (L33365), and US1205 (AF079357) and the subgroup II reference strains Wa (K02086), KU (AB022768), E210 (U36240), YO (DQ870500), and 116E (U85998), as well as 80 rotavirus strains recovered in Taiwan with various genotypes and 24 electropherotypes between 2000 and 2002, were used to design oligonucleotide primers. Oligonucleotide 6BEG.303 (5′-AAY GTR TGT ATG GAT GAR ATG-3′; nucleotides 303 to 323) was designed as the forward primer for the first and second amplification, since it is conserved among all sequences. Sequences of the region for genogroup-specific primers of the 80 Taiwanese rotavirus strains and reference strains used to design genogroup specific primers are shown in Table S1 in the supplemental material. Genogroup I-specific primer 6END.682c (5′-GTM GTT AAM ACY CTD CGG-3′; nucleotides 682 to 665) and genogroup II-specific primer 6END.1082c (5′-ATA YTC TTG ACG YAC TGC G-3′; nucleotides 1082 to 1064) were selected from the region that was divergent between strains in different genogroups and relatively conserved in strains within the same genogroup (Fig. 1).
FIG. 1.
The scheme shows the position and direction of primers and the sizes of the amplicons for VP6 genogrouping by RT-PCR. The double-stranded RNAs were reverse transcribed and amplified with 6BEG.303 and VP6-R primer pairs. After 1:10 to 1:100 dilution, the RT-PCR products were further amplified with 6BEG.303, 6END.682c, and 6END.1082c primers. VP6 genogroup I and genogroup II could be distinguished by the sizes: 380 and 780 bp, respectively.
Amplification of the VP6 genes and the NSP4 genes.
The VP6 and NSP4 genes were amplified by RT-PCR. The VP6-F (5′-GAC GGV GCR ACT ACA TGG T-3′) and VP6-R (5′-GTC CAA TTC ATN CCT GGT GG-3′) primers (18) were incorporated to amplify a 379-bp fragment of the VP6 gene for sequence analysis, and 6BEG.303 and VP6-R primers were incorporated to amplify an 824-bp fragment of the VP6 gene for PCR genogrouping. The primers 10BEG.16 and 10 END.722c (24) were incorporated to amplify a 724- or a 725-bp fragment of the NSP4 gene for sequence analysis. The RT-PCR mixture contained 5 μl of silica-purified RNA, 7% dimethyl sulfoxide, 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 1.5 mM MgCl2, 0.1% (wt/vol) gelatin, 1% Triton X-100, 200 μM deoxynucleoside triphosphates, and 500 nM primers. This mixture was heated for 10 min at 97°C, cooled on ice for 5 min, and briefly centrifuged. Human placental RNase inhibitor (22 U; HT Biotechnology, Cambridge, England), Super-RT (AMV reverse transcriptase, 5.25 U; HT Biotechnology), and Taq polymerase (1 U; HT Biotechnology) were then added. For the VP6 genes, the mixture was incubated in a thermal cycler (model 480; Perkin-Elmer, Foster City, CA) at 42°C for 1 h, followed by 30 cycles of PCR, each consisting of 94°C for 1 min, 52°C for 40 s, and 72°C for 1 min. The final extension was allowed to continue for 10 min and kept at 4°C. For the NSP4 genes, the mixture was incubated at 42°C for 1 h, followed by 30 cycles of PCR, each consisting of 95°C for 45 s, 49°C for 30 s, and 72°C for 1.5 min. The final extension was allowed to continue for 10 min and kept at 4°C.
VP6 genogroup assignment by RT-PCR.
The 824-bp RT-PCR products of the VP6 gene were used for genogrouping. The primer mixture contained 6BEG.303, 6END.682c, and 6END.1082c. The PCR mixture contained 0.5 μl of 1:10 to 1:100 diluted RT-PCR product, 20 mM Tris-HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4, 0.1% Triton X-100, 0.1 mg of bovine serum albumin (BSA)/ml, 200 μM deoxynucleoside triphosphates, 500 nM primers, and 0.5 U Taq-Plus (Taq polymerase with Pfu; Bio Basic, Inc., Canada). The mixture was incubated in a thermal cycler (GeneAmp PCR system 9700; Applied Biosystems, Foster City, CA) at 94°C for 3 min, followed by 10 cycles of PCR, each consisting of 94°C for 1 min, 46°C for 40 s, and 72°C for 1 min, and 10 cycles of 94°C for 1 min, 48°C for 40 s, and 72°C for 1 min, and finally by 15 cycles of 94°C for 1 min, 50°C for 40 s, and 72°C for 1 min. The final extension was allowed to continue for 10 min and kept at 4°C. The VP6 genogroup was defined by the sizes of the amplified products.
TA cloning.
The RT-PCR products of the VP6 gene, 379 bp in length, were purified directly or through agarose gel electrophoresis with GFX PCR DNA and a gel band purification kit (Amersham Biosciences, Piscataway, NJ) and cloned into pGEM-T Easy Vector using the pGEM-T Easy Vector system (Promega, Madison, WI) according to the manufacturer's instructions. The plasmids with the VP6 gene fragment, selected based on antibiotic resistance and lacZ disruption, were isolated by using a high-speed plasmid minikit (Geneaid, Taipei, Taiwan).
Determination of VP7, VP4, and NSP4 genotypes.
The G type and P type were determined as described previously (8, 10). The NSP4 genotype was determined by phylogenetic analysis of the NSP4 genes (4).
Sequencing.
The PCR products were purified directly or through agarose gel electrophoresis with GFX PCR DNA and the gel band purification kit. Nucleic acid sequencing was performed with a BigDye Terminator cycle sequencing kit (v3.1; Applied Biosystems). The primers used for VP6 gene sequencing are VP6-F and VP6-R; for NSP4 gene sequencing, 10BEG.16, 10END.722c, 10.374, and 10.394c (24) were used; for the plasmid with VP6 gene insert sequencing, T7 (5′-TAA TAC GAC TCA CTA TAG GG-3′) was used. The labeled products were purified by ethanol precipitation. The pellet was resuspended in the Hi-Di formamide (Applied Biosystems) and then run on an autosequencer (3100-Avant Genetic Analyzer; Applied Biosystems).
Analysis of sequences.
The sequence data were analyzed by using GeneWorks software (IntelliGenetics, Mountain View, CA). The phylogenetic relationships among strains were analyzed by the neighbor-joining method and the Tamura-Nei distance matrix listed in the MEGA analytical package (23). The robustness of the neighbor-joining trees was statistically evaluated by bootstrap analysis.
Nucleotide sequence accession numbers.
The nucleotide sequences of the partial VP6 genes of the Taiwanese genogroups I and II strains have been submitted to the GenBank sequence database. Accession numbers EU487535 to EU487561 were assigned for P[8]G1 strains with genogroup II specificity in the following order: 81TW5, 85TW516, 86TW569, 87TW914, 88TW1201, 89TW1495, 92TW3580, 92TW3672, 94TW36a, 94TW1164, 94TW1213, 95TW35, 95TW182, 95TW203a, 95TW418, 95TW481, 95TW511, 95TW803, 95TW859, 00TW124, 01TW588, 01TW863, 03TW269, 03TW1284, 04TW577, 04TW628, and 05TW2483. EU487562 was used for strain 01TW564 with P[4]G1 and genogroup I specificity. Accession numbers EU487563 to EU487575 were assigned for P[4]G2 strains with genogroup I specificity in the following order: 81TW6, 83TW278, 92TW59, 93TW111, 93TW114, 94TW133, 00TW3, 00TW469, 00TW532, 01TW499, 02TW376, 04TW360, and 05TW395. EU487576 was used for strain 01TW557 with P[8]G2 and genogroup II specificity. Accession numbers EU487577 to EU487587 were assigned for P[8]G3 strains with genogroup II specificity in the following order: 86TW606, 89TW1532, 00TW668, 01TW1274, 02TW63, 03TW270, 03TW277, 03TW596, 04TW13, 04TW151, and 05TW1320. EU487588 was used for strain 03TW322 with P[4]G3 and genogroup I specificity. Accession numbers EU833479, EU833480, DQ898131, and EU833481 were assigned for P[8]G4 strains with genogroup II specificity in the following order: 85TW425, 85TW432, 01TW964, and 03TW1902. Accession numbers DQ898123 and EU487589 to EU487594 were assigned for P[8]G9 strains with genogroup II specificity in the following order: 99TW1866, 00TW470, 01TW1306, 02TW140, 02TW240, 02TW431, and 02TW641. Additional partial VP6 sequences were assigned accession numbers EU487595 to EU487600 for reference strains in the following order: AU32 (P[8]G9, genogroup II), 95H115 (P[8]G9, genogroup II), P (P[8]G3, genogroup II), ST3 (P[8]G4, genogroup II), WI61 (P[8]G9, genogroup II), and RotaTeq. For each Taiwanese (TW) strain, the first two digits represent the year in which the sample was collected, and the last up to four digits indicate the sample number in the corresponding year.
RESULTS
Assignment of VP6 genogroup by RT-PCR.
To establish the RT-PCR assay for determination of rotavirus VP6 genogroup, reference strains, subgroup I strains including DS-1 (P[4]G2), S2 (P[4]G2), SA11 (P[2]G3), and bovine-human reassortant vaccine strains (RotaTeq), and subgroup II strains including Wa (P[8]G1), KU (P[8]G1), P (P[8]G3), YO (P[8]G3), ST3 (P[8]G4), E210 (P[4]G2), WI61 (P[8]G9), 95H115 (P[8]G9), and AU32 (P[8]G9) were subjected to RT-PCR amplification. In the first amplification, the primers, 6BEG.303 and VP6-R, were used to yield DNA products of 824 bp. In the second amplification, primer 6BEG.303 and genogroup I-specific primer 6END.682c and genogroup II-specific primer 6END.1082c were incorporated. Genogroup I and genogroup II could be distinguished according to the sizes of amplicons, 380 bp for genogroup I and 780 bp for genogroup II.
Comparison of RT-PCR, sequencing, and ELISA for determination of VP6 genogroup or subgroup specificity.
In order to understand the performance of the RT-PCR method for the determination of VP6 genogroup, 80 rotavirus strains with 24 different RNA patterns, including G1 to G4 and G9 strains recovered between 2000 and 2002 were tested, and they were also tested by nucleotide sequence analysis of the partial VP6 gene. The results obtained by these two methods were identical and 100% in agreement. Sixteen of the 80 strains belonged to genogroup I, and 64 strains belonged to genogroup II (Table 1). The correlation of VP6 genogroup determined by RT-PCR with subgroup determined by ELISA was also analyzed. Using ELISA, we found that 16 of the strains tested belonged to subgroup I and 57 of the strains belonged to subgroup II, although 7 of the strains could not be assigned to either subgroup (Table 1). The subgroup specificity correlated well with VP6 genogroup for 73 of the strains (91.3% agreement).
TABLE 1.
Determination of the subgroup and VP6 genogroup by ELISA, RT-PCR, or sequence analysis for rotavirus strains with different G types and electropherotypes recovered between 2000 and 2002a
| G type | P type | No. of RNA patterns | No. of samples | ELISA
|
Sequence analysis
|
RT-PCR
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup I | Subgroup II | ?b | Genogroup I | Genogroup II | Genogroup I | Genogroup II | ||||
| G1 | P[8] | 6 | 16 | 0 | 15 | 1 | 0 | 16 | 0 | 16 |
| P[4] | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | |
| G2 | P[4] | 5 | 15 | 15 | 0 | 0 | 15 | 0 | 15 | 0 |
| P[8] | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | |
| G3 | P[8] | 4 | 22 | 0 | 20 | 2 | 0 | 22 | 0 | 22 |
| G4 | P[8] | 1 | 3 | 0 | 3 | 0 | 0 | 3 | 0 | 3 |
| G9 | P[8] | 6 | 22 | 0 | 18 | 4 | 0 | 22 | 0 | 22 |
| Total | 24 | 80 | 16 | 57 | 7 | 16 | 64 | 16 | 64 | |
The rotavirus samples were selected based on RNA pattern; for each RNA pattern, two or three samples were selected for each year. The genogroups determined by RT-PCR and sequence analysis were in good agreement (100%), and the genogroup and subgroup determined by RT-PCR and ELISA, respectively, were in 91.3% agreement.
The “?” indicates that the sample could not be assigned to either subgroup.
In order to understand whether the test was suitable for rotavirus strains with a wide range of genetic variation, 150 rotavirus strains recovered over two periods, 1981 to 1999 and 2003 to 2005, were chosen to determine VP6 genogroup by RT-PCR and sequence analysis. The results of these two methods were identical and 100% in agreement for the 150 samples (Table 2).
TABLE 2.
Comparison of the VP6 genogroup determined by RT-PCR and sequence analysis of the rotavirus strains with different G types and electropherotypes recovered from 1981 to 1999 and from 2003 to 2005a
| G type | P type | No. of RNA patterns | Total no. of samples | No. of samples evaluated by:
|
|||
|---|---|---|---|---|---|---|---|
| Sequence analysis
|
RT-PCR
|
||||||
| Genogroup I | Genogroup II | Genogroup I | Genogroup II | ||||
| G1 | P[8] | 19 | 43 | 0 | 43 | 0 | 43 |
| P[6]+P[8] | 1 | 1 | 0 | 1 | 0 | 1 | |
| G2 | P[4] | 9 | 45 | 45 | 0 | 45 | 0 |
| P[4]+P[6] | 1 | 1 | 1 | 0 | 1 | 0 | |
| G3 | P[8] | 7 | 55 | 0 | 55 | 0 | 55 |
| P[4] | 1 | 1 | 1 | 0 | 1 | 0 | |
| G4 | P[8] | 2 | 3 | 3 | 0 | 3 | 0 |
| G9 | P[8] | 1 | 1 | 0 | 1 | 0 | 1 |
| Total | 41 | 150 | 50 | 100 | 50 | 100 | |
The rotavirus samples were selected based on RNA pattern; for each RNA pattern, two or three samples were selected for each year. These samples included G1 strains recovered from 1981 to 1989, 1992 to 1995, 1997, 1999, and 2003 to 2005; G2 strains from 1981, 1983, 1992 to 1995, 1997 to 1999, and 2003 to 2005; G3 strains from 1985, 1986, 1988 to 1991, 1995, 1997, 1999, and 2003 to 2005; G4 strains from 1985 and 2003; and a G9 strain from 1999.
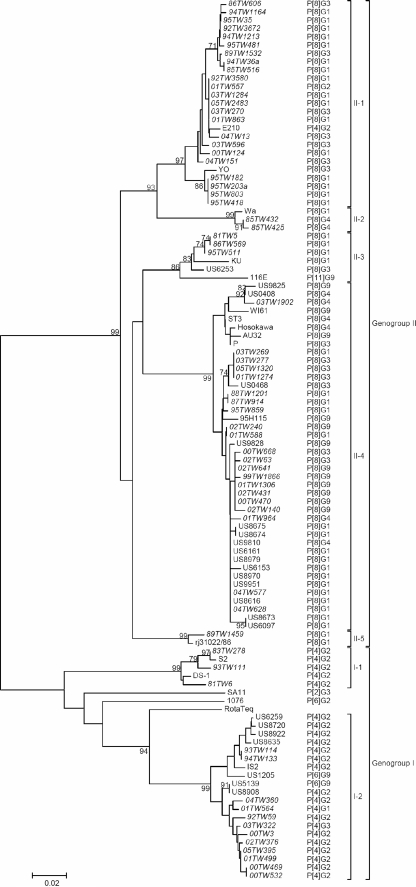
Altogether, 230 Taiwanese rotavirus strains had been subjected to sequence analysis of the partial VP6 gene. Excluding the primer sequences, 341-bp nucleotide sequences were aligned and compared, and a phylogenetic tree was constructed. Because the tree is too enormous to be shown here, 65 rotavirus strains with various genotypes and 52 RNA patterns and covering a broad range of genetic variation were selected for presentation in Fig. 2. Genogroup I could be distinguished into two genetic lineages, and genogroup II could be distinguished into five genetic lineages. The nucleotide sequence identities were 93.3 to 100% for the strains within each lineage, 83 to 91.8% between different lineages within genogroup I, 86.5 to 92.1% between different lineages within genogroup II, and 74.8 to 81.2% between the two different genogroups.
FIG. 2.
Phylogenetic analysis of the nucleotide sequences of the partial VP6 gene (nucleotides 766 to 1106). The 341-bp fragments of the VP6 gene were sequenced and a phylogenetic tree was constructed based on the neighbor-joining method within the MEGA package. Percentage bootstrap values above 70% are shown at branch nodes. Branch length for a 2% nucleotide difference is indicated at the bottom. VP6 genogroup was determined by the clustering with genogroup I or genogroup II reference strains. For each strain, the P and G genotypes are shown. Taiwanese strains are indicated by italic type.
Associations of rotavirus VP6 genogroups and G types and P types.
It has been assumed that subgroups usually segregate with P types; that is, P[4] associates with subgroup I, and P[8] associates with subgroup II (17). Therefore, in addition to the VP6 genogroup determined by RT-PCR, the G and P types of the rotavirus strains were also determined, and the associations of the genotypes of different genes were analyzed. An additional 524 rotavirus-positive fecal samples were included for this analysis. Of these, 103 strains belonged to genogroup I, 416 strains belonged to genogroup II, and 5 strains were determined as genogroup I+II. For samples with common G- and P-type combinations and most of the samples with uncommon G- and P-type combinations, the associations between VP6 genogroups and P types were that P[4] associated with genogroup I and P[8] associated with genogroup II (Tables 3 and 4). It has been suggested that there is a 100% linkage between subgroup and NSP4 genotype among human common and reassortant strains (16). For the 28 samples with uncommon G and P type combinations, the NSP4 genes were sequenced, and the NSP4 genotypes were determined by phylogenetic analysis. Except for the five samples with genogroup I+II, NSP4 genotype A associated with genogroup I, and NSP4 genotype B associated with genogroup II (Table 4).
TABLE 3.
VP6 genogroups of rotavirus strains with common G and P type combinationsa
| Rotavirus with VP7 and VP4 type | No. of strains | VP6 genogroup |
|---|---|---|
| G1P[8] | 184 | II |
| G2P[4] | 98 | I |
| G3P[8] | 4 | II |
| G9P[8] | 210 | II |
| Total | 496 |
VP6 genogroup, G type, and P type were determined by RT-PCR.
TABLE 4.
VP6 genogroups and NSP4 genotypes of the rotavirus strains with uncommon G and P type combinationsa
| No. of strains | Rotavirus
|
VP6 genogroup | No. of strains with NSP4 genotype:
|
||
|---|---|---|---|---|---|
| G type | P type | A | B | ||
| 4 | G1 | P[4] | I | 4 | 0 |
| 3 | G1 | P[4] | I+II | 1 | 2 |
| 1 | G1 | P[4]+P[8] | II | 0 | 1 |
| 14 | G2 | P[8] | II | 0 | 14 |
| 1 | G3 | P[4] | II | 0 | 1 |
| 1 | G3 | P[4]+P[8] | II | 0 | 1 |
| 1 | G4 | P[4] | I | 1 | 0 |
| 1 | G9 | P[4] | II | 0 | 1 |
| 2 | G9 | P[4] | I+II | 0 | 2 |
| 28 | Total | 6 | 22 | ||
VP6 genogroup, G type, and P type were determined by RT-PCR. NSP4 genotype was determined by sequence and phylogenetic analysis (4).
Analysis of the PCR amplicons for the rotavirus samples determined as VP6 genogroup I+II.
It was not clear whether the genogroup I+II determined by RT-PCR reflected the VP6 gene molecules containing both of the regions specific for genogroup I and genogroup II or simply resulted from a mixture of genogroup I VP6 molecules and genogroup II VP6 molecules. Therefore, for the five rotavirus samples determined as genogroup I+II, the PCR amplicons were analyzed by cloning and sequencing. Twenty clones were selected for each sample. The genogroup of the cloned VP6 gene was determined by sequence analysis. The sequences of all of these different clones belonged to either genogroup I or genogroup II, and not a single clone was found with sequences containing both of the regions specific for genogroup I and genogroup II.
DISCUSSION
Although subgrouping by ELISA has been widely used, in some cases, a subgroup determined by ELISA seems to be imprecise because of the poor reactivity between MAbs (e.g., MAb 631/9) and subgroup II rotavirus strains (18). A similar situation has been noted to occur despite the use of different MAbs. Taniguchi et al. also found that some rotavirus strains react weakly with one subgroup II-specific MAb, YO-5 (31). In addition to the problem of the poor reactivity of the monoclonal antibodies, which results in untypeable strains due to antigenic drift, the problem of limited supplies of the VP6 subgroup-specific antibodies and that of antibody titer changes during storage are also troublesome. Therefore, development of a conventional and reliable molecular method is necessary. Molecular methods, namely, sequence analysis of the VP6 gene (18) and restriction fragment length polymorphism analysis (19), have been developed to prevent the possible misclassification by ELISA. However, sequence analysis is a technique requiring sophisticated equipment and technical knowledge that might not be available at most laboratories, and the usage of restriction fragment length polymorphism might be hampered by the point mutations that frequently occur in rotavirus. In the present study, RT-PCR, another molecular method that has been extensively used in rotavirus G and P typing (8, 10), was established for assignment of VP6 genogroups. The results showed that the VP6 genogroup I and genogroup II determined by RT-PCR were identical to those determined by sequence analysis. Genetically identified VP6 genogroup I and genogroup II corresponded well to subgroup I and subgroup II determined serologically by ELISA, respectively, suggesting that RT-PCR could be an alternative method for characterization of the VP6 genes. Compared to sequence analysis, RT-PCR appeared to be simpler and more rapid.
The seven strains for which the subgroup could not be determined by ELISA were assigned to genogroup II by both sequence analysis and RT-PCR. There is no statistical significance (P = 0.167) to the finding that the seven strains whose subgroup could not be determined by ELISA belonged to genogroup II by RT-PCR and sequencing. The absorbances of these seven strains obtained from reactivity with subgroup I MAb S2-37 were near to that of a nonreactive control; however, the low absorbances were obtained either from reactivity with group A-common MAb YO-156 or with subgroup II-specific MAb YO-5. Although the reactivity was greater than that of the nonreactive control, a definite subgroup could not be assigned. These results revealed that containing a higher amount of viral particles in the sample is required for efficient determination of subgroup by ELISA compared to that for the determination of VP6 genogroup by RT-PCR. A relatively high level (4 of 22) of disagreement between ELISA and RT-PCR/sequencing on G9 strains was observed. However, there was no significance (P = 0.179) to the finding that rotavirus strains whose subgroup could not be determined by ELISA mostly belonged to the G9 strain. The low absorbances were obtained from reactivity with subgroup II-specific MAb YO-5 in three of the four G9 strains, and only one strain obtained low absorbances from reactivity with both group A-common MAb YO-156 and subgroup II-specific MAb YO-5, which suggested that some G9 strains might have antigenic drift on subgroup II-specific MAb YO-5 recognition region and caused lower reactivity. This further supported that there is a need to develop a molecular method to improve the situation that VP6 subgroup could not be determined by ELISA.
Although the primer sites for RT-PCR genogrouping were not located in the regions considered to have subgroup I or subgroup II specificity, the subgroup-defining regions were included in the partial VP6 gene for sequence analysis, the same region used in the analysis of VP6 genogroups by Iturriza-Gomara et al. (18). The genogrouping results obtained by RT-PCR in the present study were confirmed by sequence analysis of the partial VP6 genes for 230 samples, and the results correlated well and were 100% in agreement, suggesting that the degenerate primers designed for RT-PCR genogrouping were genogroup specific. The genogroup-specific primers were designed based on the VP6 sequences of reference strains from GenBank and those of 80 Taiwanese rotavirus strains with G types of G1 to G4 and G9, P genotypes of P[8] and P[4], and 24 electropherotypes. In order to understand the genogroup specificity of each of the primers, the nucleotide sequence identities within and between the genogroups were analyzed. Comparison of the VP6 gene sequences of the strains listed in Table S1 in the supplemental material, including 23 genogroup I sequences and 73 genogroup II sequences, for the primer regions, revealed that the nucleotide sequence identities of the genogroup I-specific primer within genogroup I and between the two genogroups were 83.3 to 100% and 44.4 to 61.1%, respectively. The identities of the genogroup II-specific primer within genogroup II and between the two genogroups were 89.5 to 100% and 63.2 to 73.7%, respectively.
Recently, 22 VP6 full-length sequences of the U.S. strains collected from 1996 to 2002 were submitted to the GenBank database. Based on phylogenetic analysis, Kerin et al. pointed out that rotavirus VP6 genes have greater variety than was previously suspected (21). Therefore, we included the 22 strains (10 P[8]G1, 5 P[4]G2, 2 P[8]G3, 2 P[8]G4, 1 P[6]G9, and 2 P[8]G9) in the phylogenetic analysis shown in Fig. 2 and realized that the Taiwanese strains analyzed in the present study had more genetic variation. We also compared the nucleotide sequences of these 22 U.S. strains at the regions where the genogroup-specific primers are located (Table S2 in the supplemental material) and found that 4 of the 6 genogroup I strains would be bound by our genogroup I-specific primer and that 16 genogroup II strains would be bound by our genogroup II-specific primer, suggesting that the genogroup of the 20 strains could possibly be determined by the RT-PCR method. However, the two genogroup I strains would form mismatches with our genogroup I-specific primer at the 3′ terminus, which might impede the PCR. A similar situation was observed for the VP6 gene of the 116E strain, which varied from the genogroup II-specific primer at the last nucleotide of the 3′ terminus. In the present study, reference strains, including at least four genogroup I (two P[4]G2, one P[2]G3, and RotaTeq vaccine strains) and nine genogroup II strains (two P[8]G1, one P[4]G2, two P[8]G3, one P[8]G4, and three P[8]G9), and 754 rotavirus samples with more than 52 electropherotypes recovered from a 25-year period, representing a wide range of genetic variation, could be easily genogrouped by RT-PCR. For the rare strains, if a negative result is obtained from the RT-PCR assay, we would suggest that the VP6 genogroup be determined by sequence analysis.
The association of subgroup and other genes has been discussed previously. For the associations among the VP6, VP7, and VP4 genes, it has been noticed that G1, G3, and G4 frequently are associated with P[8] and subgroup II, and G2 frequently is associated with P[4] and subgroup I (17). The association of subgroup and RNA electropherotype has also been reported previously for human rotaviruses of group A (20). However, Svensson et al. reported that the subgroup specificity could not be predicted by the migration of gene segments 10 and 11 (29). Iturriza-Gomara et al. have demonstrated the independent segregation of the VP4, VP6, and VP7 genes (18), and these authors also found a 100% linkage of the VP6 subgroup and NSP4 genotype, association of NSP4 genotype A with subgroup I and of NSP4 genotype B with subgroup II, in common and reassortant human rotaviruses (16). In the present study, excluding the five samples characterized as containing rotaviruses with genogroup I+II, all of the rotaviruses with common G- and P-type combinations, and most of those with uncommon G- and P-type combinations, had such gene associations between VP6 genogroups and P genotypes: P[8] associated with VP6 genogroup II, and P[4] associated with VP6 genogroup I. Only two P[4] strains, one P[4]G3 strain and one P[4]G9 strain, had a VP6 gene of genogroup II. Excluding the five rotavirus samples with VP6 genogroup I+II, the associations between VP6 genogroups and NSP4 genotypes, NSP4A being associated with genogroup I and NSP4B associated with genogroup II, were observed in the 23 rotavirus strains with uncommon G and P combinations.
It has been suggested that there are no true human subgroup I+II or subgroup non-I non-II strains to be found and that all such strains are from animals (9, 14, 15). It frequently happens that human rotaviruses reassort with animal strains, and it increases the possibility that subgroup I+II or subgroup non-I non-II could be found in human strains. In the present study, five rotavirus samples were determined to be genogroup I+II. However, after cloning the PCR amplicons of the VP6 gene, we found that all of the clones analyzed belonged to either genogroup I or genogroup II. These rotavirus samples appeared more likely to be the result of coinfection with two different rotavirus strains, one with genogroup I and the other with genogroup II. We also used ELISA to confirm the results and found that these samples reacted with both subgroup I-specific and subgroup II-specific MAbs. Furthermore, we did not find any sequences possessing both primer sites specific for the genogroup I and genogroup II. Therefore, there was no true genogroup I+II in the rotavirus samples tested in the present study.
The RT-PCR assay established in the present study to determine the VP6 genogroup was successfully applied to reference rotavirus strains, including RotaTeq vaccine strains, and 754 Taiwanese rotavirus strains recovered between 1981 and 2005. The assay appears to be a reliable and convenient method for determining the VP6 genogroups of human rotaviruses with a wide range of genetic variation.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Science Council (grants NSC-92-2314-B-002-355, NSC-94-3112-B-002-022, and NSC-95-3112-B-002-004) and the Department of Health (grant DOH92-DC-1203) of Taiwan, Republic of China.
Footnotes
Published ahead of print on 30 July 2008.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Au, K. S., W. K. Chan, J. W. Burns, and M. K. Estes. 1989. Receptor activity of rotavirus nonstructural glycoprotein NS28. J. Virol. 634553-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ball, J. M., P. Tian, C. Q. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272101-104. [DOI] [PubMed] [Google Scholar]
- 3.Ciarlet, M., F. Liprandi, M. E. Conner, and M. K. Estes. 2000. Species specificity and interspecies relatedness of NSP4 genetic groups by comparative NSP4 sequence analyses of animal rotaviruses. Arch. Virol. 145371-383. [DOI] [PubMed] [Google Scholar]
- 4.Cunliffe, N. A., P. A. Woods, J. P. Leite, B. K. Das, M. Ramachandran, M. K. Bhan, C. A. Hart, R. I. Glass, and J. R. Gentsch. 1997. Sequence analysis of NSP4 gene of human rotavirus allows classification into two main genetic groups. J. Med. Virol. 5341-50. [PubMed] [Google Scholar]
- 5.Estes, M. K. 2001. Rotaviruses and their replication, p. 1747-1785. In D. M. Knipe, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott/Williams & Wilkins, Philadelphia, PA.
- 6.Estes, M. K. 1996. Rotaviruses and their replication, p. 1625-1655. In B. N. Fields, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology, 3rd ed. Lippincott/Raven Press, Philadelphia, PA.
- 7.Estes, M. K., and J. Cohen. 1989. Rotavirus gene structure and function. Microbiol. Rev. 53410-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bhan. 1992. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 301365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorziglia, M., Y. Hoshino, K. Nishikawa, W. L. Maloy, R. W. Jones, A. Z. Kapikian, and R. M. Chanock. 1988. Comparative sequence analysis of the genomic segment 6 of four rotaviruses each with a different subgroup specificity. J. Gen. Virol. 69(Pt. 7)1659-1669. [DOI] [PubMed] [Google Scholar]
- 10.Gouvea, V., R. I. Glass, P. Woods, K. Taniguchi, H. F. Clark, B. Forrester, and Z. Y. Fang. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 28276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg, H., V. McAuliffe, J. Valdesuso, R. Wyatt, J. Flores, A. Kalica, Y. Hoshino, and N. Singh. 1983. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect. Immun. 3991-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulati, B. R., R. Deepa, B. K. Singh, and C. D. Rao. 2007. Diversity in Indian equine rotaviruses: identification of genotype G10,P6[1] and G1 strains and a new VP7 genotype (G16) strain in specimens from diarrheic foals in India. J. Clin. Microbiol. 45972-978. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Horie, Y., O. Masamune, and O. Nakagomi. 1997. Three major alleles of rotavirus NSP4 proteins identified by sequence analysis. J. Gen. Virol. 78(Pt. 9)2341-2346. [DOI] [PubMed] [Google Scholar]
- 14.Hoshino, Y., M. Gorziglia, J. Valdesuso, J. Askaa, R. I. Glass, and A. Z. Kapikian. 1987. An equine rotavirus (FI-14 strain) which bears both subgroup I and subgroup II specificities on its VP6. Virology 157488-496. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino, Y., R. G. Wyatt, H. B. Greenberg, A. R. Kalica, J. Flores, and A. Z. Kapikian. 1983. Isolation, propagation, and characterization of a second equine rotavirus serotype. Infect. Immun. 411031-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iturriza-Gomara, M., E. Anderton, G. Kang, C. Gallimore, W. Phillips, U. Desselberger, and J. Gray. 2003. Evidence for genetic linkage between the gene segments encoding NSP4 and VP6 proteins in common and reassortant human rotavirus strains. J. Clin. Microbiol. 413566-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iturriza-Gomara, M., B. Isherwood, U. Desselberger, and J. Gray. 2001. Reassortment in vivo: driving force for diversity of human rotavirus strains isolated in the United Kingdom between 1995 and 1999. J. Virol. 753696-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iturriza-Gomara, M., C. Wong, S. Blome, U. Desselberger, and J. Gray. 2002. Molecular characterization of VP6 genes of human rotavirus isolates: correlation of genogroups with subgroups and evidence of independent segregation. J. Virol. 766596-6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iturriza-Gomara, M., C. Wong, S. Blome, U. Desselberger, and J. Gray. 2002. Rotavirus subgroup characterisation by restriction endonuclease digestion of a cDNA fragment of the VP6 gene. J. Virol. Methods 10599-103. [DOI] [PubMed] [Google Scholar]
- 20.Kalica, A. R., H. B. Greenberg, R. T. Espejo, J. Flores, R. G. Wyatt, A. Z. Kapikian, and R. M. Chanock. 1981. Distinctive ribonucleic acid patterns of human rotavirus subgroups 1 and 2. Infect. Immun. 33958-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerin, T. K., E. M. Kane, R. I. Glass, and J. R. Gentsch. 2007. Characterization of VP6 genes from rotavirus strains collected in the United States from 1996-2002. Virus Genes 35489-495. [DOI] [PubMed] [Google Scholar]
- 22.Khamrin, P., N. Maneekarn, S. Peerakome, W. Chan-it, F. Yagyu, S. Okitsu, and H. Ushijima. 2007. Novel porcine rotavirus of genotype P[27] shares new phylogenetic lineage with G2 porcine rotavirus strain. Virology 361243-252. [DOI] [PubMed] [Google Scholar]
- 23.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 5150-163. [DOI] [PubMed] [Google Scholar]
- 24.Lee, C. N., Y. L. Wang, C. L. Kao, C. L. Zao, C. Y. Lee, and H. N. Chen. 2000. NSP4 gene analysis of rotaviruses recovered from infected children with or without diarrhea. J. Clin. Microbiol. 384471-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, Y. P., S. Y. Chang, C. L. Kao, L. M. Huang, M. Y. Chung, J. Y. Yang, H. Y. Chen, K. Taniguchi, K. S. Tsai, and C. N. Lee. 2006. Molecular epidemiology of G9 rotaviruses in Taiwan between 2000 and 2002. J. Clin. Microbiol. 443686-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathieu, M., I. Petitpas, J. Navaza, J. Lepault, E. Kohli, P. Pothier, B. V. Prasad, J. Cohen, and F. A. Rey. 2001. Atomic structure of the major capsid protein of rotavirus: implications for the architecture of the virion. EMBO J. 201485-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsui, S. M., E. R. Mackow, and H. B. Greenberg. 1989. Molecular determinant of rotavirus neutralization and protection. Adv. Virus Res. 36181-214. [DOI] [PubMed] [Google Scholar]
- 28.Mori, Y., M. A. Borgan, N. Ito, M. Sugiyama, and N. Minamoto. 2002. Diarrhea-inducing activity of avian rotavirus NSP4 glycoproteins, which differ greatly from mammalian rotavirus NSP4 glycoproteins in deduced amino acid sequence in suckling mice. J. Virol. 765829-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svensson, L., L. Grahnquist, C. A. Pettersson, M. Grandien, G. Stintzing, and H. B. Greenberg. 1988. Detection of human rotaviruses which do not react with subgroup I- and II-specific monoclonal antibodies. J. Clin. Microbiol. 261238-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang, B., J. M. Gilbert, S. M. Matsui, and H. B. Greenberg. 1997. Comparison of the rotavirus gene 6 from different species by sequence analysis and localization of subgroup-specific epitopes using site-directed mutagenesis. Virology 23789-96. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi, K., T. Urasawa, S. Urasawa, and T. Yasuhara. 1984. Production of subgroup-specific monoclonal antibodies against human rotaviruses and their application to an enzyme-linked immunosorbent assay for subgroup determination. J. Med. Virol. 14115-125. [DOI] [PubMed] [Google Scholar]
- 32.Tian, P., M. K. Estes, Y. Hu, J. M. Ball, C. Q. Zeng, and W. P. Schilling. 1995. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J. Virol. 695763-5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyatt, R. G., H. B. Greenberg, W. D. James, A. L. Pittman, A. R. Kalica, J. Flores, R. M. Chanock, and A. Z. Kapikian. 1982. Definition of human rotavirus serotypes by plaque reduction assay. Infect. Immun. 37110-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.