Abstract
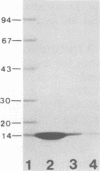
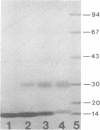
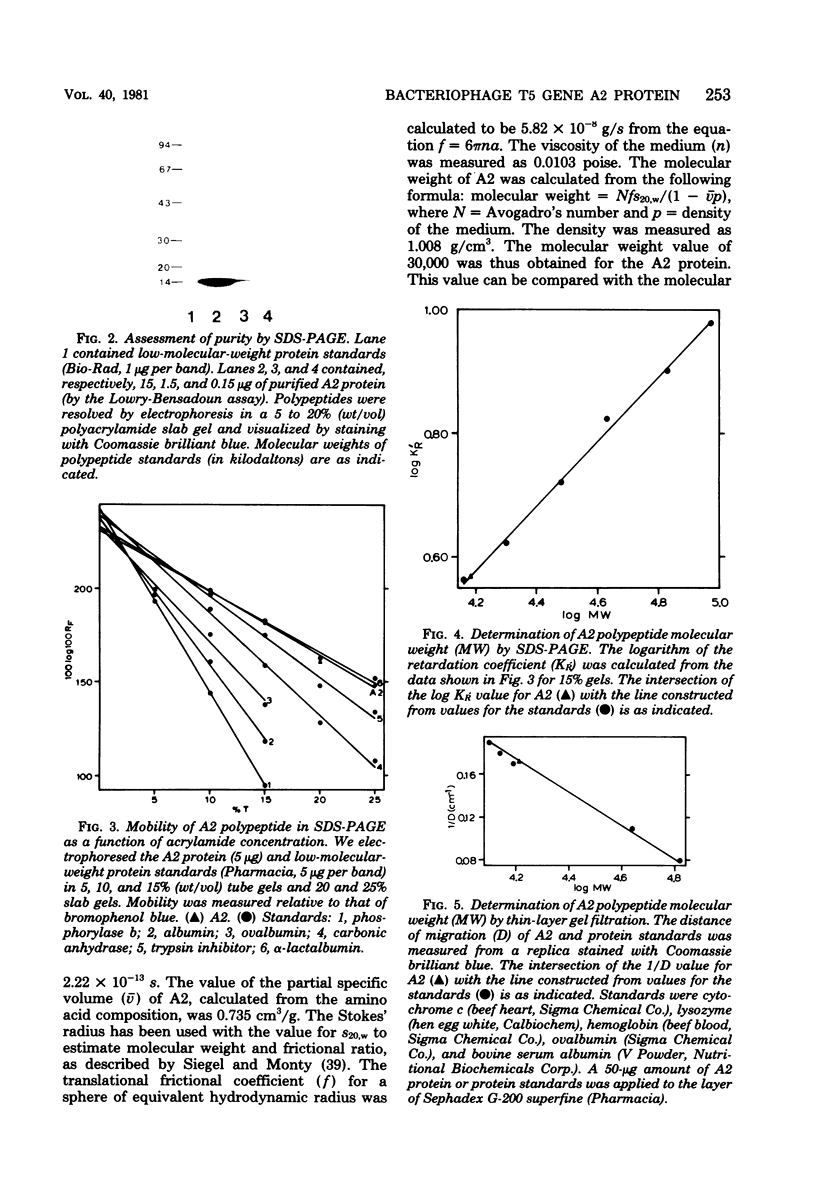
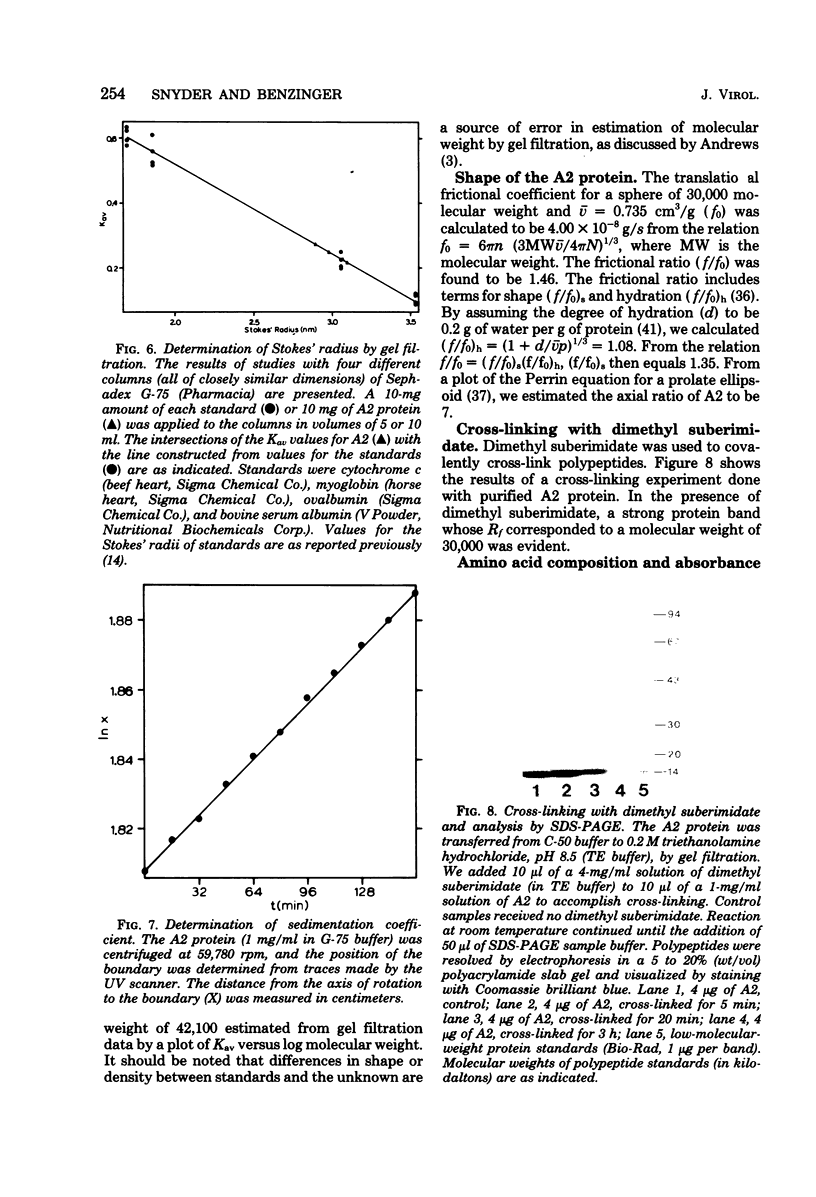
Second-step transfer of bacteriophage T5 DNA requires the function of the T5 pre-early proteins A1 and A2. We have isolated and characterized the gene A2 protein as part of an effort to determine the mechanism of second-step transfer. The A2 protein was purified by DNA-cellulose column chromatography followed by gel filtration and ion-exchange column chromatography. The A2 protein's identity was confirmed by two-dimensional gel electrophoresis. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and thin-layer gel filtration in 6 M guanidine hydrochloride demonstrated a molecular weight of 15,000 for the A2 polypeptide. Migration of the A2 protein through gel filtration columns under nondenaturing conditions, in combination with sedimentation behavior, indicated dimerization of the A2 polypeptide. The existence of the A2 dimer was confirmed by protein cross-linking with dimethyl suberimidate and analysis of the cross-linked proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The amino acid composition, degree of polymerization, DNA-binding ability, and physical characteristics of the T5 gene A2 protein are consistent with a function of the A2 protein in DNA transfer.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Beckman L. D., Hoffman M. S., McCorquodale D. J. Pre-early proteins of bacteriophage T5: structure and function. J Mol Biol. 1971 Dec 28;62(3):551–564. doi: 10.1016/0022-2836(71)90155-0. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Berthold V., Geider K. Interaction of DNA with DNA-binding proteins. The characterization of protein HD from Escherichia coli and its nucleic acid complexes. Eur J Biochem. 1976 Dec 11;71(2):443–449. doi: 10.1111/j.1432-1033.1976.tb11132.x. [DOI] [PubMed] [Google Scholar]
- Brunel F., Davison J. Restriction insensitivity in bacteriophage T5. III. Characterization of EcoRI-sensitive mutants by restriction analysis. J Mol Biol. 1979 Mar 15;128(4):527–543. doi: 10.1016/0022-2836(79)90291-2. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delincée H., Radola B. J. Determination of isoelectric points in thin-layer isoelectric focusing: the importance of attaining the steady state and the role of CO2 interference. Anal Biochem. 1978 Oct 15;90(2):609–623. doi: 10.1016/0003-2697(78)90154-9. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Dunn G. B., McCorquodale D. J. Identification of the gene controlling the synthesis of the major bacteriophage T5 membrane protein. J Virol. 1976 May;18(2):542–549. doi: 10.1128/jvi.18.2.542-549.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Felgenhauer K. Evaluation of molecular size by gel electrophoretic techniques. Hoppe Seylers Z Physiol Chem. 1974 Oct;355(10):1281–1290. doi: 10.1515/bchm2.1974.355.2.1281. [DOI] [PubMed] [Google Scholar]
- Glenn J., Cheung A. K., Duckworth D. H. Are class I (pre-early) proteins of bacteriophage T5 sufficient to induce abortive infection of ColIb+ Escherichia coli? J Virol. 1979 May;30(2):431–437. doi: 10.1128/jvi.30.2.431-437.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Desai S. M., Vaughan J., Weiss S. B. Bacteriophage T5 transfer RNA. Isolation and characterization of tRNA species and refinement of the tRNA gene map. J Biol Chem. 1980 Apr 10;255(7):3164–3173. [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Nitecki D. E., Goodman J. W. Estimation of molecular weights and Stokes' radii of polypeptide chains by thin-layer gel filtration in guanidine hydrochloride. Anal Biochem. 1972 Jan;45(1):286–297. doi: 10.1016/0003-2697(72)90029-2. [DOI] [PubMed] [Google Scholar]
- Kratky O., Leopold H., Stabinger H. The determination of the partial specific volume of proteins by the mechanical oscillator technique. Methods Enzymol. 1973;27:98–110. doi: 10.1016/s0076-6879(73)27007-6. [DOI] [PubMed] [Google Scholar]
- LANNI Y. T. Invasion by bacteriophage T5. II. Dissociation of calcium-independent and calcium-dependent processes. Virology. 1960 Apr;10:514–529. doi: 10.1016/0042-6822(60)90133-1. [DOI] [PubMed] [Google Scholar]
- LANNI Y. T., MCCORQUODALE D. J., WILSON C. M. MOLECULAR ASPECTS OF DNA TRANSFER FROM PHAGE T5 TO HOST CELLS. II. ORIGIN OF FIRST-STEP-TRANSFER DNA FRAGMENTS. J Mol Biol. 1964 Oct;10:19–27. doi: 10.1016/s0022-2836(64)80024-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanni Y. T. DNA transfer from phage T5 to host cells: dependence on intercurrent protein synthesis. Proc Natl Acad Sci U S A. 1965 May;53(5):969–973. doi: 10.1073/pnas.53.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni Y. Functions of two genes in the first-step-transfer DNA of bacteriophage T5. J Mol Biol. 1969 Aug 28;44(1):173–183. doi: 10.1016/0022-2836(69)90412-4. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McCorquodale D. J., Buchanan J. M. Patterns of protein synthesis in T5-infected Escherichia coli. J Biol Chem. 1968 May 25;243(10):2550–2559. [PubMed] [Google Scholar]
- McCorquodale D. J., Shaw A. R., Shaw P. K., Chinnadurai G. Pre-early polypeptides of bacteriophages T5 and BF23. J Virol. 1977 May;22(2):480–488. doi: 10.1128/jvi.22.2.480-488.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Rice A. C., Ficht T. A., Holladay L. A., Moyer R. W. The purification and properties of a double-stranded DNA-binding protein encoded by the gene D5 of bacteriophage T5. J Biol Chem. 1979 Aug 25;254(16):8042–8051. [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Gros F. Characterization of a novel, low-molecular-weight DNA-binding protein from Escherichia coli. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3428–3432. doi: 10.1073/pnas.72.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. R., Lang D., McCorquodale D. J. Terminally redundant deletion mutants of bacteriophage BF23. J Virol. 1979 Jan;29(1):220–231. doi: 10.1128/jvi.29.1.220-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]