Abstract
Study objective
To investigate the possible relation between bladder cancer mortality among white men and women and private water use in New England, USA, where rates have been persistently raised and use of private water supplies (wells) common.
Design
Ecological study relating age adjusted cancer mortality rates for white men and women during 1985–1999 and proportion of persons using private water supplies in 1970. After regressing mortality rates on population density, Pearson correlation coefficients were computed between residual rates and the proportion of the population using private water supplies, using the state economic area as the unit of calculation. Calculations were conducted within each of 10 US regions.
Setting
The 504 state economic areas of the contiguous United States.
Participants
Mortality analysis of 11 cancer sites, with the focus on bladder cancer.
Main results
After adjusting for the effect of population density, there was a statistically significant positive correlation between residual bladder cancer mortality rates and private water supply use among both men and women in New England (men, r = 0.42; women, r = 0.48) and New York/New Jersey (men, r = 0.49; women, r = 0.62).
Conclusions
Use of well water from private sources, or a close correlate, may be an explanatory variable for the excess bladder cancer mortality in New England. Analytical studies are underway to clarify the relation between suspected water contaminants, particularly arsenic, and raised bladder cancer rates in northern New England.
Keywords: bladder cancer, drinking water, private water supply, New England
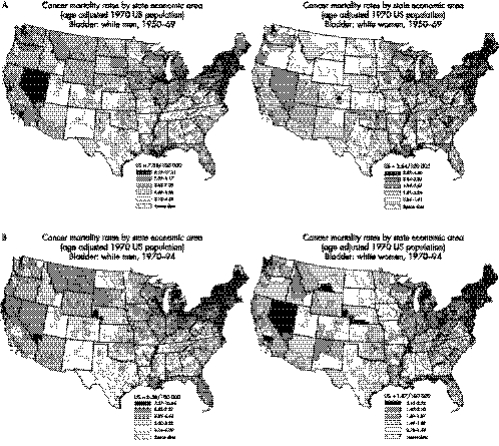
The Atlas of Cancer Mortality in the United States, 1950–1994 shows persistently raised rates for bladder cancer among both men and women in the north eastern states (fig 1A and 1B), most notably in northern New England.1 (A colour version of the figure is available on line http://www.jech.com/supplemental). A number of established bladder cancer risk factors, such as smoking and occupational exposures, may have contributed to these increased rates.2 Recent smoking prevalence rates in northern New England are similar to national rates3; however, data on per capita cigarette sales tax from 1950 to 1975 show that cigarette sales in this region were higher than those for the United States overall.4 Although this finding suggests that smoking may explain part of the regional excess in bladder cancer mortality, the geographical patterns for lung cancer mortality do not show a similar persistent excess in New England,1 suggesting that other factors may be involved. Although occupational exposures in the leather and textile industries, and from truck driving, have been linked to bladder cancer in New England, they account for only a small fraction of the excess mortality in this region and would not explain the continued increase in recent years.5,6
Figure 1 Bladder cancer mortality rated by state economic areas (A) 1950–1969 and (B) 1970–1994.
Private supplies served as the primary source of drinking water for 15.1% of the New England population in 1970, increasing to 20.8% in 1990.7 The US census did not collect these data in 2000. In the three northern states of New England (Maine, New Hampshire, Vermont), 34.3% of the 1970 population was served by private supplies. Unlike public water supplies, private wells are not subject to federal drinking water standards. In New England, private wells are most commonly drilled into fractured crystalline bedrock aquifers where geochemical conditions may promote the mobilisation of natural contaminants. Thus, the use of private wells may provide a key to the raised bladder cancer mortality in northern New England.
To investigate whether the bladder cancer excess in northern states of New England may be related to private well use, we examined the correlation between the prevalence of private well use and bladder cancer mortality rates in the region, using the state economic area (SEA) (a group of similar counties) as the unit of analysis. For comparative purposes, we performed this analysis for other selected cancer sites within each of 10 standard regions of the contiguous United States, as defined by the US Office of Management and Budget, and used by many Federal agencies in the United States.8 Each region includes several states, and between 23 and 107 SEAs (see table 1).
Table 1 Correlation coefficients between 1985 and 1999, age adjusted cancer mortality rates (adjusted for population density), and proportion of the population using self supplied water, by state economic area (SEA).
| Region† | Number | Bladder | Lung | Kidney | Oesophagus | Stomach | Colon | Rectum | Pancreas | Leukaemia | Prostate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | |||||||||||
| 1 | 25 | 0.42* | 0.41* | 0.59* | 0.38 | −0.27 | 0.09 | 0.09 | 0.14 | −0.01 | −0.04 |
| 2 | 26 | 0.49* | 0.58* | 0.68* | 0.31 | −0.55* | 0.40* | 0.38 | −0.03 | 0.17 | 0.32 |
| 3 | 53 | 0.02 | 0.39* | 0.17 | 0.14 | −0.43* | −0.18 | −0.09 | 0.15 | −0.04 | 0.03 |
| 4 | 107 | 0.06 | 0.31* | −0.03 | −0.15 | 0.08 | 0.02 | −0.05 | 0.12 | 0.03 | −0.16 |
| 5 | 99 | 0.12 | −0.20 | −0.05 | 0.07 | 0.09 | 0.02 | 0.13 | −0.08 | 0.11 | 0.34* |
| 6 | 72 | 0.09 | 0.43* | −0.24* | −0.15 | 0.03 | 0.17 | 0.00 | 0.06 | 0.04 | 0.03 |
| 7 | 44 | 0.03 | 0.10 | −0.22 | −0.05 | −0.06 | 0.27 | 0.18 | −0.40* | −0.14 | −0.03 |
| 8 | 30 | 0.41* | 0.51* | 0.56* | 0.42* | 0.43* | 0.79* | 0.40* | 0.51* | 0.39* | 0.29 |
| 8‡ | 25 | 0.18 | 0.11 | 0.48* | 0.11 | 0.15 | 0.70* | 0.09 | 0.29 | 0.28 | 0.39 |
| 9 | 25 | 0.27 | 0.60* | 0.74* | 0.38 | 0.08 | 0.27 | 0.42* | 0.31 | −0.10 | 0.52* |
| 10 | 23 | −0.04 | 0.01 | −0.15 | 0.09 | −0.14 | 0.34 | 0.25 | −0.19 | 0.12 | 0.08 |
| Women | |||||||||||
| Number | Bladder | Lung | Kidney | Oesophagus | Stomach | Colon | Rectum | Pancreas | Leukaemia | Breast | |
| 1 | 25 | 0.48* | 0.33 | 0.02 | 0.24 | −0.42* | 0.41* | 0.12 | 0.07 | 0.19 | −0.05 |
| 2 | 26 | 0.62* | 0.79* | 0.40* | 0.36 | −0.42* | 0.65* | 0.20 | 0.11 | 0.04 | −0.02 |
| 3 | 53 | −0.09 | 0.26 | 0.16 | −0.23 | −0.47* | −0.11 | −0.01 | −0.45* | −0.28* | −0.03 |
| 4 | 107 | 0.03 | −0.18 | −0.13 | −0.24* | 0.08 | −0.03 | −0.06 | −0.11 | 0.07 | −0.12 |
| 5 | 99 | 0.01 | −0.16 | 0.03 | 0.12 | −0.05 | 0.07 | 0.05 | 0.09 | 0.03 | −0.05 |
| 6 | 72 | 0.33 | 0.15 | −0.06 | −0.14 | −0.03 | 0.37* | −0.12 | 0.14 | −0.04 | 0.12 |
| 7 | 44 | 0.00 | −0.20 | 0.11 | −0.17 | 0.11 | 0.44* | 0.33* | −0.34 | 0.16 | 0.34* |
| 8 | 30 | 0.35 | 0.34 | 0.13 | 0.29 | 0.19 | 0.52* | 0.52* | 0.46* | −0.03 | 0.52* |
| 8‡ | 25 | 0.07 | −0.17 | −0.15 | −0.10 | 0.02 | 0.27 | 0.31 | 0.16 | −0.13 | 0.31 |
| 9 | 25 | 0.16 | 0.44* | 0.50* | 0.18 | 0.08 | 0.30 | 0.22 | 0.13 | 0.37 | 0.19 |
| 10 | 23 | −0.13 | 0.13 | 0.18 | −0.29 | 0.25 | 0.51* | 0.38 | −0.13 | −0.17 | −0.10 |
* p<0.05. †US regions: (1) CT, ME, MA, NH, RI, VT; 2) NJ, NY; (3) DE, MD, PA, VA, WV, DC; (4) AL, FL, GA, KY, MS, NC, SC, TN; (5) IL, IN, MI, MN, OH, WI; (6) AR, LA, NM, OK, TX; (7) IA, KS, MO, NE; (8) CO, MT, ND, SD, UT, WY; (9) AZ, CA, NV; (10) ID, OR, WA. ‡Region 8, after omitting the five SEAs in Utah.
Methods
Sources of data
We obtained age adjusted bladder cancer mortality rates (1970 US standard) for white men and white women for the period 1985–1999, for counties and SEAs, based on data from the Atlas of Cancer Mortality (fig 1A and 1B), updated through 1999.1 There are 504 SEAs in the contiguous United States, each composed of one or more counties within a state with similar economic and cultural characteristics. We conducted the analysis at the SEA level because SEAs have larger populations and more stable mortality rates than individual counties. The median population size of SEAs in 1970 was 231 350 (interquartile range = 144 450−377 400. The mean population size was 379 548 (standard deviation = 644 454).
We obtained water use data for 1970 from the US Bureau of the Census,9 which reports the number of housing units in each county served by public and by private drinking water supplies. Data from 1970 were chosen because it was the first census year to distinguish between private and public supply on a county level, and represents a period at least 15 years before the cancer mortality data used in the analysis. In the United States, and New England specifically, almost all (98%) domestic water supplied by private sources is derived from groundwater obtained from private wells.10 We aggregated county level data to the SEA level. For each SEA, we calculated the proportion of the 1970 housing units using private water supplies under the assumption that the proportion of housing units on private sources in each SEA was a good approximation of the population proportion using this type of water supply. A parallel calculation was made for population density, in this case summing county populations and land areas.
Statistical analyses
We first adjusted the regional mortality rates for the effect of population density at the national level, because population density is positively associated with bladder cancer mortality rates in the United States.2,11,12 We regressed the SEA specific bladder cancer mortality rates (per 100 000) on the logarithms of population density (1970, in persons per square mile) for all SEAs of the contiguous United States. The Pearson correlation coefficient for the association between log population size and bladder cancer rate (1970) was 0.40 for men and 0.35 for women. Subsequent analyses used residuals (observed minus predicted rates) from this regression. Residuals represent bladder cancer rates after adjustment for effects related to population density. Pearson correlation coefficients of bladder cancer mortality rate residuals and the percentage of people served by private wells were then computed within New England and the other US regions, using SEA level data.9
For comparative purposes, we carried out similar analyses for other sites of cancer. We selected several cancers other than the urinary bladder that are related to smoking or associated with drinking water contaminants, as well as cancer sites that have not been associated with these exposures.2,13,14,15
Results
Table 1 shows the Pearson correlation coefficients for bladder cancer mortality rate residuals and percentage private well use. Results for rate residuals for 10 other cancers are also presented (lung, kidney, oesophagus, stomach, colon, rectum, pancreas, leukaemia, prostate, and female breast). Among men, bladder cancer rate residuals were significantly correlated with private well use in New England (region 1), as well as in regions 2 (NY and NJ) and 8 (six Western states). Among women, significant associations for bladder cancer mortality residuals were found in New England and region 2. Lung and kidney cancers in both sexes were positively correlated with private well use within regions 2 and 9, and among men in region 1. The other significant results within New England were a positive association for colon cancer and a negative association for stomach cancer among women. There was also a positive association for colon cancer in both sexes in region 2. Other findings varied. Notable was the lack of association for leukaemia in any region, with the exception of a significant negative finding among women in region 3 and a positive association among men in region 8 (including Utah). In region 8, we found significant positive associations for 9 of 10 cancer sites among men and 4 of 10 among women. Utah, with 5 of the 30 SEAs in region 8, has long been recognised for its low mortality rates for bladder and other cancers, attributable primarily to low prevalence of smoking and drinking among members of the Church of Latter‐day Saints (Mormons).16 All Utah SEAs were below the median for both bladder cancer residual rates and proportion using a private water source. Residual bladder cancer rates in four of five Utah SEAs were negative, and in the remaining Utah SEA, the rate was close to zero. In addition, private well use in Utah is low: In three SEAs, less than 4% of the population used a private water source and less than 15% in the remaining two. Thus, when we omitted the five Utah SEAs from the calculation for region 8, male bladder cancer mortality was not correlated with private well use. Overall, there were only two significant findings among men and none among women.
Discussion
A link between bladder cancer mortality and population density (urbanicity) in the United States was described by Hoover et al12 and by Blot and Fraumeni,11 based on data for 1950–1969. The urban excess of bladder cancer, among men and women, is also evident in more recent data (1970–1994) and in the 1985–1999 data from the Atlas of Cancer Mortality in the United States, 1950–1994, as updated through 1999 and analysed in our study.1 Blot and Fraumeni also saw a rural excess of bladder cancer in the north eastern United States in 1950–1969 that was also apparent in our more recent data.11
After adjusting for the potential influence of population density on bladder cancer mortality rates, we found a significant correlation between bladder cancer mortality and the prevalence of private well use among both men and women in the north east (regions 1 and 2). A significant correlation was also seen among men in region 8 (six Rocky Mountain and northern Great Plains states), which disappeared with the omission of Utah SEAs. These correlations could be related to consumption of ground water from private wells containing increased levels of a bladder cancer carcinogen(s). Alternatively, some other factor that is more prevalent among private well users might pose an increased risk of bladder cancer mortality in these regions.
In the north east, it has been common practice to drill private wells into fractured crystalline bedrock aquifers. The proportion of private wells drilled into bedrock, as compared with unconsolidated surficial deposits, has increased over time, reaching 80% in New England in 1990.7 Although some anthropogenic contaminants such as nitrate are found in bedrock aquifer wells, natural contaminants such as inorganic arsenic, fluoride, iron, manganese, and radionuclides (for example, dissolved radon gas, radium isotopes, and uranium) are more common.17,18 Raised arsenic levels have been observed in private wells in parts of region 1, particularly in northern and eastern New England, where the prevalence of private well use is high relative to the rest of the country.17,19,20
In region 1 (New England), significant positive correlations with private well use were also found for cancers of the lung and kidney (men only). These cancers have been associated with smoking, raising the possibility that smoking, rather than drinking water contamination, is the important underlying factor. This would be plausible if smoking were more common among private well users (that is, rural residents) than people served by public water supplies, specifically in New England. This seems not to be the case, based on data for 615 control subjects from an ongoing population based case‐control study of bladder cancer in New Hampshire showing that smokers tend to live in more densely populated areas than non‐smokers (Karagas, personal communication). We were unable to adjust directly for smoking in our analysis because scale appropriate, sex specific data on cigarette consumption are unavailable. However, the correlation between bladder cancer mortality and intensity of private well use was seen after adjusting for population density, which is a partial surrogate for smoking, based on national data.21,22 Cancers of the bladder, lung, and kidney have been linked to arsenic in drinking water in studies from Taiwan, Chile, Argentina, and elsewhere.23 An interaction between smoking and ingested arsenic in the aetiology of bladder cancer is suggested by several recent studies, and may help explain our findings, even in the absence of increased smoking rates.24,25,26
What this paper adds
Ever since the 1950s, when the National Cancer Institute started enumerating cancer mortality by county in the United States, an excess of bladder cancer mortality has been noted in northern New England. The reasons for this excess are unknown, and are deserving of investigation.
In seeking possible explanations for this phenomenon, in this paper we examined the association between cancer mortality rates in New England (by state economic area, aggregations of similar counties) and the proportion of the population that used private wells. We found a strong correlation between population density adjusted bladder cancer mortality and the extent of private well use in New England among both men and women. This finding is consistent with the notion that inorganic arsenic or other water contaminants found predominantly in private water supplies may be related to the excess cancer mortality noted. For comparative purposes, we also examined the association of private well use with bladder cancer in nine other regions of the United States, as well as associations for 10 other cancer sites in each of the 10 US regions.
In region 2 (New York and New Jersey), the correlation between increased bladder cancer mortality rates and private water supply use is more difficult to interpret. Unlike region 1, region 2 has a smaller proportion of private supply users than the country overall (13% compared with 18%).9 However, there is an excess of bladder cancer mortality as in northern New England. Kidney, lung, and colon cancers were also significantly correlated with private well use in both men and women in region 2. The factor(s) underlying these associations are unclear.
In region 8, mortality rates for 9 of 10 cancer sites among men and 4 of 10 among women were significantly associated with private well use. Region 8 includes the state of Utah. Utah is unusual in having the lowest cancer mortality rates, for many anatomic sites, of any state in the United States, because of the religious prohibition of tobacco and alcohol use among its predominantly Morman population.16 Private well use in the Utah SEAs happens also to be low. Inclusion of the Utah SEAs in the calculation for region 8 thus resulted in strong positive, but probably spurious, associations with private well use. When data from the five SEAs of Utah were omitted from the region 8 calculation, only two cancer sites among men, and none among women, were significantly associated with private well use, suggesting that inclusion of the Utah SEAs, with their unusually low rates and low private well use, indeed resulted in specious findings.
In summary, we found positive significant associations between excess mortality from bladder cancer and private well use within US regions 1, 2 (both sexes), and 8 (men only, including Utah data) after adjustment for population density. Whether these correlations are attributable to the presence of a bladder cancer carcinogen(s) in private wells in these regions, or to another factor correlated with private well use, cannot be determined from this ecological study. Private well use by SEA is a crude indicator of exposure and it is not possible to identify the specific contaminant in well water (for example, inorganic arsenic, manganese, radionuclides, nitrate) that may be related to bladder cancer mortality, or to exclude the influence of an unknown correlate. Our study shares limitations common to ecological studies,27 particularly our inability to directly control for the potential confounding effect of smoking. This study is based on mortality, not incidence, and access to medical care could be an issue. Individual information is needed to fully evaluate the effects of drinking private well water and other risk factors on bladder cancer risk in northern New England. Ongoing studies in New Hampshire and in three New England states (Maine, New Hampshire, Vermont) are designed to evaluate whether consumption of private well water contributes to the excess bladder cancer mortality in northern New England, and if so, to identify the specific carcinogens responsible.28,29
A colour version of the figure is available on line (http://www.jech.com/supplemental).
Supplementary Material
Footnotes
Funding: this research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Division of Cancer Epidemiology and Genetics.
Conflicts of interest: none declared.
A colour version of the figure is available on line (http://www.jech.com/supplemental).
References
- 1.Devesa S S, Grauman D J, Blot W J.et al Atlas of cancer mortality in the United States: 1950–1994: http://www3.cancer.gov/atlasplus (updated using data through 1999 from the US National Center for Health Statistics) Bethesda, MD: National Institutes of Health, National Cancer Institute, 1999
- 2.Silverman D T, Morrison A S, Devesa S S. Bladder Cancer. In: Schottenfeld D, Fraumeni JF Jr , eds. Cancer epidemiology and prevention. 2nd ed. New York: Oxford University Press, 19961156–1179.
- 3.Shopland D R, Hartman A M, Gibson J T.et al Cigarette smoking among US adults by state and region: estimates from the current population survey. J Natl Cancer Inst 1996881748–1758. [DOI] [PubMed] [Google Scholar]
- 4.The Tobacco Institute The tax burden on tobacco: historical compilation. New York: Wiley, 1994
- 5.Brown L M, Zahm S H, Hoover R N.et al High bladder cancer mortality in rural New England (United States): an etiologic study. Cancer Causes Control 19956361–368. [DOI] [PubMed] [Google Scholar]
- 6.Hoar S K, Hoover R. Truck driving and bladder cancer mortality in rural New England. J Natl Cancer Inst 198574771–774. [PubMed] [Google Scholar]
- 7.US Census Bureau Historical census of housing tables: sources of water. Washington: US Census Bureau, 1999
- 8.Executive Office of the President, United States Office of Management and Budget Standard Federal Regions, Circular No. A‐105. 4‐4‐1974. Washington, DC
- 9.USCensus Bureau Census of housing: 1970. Vol 1. Housing characteristics for states, cities, and counties. Parts 2‐52. Washington: US Census Bureau, 1972
- 10.Solley W B, Merk C F, Pierce R R. Estimated use of water in the United States in 1985. Reston, VA: US Geological Survey, 198882
- 11.Blot W J, Fraumeni J F., Jr Geographic patterns of bladder cancer in the United States. J Natl Cancer Inst 1978611017–1023. [PubMed] [Google Scholar]
- 12.Hoover R N, Mason T J, McKay F W.et al Geographic patterns of mortality in the United States. In: Fraumeni JFJ, ed. Persons at high risk of cancer: an approach to cancer etiology and control. New York: Academic Press, 1975343–360.
- 13.Smith A H, Goycolea M, Haque R.et al Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol 1998147660–669. [DOI] [PubMed] [Google Scholar]
- 14.Wu M M, Kuo T L, Hwang Y H.et al Dose‐response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol 19891301123–1132. [DOI] [PubMed] [Google Scholar]
- 15.Villanueva C M, Cantor K P, Cordier S.et al Disinfection byproducts and bladder cancer: a pooled analysis. Epidemiology 200415357–367. [DOI] [PubMed] [Google Scholar]
- 16.Merrill R M, Lyon J L. Cancer incidence among Mormons and non‐Mormons in Utah (United States) 1995–1999. Prev Med 200540535–541. [DOI] [PubMed] [Google Scholar]
- 17.Ayotte J D, Nielsen M G, Robinson G R., Jret alRelation of arsenic, iron, and manganese in ground water to aquifer type, bedrock lithogeochemistry, and land use in the New England Coastal Basins. Reston, VA: US Geological Survey, 199930
- 18.Morrissey D J. New Hampshire ground‐water quality. In: Moody DW, Carr J, Chase EB, et al, eds. National Water Summary 1986: hydrologic events and ground water quality. Reston, VA: US Geologic Survey, 1988363–368.
- 19.Ayotte J D, Montgomery D L, Flanagan S M.et al Arsenic in groundwater in eastern New England: occurrence, controls, and human health implications. Environ Sci Technol 2003372075–2083. [DOI] [PubMed] [Google Scholar]
- 20.Peters S C, Blum J D, Klaue B.et al Arsenic occurrence in New Hampshire drinking water. Environ Sci Technol 1999331328–1333. [Google Scholar]
- 21.Goldsmith J R. Lung cancer's persistent association with population density in Los Angeles County communities. Public Health Rev 199119147–161. [PubMed] [Google Scholar]
- 22.Walter S D, Meigs J W, Heston J F. The relationship of cancer incidence to terrestrial radiation and population density in Connecticut, 1935–1974. Am J Epidemiol 19861231–14. [DOI] [PubMed] [Google Scholar]
- 23.National Research Council Subcommittee on arsenic in drinking water Arsenic in drinking water. Washington, DC: National Academy Press, 1999
- 24.Bates M N, Smith A H, Cantor K P. Case‐control study of bladder cancer and arsenic in drinking water. Am J Epidemiol 1995141523–530. [DOI] [PubMed] [Google Scholar]
- 25.Bates M N, Rey O A, Biggs M L.et al Case‐control study of bladder cancer and exposure to arsenic in Argentina. Am J Epidemiol 2004159381–389. [DOI] [PubMed] [Google Scholar]
- 26.Steinmaus C, Yuan Y, Bates M N.et al Case‐control study of bladder cancer and drinking water arsenic in the western United States. Am J Epidemiol 20031581193–1201. [DOI] [PubMed] [Google Scholar]
- 27.Piantadosi S, Byar D P, Green S B. The ecological fallacy. Am J Epidemiol 1988127893–904. [DOI] [PubMed] [Google Scholar]
- 28.Karagas M R, Tosteson T D, Blum J.et al Design of an epidemiologic study of drinking water arsenic exposure and skin and bladder cancer risk in a US population. Environ Health Perspect 1998106(suppl 4)1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karagas M R, Tosteson T D, Morris J S.et al Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire. Cancer Causes Control 200415265–272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.